Abstract
Objective
Although international guidelines recommend initiating antiretroviral therapy (ART) when a patient’s CD4 cell count is ≤350 cells/μL, most patients in resource-limited settings present with much lower CD4 cell counts. The lowest level that their CD4 cell count reaches, the nadir, may have long-term consequences in terms of mortality. We examined this health state in a large cohort of HIV+ patients in Uganda.
Design
This was an observational study of HIV patients in Uganda aged 14 years or older, who were enrolled in 10 major clinics across Uganda.
Methods
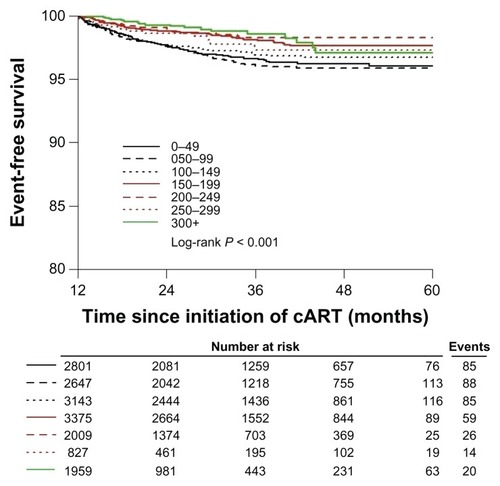
We assessed the CD4 nadir of patients, using their CD4 cell count at initