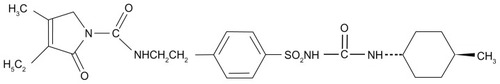
Abstract
Type 2 diabetes mellitus is characterized by insulin resistance and progressive β cell failure; therefore, β cell secretagogues are useful for achieving sufficient glycemic control. Glimepiride is a second-generation sulfonylurea that stimulates pancreatic β cells to release insulin. Additionally, is has been shown to work via several extra pancreatic mechanisms. It is administered as monotherapy in patients with type 2 diabetes mellitus in whom glycemic control is not achieved by dietary and lifestyle modifications. It can also be combined with other antihyperglycemic agents, including metformin and insulin, in patients who are not adequately controlled by sulfonylureas alone. The effective dosage range is 1 to 8 mg/day; however, there is no significant difference between 4 and 8 mg/day, but it should be used with caution in the elderly and in patients with renal or hepatic disease. In clinical studies, glimepiride was generally associated with lower risk of hypoglycemia and less weight gain compared to other sulfonylureas. Glimepiride use may be safer in patients with cardiovascular disease because of its lack of detrimental effects on ischemic preconditioning. It is effective in reducing fasting plasma glucose, post-prandial glucose, and glycosylated hemoglobin levels and is a useful, cost-effective treatment option for managing type 2 diabetes mellitus.
Introduction
Diabetes is a major public health problem affecting 285 million people worldwide.Citation1 The prevalence of diabetes is projected to double globally by 2030.Citation2 Complications of diabetes include renal failure, neuropathy and peripheral vascular disease with potential for loss of limbs, retinopathy with increased risk of blindness, and an increased risk of cardiovascular disease and stroke, which are related to poorly controlled diabetes.Citation3 Good glycemic control can prevent or delay chronic disease-related microvascular complications as shown by the United Kingdom Prospective Diabetes Study (UKPDS) and the landmark Diabetes Control and Complications Trial.Citation4,Citation5
The pathophysiology of type 2 diabetes mellitus (T2DM) is characterized by relative decrease in insulin secretion and/or insulin resistance. Insulin resistance is a complex phenomenon exacerbated by obesity, particularly central obesity, and is believed to start at a young age because hyperinsulinemia is observed in preteens when both parents have diabetes.Citation6
T2DM results in progressive loss of insulin secretion and the UKPDS showed that ≥50% loss of β cells had occurred by the time of diagnosis; therefore, β cell secretagogues are useful for achieving sufficient glycemic control.Citation7,Citation8
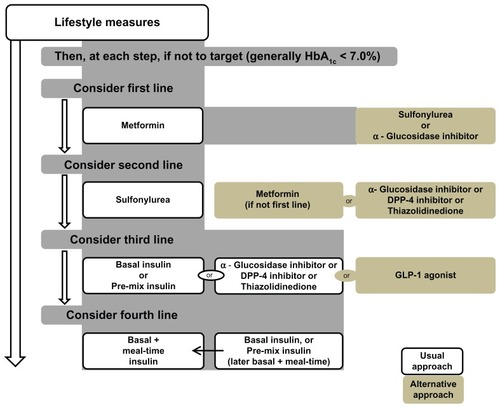
The American Diabetes Association/European Association for the study of Diabetes presented a consensus algorithm for managing T2DM () based on expected glycosylated hemoglobin (HbA1c) levels.Citation9 International Diabetes Federation guidelines for the management of T2DM also recommend lifestyle modifications in the initial stages, and addition of metformin or sulfonylurea is then recommended if additional therapy is required ().Citation10
Figure 1 Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association and the European Association for the Study of Diabetes. © 2012, Springer Science and Business Media. Reproduced with kind permission from Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596.Citation9
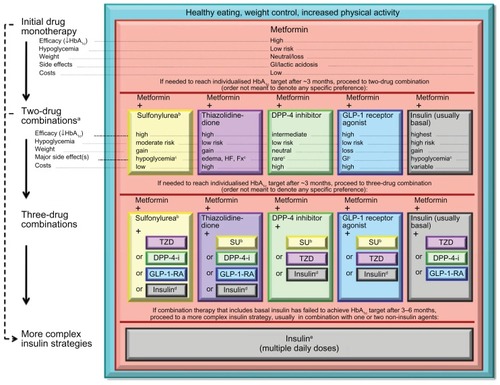
Figure 2 International Diabetes Federation treatment algorithm for people with type 2 diabetes.
© 2005, International Diabetes Federation. Reproduced with kind permission from International Diabetes Federation Clinical Guidelines Task Force. Global guidelines for type 2 diabetes. 2005. Available from: http://www.idf.org/Global_guideline. Accessed on March 29, 2012.Citation10
The goals of pharmacologic therapy in diabetes are to achieve good glycemic control while avoiding hypoglycemia and weight gain so as to decrease the risk of future micro- and macrovascular complications.
Clinicians have a choice of a number of available glucose-lowering agents for managing T2DM which have been shown to be effective and well-tolerated in clinical practice ().
Table 1 Comparison of oral hypoglycemic agents used in the management of type 2 diabetes mellitus
Methodology
The primary objective of this review is to assess the efficacy and safety of glimepiride, primarily from a clinical viewpoint, while considering clinically relevant end points.
A MEDLINE database search (January 1994 to December 2011) was performed to identify relevant published articles, including reviews and abstracts evaluating glimepiride for treating patients with T2DM (). Data from animal studies were also included if human data were not available. Reference lists of identified articles were also consulted. An occasional systematic review article and/or meta-analysis summarizing numerous clinical trials were selected for summarizing key data. Pharmacology information was taken from representative original articles.
Table 2 Pharmacokinetic properties of glimepiride
Initially, all articles mentioning the drug glimepiride were considered for review. Forty-five clinical trials were used to describe the clinical efficacy and safety of glimepiride. Clinical studies were selected for analysis based on methodological quality, appropriate study design, and publication of results.
Introduction of compounds
Sulfonylureas (SUs) are widely used in the management of T2DM as insulin secretagogues and are named for their common core configuration. They are classified as first- and secondgeneration SUs. First-generation SUs include long-acting chlorpropamide, tolbutamide, tolazamide, and acetohexamide. Substitutions at either end of the compound result in pharmacologic and pharmacokinetic differences among SUs.Citation11
Second-generation SUs include glyburide (glibenclamide), glipizide, gliquidone, and glimepiride, which vary in duration of action. Glimepiride and glyburide are longer-acting agents than glipizide. Glimepiride is the newest second-generation SU and is sometimes classified as a third-generation SU because it has larger substitutions than other second-generation SUs (). It was first introduced into clinical practice in Sweden. The United States Food and Drug Administration (FDA) approved glimepiride in 1995 for the treatment of T2DM as monotherapy as well as in combination with metformin or insulin.
Although other SUs are used with insulin, glimepiride is the only SU approved by FDA for use in combination with insulin. It is used in more than 60 countries worldwide. Treatment with glimepiride as monotherapy results in a 1.5%–2.0% reduction in HbA1c.Citation11,Citation12 Pharmacokinetic properties of glimepiride are shown in .
Pharmacodynamics
Pancreatic effects
Glimepiride acts at ATPase-dependent potassium channels in β cells of the pancreas to stimulate insulin release.Citation14 using euglycemic and hyperglycemic clamp studies it has been shown to improve both first- and second-phase insulin secretion.Citation15
Glimepiride binds to 65-kD proteins on β cells. In healthy volunteers, a linear relationship was shown between serum glimepiride concentrations and insulin release during euglycemia and a nearly linear relationship under hyperglycemic conditions.Citation16,Citation17
Maximal glucose-lowering activity and insulin level in T2DM patients is achieved within 2–3 hours of taking glimepiride and can last for 24 hours.Citation16 In a 14-week clinical study, peak concentrations 2 hours after administration of 1, 4, and 8 mg doses of glimepiride were associated with decreases in median fasting plasma glucose (FPG) of 43, 70.5, and 74 mg/dL, respectively.Citation12
Glimepiride reduces blood glucose levels and increases insulin levels in blood. A 3-day study of 14 T2DM patients found greater reductions in blood glucose (4.1 vs 1.9 mmol/L) and increase in C-peptide (1.8 vs 1.4 mg/L) and plasma insulin (41 vs 25 mu/L) with 2 mg/day glimepiride compared to placebo (P < 0.05).Citation18
Hypoglycemia after exercise while taking glimepiride was observed in 167 patients with T2DM.Citation19 This was associated with a greater reduction in insulinemia than glibenclamide during exercise, despite similar reductions in blood glucose.
Glimepiride may be taken before or after breakfast with similar results. The efficacy of 2 mg/day glimepiride for 2 weeks on blood glucose levels was not significantly different over a period of 0–4 hours when the drug was given either immediately before breakfast or 30 minutes after breakfast.Citation20
Extrapancreatic effects
The extrapancreatic effects of glimepiride are similar to those of other sulfonylureas. Although peripheral tissue response to insulin is potentiated like other SUs, the clinical relevance of this is not yet clear.Citation21,Citation22 In in vitro studies, glimepiride was found to be two times as potent as glibenclamide in stimulating lipogenesis and glycogenesis.Citation23 Studies in cultured skeletal muscle also suggest a sensitizing effect of glimepiride.Citation24 Possible mechanisms include promotion of GLUT4 transport protein activation and/or translocation in fat and muscle.Citation16,Citation22 Glimepiride reduced insulin resistance and increased hepatic glucose disposal in animal models, but showed no effect in glucose utilization in patients with type 1 diabetes.Citation25
Cardiovascular effects
Glimepiride appears to cause fewer cardiovascular effects than other SUs.Citation16 It was found to be associated with few cardiac changes, fewer ventricular arrhythmias, and little or no effect on blood pressure compared to glyburide and glipizide in animal studies.Citation23 The exact mechanism of this difference in cardiovascular activity is not clear; however, involvement of adenosine triphosphate-sensitive potassium (KATP) channels are thought to play an important role.Citation24,Citation25
Table 3 Comparative efficacy of glimepiride in patients with type 2 diabetes
Unlike other SUs, glimepiride does not impair ischemic preconditioning of cardiac myocytes. Ischemic preconditioning is an adaptive phenomenon which occurs in response to an ischemic event and delays infarct development during subsequent ischemic episodes, which may help limit tissue damage.Citation26 The postulated mechanism involves selective interaction of glimepiride with sacrolemmal ATP dependent potassium channels in cardiac myocytes rather than mitochondrial channels.Citation27 Evidence suggests that glimepiride preserves myocardial preconditioning, a protective mechanism that limits damage in the event of an ischemic event.Citation14
Data from animal studies suggests that the effects of glimepiride on KATP channels, cardiac vessels, or blood vessels were insignificant compared to that caused by the same dosage of glyburide.Citation28 Similarly, glimepiride has less of an effect in promoting ST segment elevation, enhancing coronary resistance and reducing coronary blood flow compared to glyburide or gliclazide.Citation29
Thus, using glimepiride may be safer than other SUs in cardiac patients due to its lack of detrimental effects on cardiac preconditioning.Citation26
Clinical efficacy
The drug has been assessed in placebo-controlled studies as monotherapy and compared with other SUs and insulin in T2DM patients. Most studies examined FPG, post-prandial glucose (PPG), and HbA1c. Some studies included plasma lipids, serum insulin, or fasting C-peptide levels.
Glimepiride as monotherapy
To assess the efficacy of glimepiride in T2DM, Goldberg et al randomized 304 patients to receive either placebo or one of the three doses (1, 4, or 8 mg) of glimepiride during a 14-week study period.Citation29 All glimepiride regimens significantly reduced FPG, PPG, and HbA1c values (P < 0.001) compared to placebo by the end of the study period. Median changes in FPG levels were 43, 70, and 74 mg/dL at glimepiride doses of 1, 4, and 8 mg, respectively. HbA1c levels were lowered by 1.2%, 1.8%, and 1.9%, and the corresponding decreases in PPG were 63, 92, and 94 mg/dL, respectively. The 4- and 8-mg doses of glimepiride were more effective than the 1-mg dose; however, the 4-mg dose provided a nearly maximal antihyperglycemic effect.
Another study showed equal effects on FPG, PPG, HbA1c, C-peptide, and insulin levels in a cross-over study of 98 patients treated with glimepiride.Citation31 The only significant difference was observed in glucose levels throughout the day, which were lower with a once daily dose compared to a twice daily dosage. The opposite results were observed by Rosenstock et alCitation31 who found a significant decrease in FPG by 0.6 mmol/L with glimepiride when it was given twice daily compared to once daily dosage.
Another multicenter, randomized, placebo-controlled clinical trial by Schade et al studied glimepiride (1–8 mg) titrated over 10 weeks compared with placebo in T2DM subjects who were not controlled by diet alone.Citation32 In this study, glimepiride lowered FPG by 46 mg/dL, PPG by 72 mg/dL, and HbA1c by 1.4% more than the placebo (P < 0.001). Good glycemic control (HbA1c < 7.2%) was achieved in 69% of glimepiride subjects compared to 32% of controls. C-peptide levels and non-fasting insulin levels were also increased in the study subjects.
Glimepiride monotherapy reduced both FPG and PPG levels more than placebo and once daily administration is equivalent to twice daily dosing. Studies also suggest that glimepiride controls blood glucose level throughout the day through its effect on stimulating insulin release, which appears to be greater 2 h after meals than under fasting conditions. These findings suggest that glimepiride enhances insulin and C-peptide secretion under physiologic conditions.
Combination therapy for treating T2DM is now a recommended practice as the disease progresses.Citation10,Citation33 Several studies have examined the combination of glimepiride with other oral hypoglycemic agents with different mechanisms of action for good glycemic control when monotherapy fails.Citation33–Citation35
In a study involving 372 patients with poorly controlled T2DM, glimepiride was added to metformin monotherapy. Study subjects were divided into three groups: metformin group, glimepiride group, metformin plus glimepiride group. In this study, a combination of glimepiride and metformin was shown to be more effective for controlling blood glucose levels compared to the use of either drug alone.Citation33
Combination treatment was significantly more effective in controlling HbA1c (% change +0.07 ± 1.20 for metformin, +0.27 ± 1.10 for glimepiride, −0.74 ± 0.96 for combination treatment, P < 0.001). No significant difference was observed between metformin or glimepiride monotherapy with respect to change in HbA1c or fasting blood glucose; however, glimepiride was significantly more effective than metformin in reducing postprandial blood glucose. Episodes of symptomatic hypoglycemia was also higher in the combination group than in either monotherapy group (P = 0.039).
Comparison with thiazolidinediones
Combination therapy with rosiglitazone plus glimepiride versus rosiglitazone plus placebo was evaluated in a multicenter, double-blind, placebo-controlled study.Citation34 A target HbA1c of <7% was achieved in the glimepiride group and no significant difference was observed in adverse events between the two groups. Metformin plus glimepiride versus metformin plus pioglitazone was studied in another study by Umpierrez.Citation35 In both treatment groups, a similar decrease in mean HbA1c (P = 0.000) and FPG (P < 0.05) compared to baseline was observed; however, a more rapid decline in HbA1c levels (P < 0.05) was achieved with glimepiride (80–90 days) compared to pioglitazone (140–150 days). The study concluded that in poorly controlled T2DM patients on metformin monotherapy, addition of glimepiride was associated with faster glycemic control, lower total cholesterol, and low-density lipoprotein (LDL) as well as reduced short-term health care costs compared to the addition of pioglitazone, which was associated with a higher rate of peripheral edema (4% vs 1% with glimepiride).
Comparison with other sulfonylureas
Glimepiride has been compared to other SUs, including glibenclamide, glipizide, and gliclazide in several clinical trials.
Glimepiride 1–8 mg/day was found to be as effective as glibenclamide 1.26–20 mg/day in lowering FPG, PPG, and HbA1c. Dills et al evaluated the efficacy of glimepiride (≤16 mg) and glyburide (≤20 mg) as monotherapy in 577 patients with T2DM.Citation36 There was no significant glycemic difference between FPG, PPG, or HbA1c in both study groups after the 1-year treatment period. However, the incidence of hypoglycemia was lower with glimepiride (1.7%) than with glibenclamide (5.0%) (P < 0.015).
Another multicenter, prospective, double-blind study comparing glimepiride (1 mg daily, n = 524) and glibenclamide (2.5 mg daily, n = 520) by Draeger et al showed similar results.Citation37 Glimepiride provided equal glycemic control compared to glyburide, with mean FPG and HbA1c of 174 mg/dL and 8.4% for glimepiride and 168 mg/dL and 8.3% for glibenclamide. Additionally, in this study, glimepiride caused fewer hypoglycemic symptoms compared to glibenclamide. Glimepiride was associated with significantly smaller increases in fasting insulin (P = 0.04) and C-peptide (P = 0.03) concentrations than glyburide. In this trial, 11% of glimepiride-treated patients experienced 105 hypoglycemic episodes, and 14% of the glibenclamide treated patients experienced 150 such episodes.Citation16
Schernthaner et al compared once daily gliclazide MR and glimepiride in patients with T2DM.Citation38 In this double-blind, 27-week parallel group study, 845 subjects were randomized to either gliclazide modified release (MR) 30–120 mg daily or glimepiride 1–16 mg daily as monotherapy or in combination with their current treatment (metformin or α glucosidase inhibitor). Efficacy was evaluated based on HbA1c and safety by hypoglycemic episodes using the European Agency definition. HbA1c decreased similarly in both groups from 8.4% to 7.2% in patients on gliclazide MR and from 8.2% to 7.2% in patients receiving glimepiride. The study concluded that glimepiride is as effective as gliclazide MR either as monotherapy or in combination therapy; however, the safety of gliclazide MR was significantly better in terms of hypoglycemic episodes compared with glimepiride.Citation38 Another study using glimepiride or metformin as monotherapy observed changes in serum sialic acid in patients with T2DM over a period of 12 months. The study concluded that there were no statistically significant differences between groups.Citation39
Glimepiride in combination with insulin
Patients who fail to achieve good glycemic control on combination therapy may require insulin.Citation40 Glimepiride is the only SU currently approved by the FDA for combination therapy with insulin. Several studies have demonstrated that a combination of insulin and glimepiride results in a decreased requirement of insulin and good glycemic control.Citation38–Citation42
In a 24-week study of obese patients not adequately controlled by maximum doses of SUs, addition of insulin was compared to insulin + placebo.Citation41 Subjects were randomized to receive insulin and either glimepiride 16 mg/day or placebo, and the insulin dosage was titrated to achieve FPG of 100–120 mg/dL. The two groups showed similar HbA1c and FPG at the end of the study period. However, the group receiving insulin + glimepiride required less insulin (48 vs 78 U/day) and FPG was lowered more rapidly after 2 and 4 weeks of treatment than in the insulin/placebo group.Citation41 Thus, insulin sparing properties are greater with glimepiride than with other SUs.
Another study conducted in 695 poorly controlled patients with T2DM assessed the safety and efficacy of glimepiride with NPH or glargine. Patients were divided into three groups to receive bedtime NPH, bedtime glargine, or morning glargine for 24 weeks in addition to 3 mg of glimepiride. HbA1c improvement was observed more with morning insulin glargine than with NPH insulin (P = 0.001) or bedtime insulin glargine (P = 0.008). The study concluded that the risk for nocturnal hypoglycemia was lower with glimepiride in combination with morning and bedtime insulin glargine than with glimepiride in combination with bedtime NPH insulin.Citation43
Combination of glimepiride with dipeptidyl peptidase-4 inhibitors
Recently, several new classes of hypoglycemic agents have been introduced, including glucagon like peptide-1 and dipeptidyl peptidase-4 (DDP-4) inhibitors. These agents improved glycemic control in T2DM patients either as monotherapy or in combination with SU, metformin, thiazolidinedione, or insulin.Citation44–Citation46 Glimepiride can be used in combination with metformin and DDP-4 inhibitors if glycemic control is not achieved with a single or with two agents (). Studies have reported an equal efficacy for glimepiride plus metformin vs vildagliptin/sitagliptin plus metformin in terms of HbA1c reduction.Citation47–Citation49
Although DDP-4 induces less weight gain and hypoglycemia compared to glimepiride, further long-term follow-up studies are needed to determine their safety and efficacy.
Advantages of glimepiride compared to other SUs
Hypoglycemia and weight gain are two important disadvantages of SU therapy; however, the unique properties of glimepiride may provide advantages over other currently available insulin secretagogues.
Glimepiride is generally well-tolerated, and its safety has been reviewed in various randomized clinical studies involving more than 5000 patients. Data from these clinical trials indicate that the overall incidences of adverse events associated with glimepiride are generally lower compared with other SUs.Citation15,Citation16,Citation36,Citation37,Citation50
Hypoglycemia
Severe hypoglycemia is a potentially life-threatening condition and is typically associated with SUs; however, glimepiride differs from older agents in this class, as it is associated with equivalent metabolic control and lower stimulation of insulin secretion.
In a prospective analysis, frequency of severe hypoglycemia with glimepiride was compared with glibenclamide in T2DM patients.Citation51 In this 4-year population-based study, blood glucose levels of all 30,768 patients who attended the emergency department of the region’s central hospital were determined to identify severe hypoglycemia, which was defined as blood glucose level of <2.8 mmol/L or a requirement for intravenous glucose or glucagon injection.
The results showed that although glimepiride was prescribed more frequently than glibenclamide (6976 vs 6789 persons-years), glimepiride induced fewer episodes of hypoglycemia compared to glibenclamide (6 vs 38 episodes). The study concluded that in routine clinical practice, glimepiride is associated with fewer episodes of severe hypoglycemia; the risk can be minimized if individual targets are determined before prescribing this medicine. Glimepiride has been shown to induce a statistically significant decrease in C-peptide and insulin levels compared with glibenclamide, which may explain the reduction of hypoglycemia during and after physical exercise;Citation52 however, the risk of hypoglycemia is increased with concomitant use of other antihyperglycemic agents. Similarly, advanced age, renal, hepatic, and/or cardiovascular comorbidities may increase hypoglycemia risk; this drug should be used with caution in these patients.Citation53
Weight gain
Most patients with T2DM are overweight.Citation54 In these patients, weight reduction results in considerable improvements in their clinical and metabolic profiles, including HbA1c. Weight gain is considered a disadvantage of SUs, thiazolidinediones, and insulin; however, studies suggest that glimepiride has a weight-neutral effect on patients with T2DM.Citation55,Citation56
Several observational cohort studies have shown considerable weight loss with glimepiride. In one study, an average weight loss of 3 kg was reported after 1–5 years of glimepiride,Citation56 while in another study, treatment with glimepiride resulted in weight loss of up to 2.2 kg within 8 weeks.Citation55
The effects of glimepiride or glibencalmide treatment on body weight in patients with T2DM were observed over a 12-month period in a retrospective observational cohort study.Citation57 In this study, mean weight loss and reduction in body mass index from baseline to the end of the study period were greater with glimepiride compared to glibenclamide ([−2.01 ± 4.01 kg/−0.7 ± 1.4 kg/m2] vs [−0.58 ± 3.7 kg/−0.2 ± 1.3 kg/m2]; P < 0.001). The study concluded that initial treatment of T2DM with glimepiride was associated with a significantly greater decrease in body weight and body mass index than treatment with glibenclamide, while providing equivalent glycemic control.Citation57
Weight gain associated with therapies for managing T2DM is an important consideration in clinical practice and a major limitation in achieving good glycemic control. Glimepiride differs from other agents in this class in that it is associated with equivalent metabolic control with weight-neutral effects on patients with T2DM.
The exact mechanism of the weight-neutral effects of glimepiride has not been established; however, lower stimulation of insulin secretion in response to glimepiride compared to other SUs have been implicated.Citation52,Citation58,Citation59 Additionally, glimepiride has many extra-pancreatic glucose-lowering effects,Citation52,Citation59,Citation60 including decreased endogenous glucose production as well as improved peripheral glucose uptake.Citation21 These effects may explain the weight loss or weight neutrality associated with glimepiride use.
Dosage and administration
The starting dose of glimepiride is 1–2 mg typically taken before breakfast. The dose is adjusted according to self-monitoring of blood glucose levels and is gradually increased until glycemic control is achieved. The maximum recommended dosage is 8 mg/day,Citation61 although doses up to 32 mg/day have been used in clinical trials. Typical maintenance dosages are 1–4 mg/day. However, higher dosages (6–8 mg/day) have been found to be associated with reduced mean HbA1c before and after treatment.62 It may also be combined with other treatment modalities for T2DM, including insulin in patients who are not controlled with SUs. However, the combination of insulin and glimepiride requires a lower initial dose of insulin.Citation63
Glimepiride in special situations
Glimepiride appears to be well-tolerated in patients with T2DM, including the elderly. However, it should be used cautiously in elderly, debilitated or malnourished patients. Although it can be used in renal insufficiency, patients should be monitored for signs and symptoms of hypoglycemia and lower doses of glimepiride should be used in these situations.
Conclusion
Glimepiride is a second-generation sulfonylurea which can be used as monotherapy or in combination with other antihyperglycemic agents, including insulin. It is the only SU currently recommended for use with insulin. The safety and efficacy of glimepiride has been confirmed in various controlled studies and it is associated with a lower risk of hypoglycemia and weight gain compared to other SUs.
Glimepiride is effective in reducing FPG, PPG, and HbA1c levels and is a useful, cost-effective treatment option for managing T2DM.
Acknowledgments
We acknowledge the support of Getz Pharma (Pvt) Ltd, for providing educational support to the research department of Baqai Institute of Diabetology and Endocrinology (BIDE).
Disclosure
The authors have no conflicts of interest to declare.
References
- SchwatzPDiabetes Prevention in PracticeDresden, GermanyWCPD2010
- WildSRoglicGGreenASicreeRKingHGlobal prevalence of diabetes: estimates for the year 2000 and projections for 2030Diabetes Care20042751047105315111519
- World Health OrganizationFact sheet number 312: Diabetes. Media centre fact sheet2008 Available from: http://www.who.int/mediacentre/factsheets/fs312/en/Accessed on March 29, 2012
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318378539742976
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research GroupN Engl J Med1993329149779868366922
- FujimotoWYBergstromRWLeonettiDLNewell-MorrisLLShumanWPWahlPWMetabolic and adipose risk factors for NIDDM and coronary disease in third-generation Japanese-American men and women with impaired glucose toleranceDiabetologia19943755245328056192
- RobertsonRPPorteDJrThe glucose receptor a defective mechanism in diabetes mellitus distinct from the beta adrenergic receptorClin Invest1973524870876
- ReavenGMBanting lecture 1988. Role of insulin resistance in human diseaseDiabetes19883712159516073056758
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetologia20125561577159622526604
- International Diabetes Federation Clinical Guidelines Task ForceGlobal guidelines for type 2 diabetes2005 Available from: http://www.idf.org/Global_guidelineAccessed on March 29, 2012
- ShuklaUAChiEMLehrKHGlimepiride pharmacokinetics in obese versus non-obese diabetic patientsAnn Pharmacother2004381303514742789
- Massi-BenedettiMGlimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experienceClin Ther200325379981612852703
- KorytkowskiMThomasAReidLTedescoMBGoodingWEGerichJGlimepiride improves both first and second phases of insulin secretion in type 2 diabetesDiabetes Care20022591607161112196435
- CampbellRKGlimepiride: role of a new sulfonylurea in the treatment of type 2 diabetes mellitusAnn Pharmacother19983210104410529793597
- RosenkranzBPharmacokinetic basis for the safety of glimepiride in risk groups of NIDDM patientsHorm Metab Res19962894344398911979
- GoldbergRBHolveySMSchneiderJThe Glimepiride Protocol #201 Study Group. A dose response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agentsDiabetes Care199619847856
- Wernicke-PantenKHauptEPfeifferCEarly onset of pharmacodynamic effects of glimepiride in type II diabetic patients [abstract]Diabetologia199437Suppl 1A163
- Massi-BenedettiMHerzMPfeifferCThe effects of acute exercise on metabolic control in type II diabetic patients treated with glimepiride or glibenclamideHorm Metab Res1996284514558911982
- RosskampRHerzMEffect of the time of ingestion of the sulfonylurea glimepiride on the daily blood glucose profile in NIDDM patients [abstract]15th Int Diab Fed Congr1994416
- OverkampDVolkAMaerkerEAcute effect of glimepiride on insulin-stimulated glucose metabolism in glucose-tolerant insulin-resistant offspring of patients with type 2 diabetesDiabetes Care200225112065207312401758
- MüllerGThe molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug AmarylMol Med200061190793311147570
- MüllerGWiedSWetekamEMCreceliusAUnkelbachAPünterJStimulation of glucose utilization in 3T3 adipocytes and rat diaphragm in vitro by the sulphonylureas, glimepiride and glibenclamide, is correlated with modulations of the cAMP regulatory cascadeBiochem Pharmacol19943048985996
- VéghAPappJGHaemodynamic and other effects of sulphonylurea drugs on the heartDiabetes Res Clin Pract1996Suppl 31S43538864640
- MüllerGWiedSStraubJJungCCoordinated regulation of esterification and lipolysis by palmitate, H2O2 and the anti-diabetic sulfonylurea drug, glimepiride, in rat adipocytesEur J Pharmacol20085971–361818789917
- BriscoeVJGriffithMLDavisSNThe role of glimepiride in the treatment of type 2 diabetes mellitusExpert Opin Drug Metab Toxicol20106222523520055691
- MocanuMMMaddockHLBaxterGFLawrenceCLStandenNBYellonDMGlimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxideCirculation2001103253111311611425777
- LangtryHDBalfourJAGlimepiride: a review of its use in the management of type 2 diabetes mellitusDrugs19985545635849561345
- GeisenKVeghAKrauseEPappJGCardiovascular effects of conventional sulfonylureas and glimepirideHorm Metab Res1996284965078911987
- GoldbergRBHolveySMSchneiderJA dose-response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agents. The Glimepiride Protocol #201 Study GroupDiabetes Care19961988498568842603
- SonnenbergGEGargDCWeidlerDJShort-term comparison of once - versus twice-daily administration of glimepiride in patients with non-insulin-dependent diabetes mellitusAnn Pharmacother1997316716769184703
- RosenstockJSamolsEMuchmoreDBSchneiderJGlimepiride, a new once-daily sulphonylurea. a double-blind placebo-controlled study of NIDDM patients. Glimepiride Study GroupDiabetes Care199619119411998908379
- SchadeDSJovanovicLSchneiderJA placebo-controlled, randomized study of glimepiride in patients with type 2 diabetes mellitus for whom diet therapy is unsuccessfulJ Clin Pharmacol19983876366419702849
- CharpentierGFleuryFKabirMVaurLHalimiSImproved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patientsDiabet Med2001181082883411678974
- McCluskeyDTougerMSMelisRSchleusenerDSResults of a randomized, double-blind, placebo-controlled study administering glimepiride to patients with type 2 diabetes mellitus inadequately controlled with rosiglitazone monotherapyClin Ther200426111783179015639690
- UmpierrezGIssaMVlajnicAGlimepiride versus pioglitazone combination therapy in subjects with type 2 diabetes inadequately controlled on metformin monotherapy: results of a randomized clinical trialCurr Med Res Opin200622475175916684436
- DillsDGSchneiderJClinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. Glimepiride/Glyburide Research GroupHorm Metab Res19962894264298911977
- DraegerKEWernicke-PantenKLompHJSchulerERosskampRLong-term treatment of type 2 diabetic patients with the new oral antidiabetic agent glimepiride (Amaryl): a double-blind comparison with glibenclamideHorm Metab Res19962894194258911976
- SchernthanerGGrimaldiADi-MarioUGUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patientsEur J Clin Invest20043453554215305887
- RahmanIUMalikSABashirMKhanRUIdreesMMonotherapy with metformin or glimepiride and changes in serum sialic acid in type 2 diabetes mellitusBrit J Diab Vas Dis2011113137140
- GarberAJBenefits of combination therapy of insulin and oral hypoglycemic agentsArch Intern Med2003163151781178212912710
- RiddleMCCombined therapy with a sulfonylurea plus evening insulin: safe, reliable, and becoming routineHorm Metab Res1996284304338911978
- RiddleMCSchneiderJBeginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Glimepiride Combination GroupDiabetes Care1998217105210579653594
- FritscheASchweitzerMAHäringHUGlimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trialAnn Intern Med20031381295295912809451
- SchneiderJAn overview of the safety and tolerance of glimepirideHorm Metab Res1996284134188911975
- HolsteinAPlaschkeAEgbertsEHLower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamideDiabetes Metab Res Rev200117646747311757083
- MüllerGThe molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug AmarylMol Med200061190793311147570
- HeineRJRole of sulfonylureas in non-insulin-dependent diabetes mellitus: part II – ‘the cons’Horm Metab Res1996285225268911991
- TrembleJMDonaldsonDIs continued weight gain inevitable in type 2 diabetes mellitus?J R Soc Promot Health199911923523910673844
- ScholzGSchneiderKKnirschWBeckerGEfficacy and tolerability of glimepiride in daily practice: a non-interventional observational cohort studyClin Drug Invest2001219597604
- WeitgasserRLechleitnerMLugerAKlinglerAEffects of glimepiride on HbA1c and body weight in type 2 diabetes: results of a 1.5-year follow-up studyDiabetes Res Clin Pract200361131912849919
- MartinSKolbHBeuthJvan LeendertRSchneiderBScherbaumWAChange in patients’ body weight after 12 months of treatment with glimepiride or glibenclamide in type 2 diabetes: a multicentre retrospective cohort studyDiabetologia200346121611161714600814
- DraegerKWernicke-PantenKLompHJSchulerERosskampRLong-term treatment of type 2 diabetic patients with the new oral antidiabetic agent glimepiride (Amaryl): a double-blind comparison with glibenclamideHorm Metab Res1996284194258911976
- MüllerGHartzDPünterJOkonomopulosRKramerWDifferential interaction of glimepiride and glibenclamide with the beta-cell sulfonylurea receptor. I. Binding characteristicsBiochim Biophys Acta199411912672778172912
- MüllerGSatohYGeisenKExtrapancreatic effects of sulfonylureas – a comparison between glimepiride and conventional sulfonylureasDiabetes Res Clin Pract1995Suppl 28S1151378529504
- Hoechst-Roussel Pharmaceuticals, Inc Amaryl (glimepiride) prescribing informationSomerville, NJHoechst-Roussel Pharmaceuticals, Inc1995
- HydrieMZIGulAHakeemRAhmadaniMYBasitAGlimepiride study on type-2 diabetic subjectsPak J Med Sci200622132135
- BugosCAustinMAthertonTViereckCLong-term treatment of type 2 diabetes mellitus with glimepiride is weight neutral: a meta-analysisDiabetes Res Clin Pract200050Suppl 1S4711080562
- RosenstockJFitchetMVildagliptin: clinical trials programme in monotherapy and combination therapy for type 2 diabetesInt J Clin Pract Suppl2008159152318269437
- GarberAJFoleyJEBanerjiMAEffects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylureaDiabetes Obes Metab200810111047105618284434
- FonsecaVBaronMShaoQDejagerSSustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitusHorm Metab Res20084042743018401832
- MatthewsDRDejagerSAhrenBVildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year studyDiabetes Obes Metab20101278078920649630
- DejagerSRazacSFoleyJESchweizerAVildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose studyHorm Metab Res20073921822317373638
- JeonHJOhTKComparison of Vildagliptin-metformin and glimepiridemetformin treatments in type 2 diabetic patientsDiabetes Metab J20113552953522111045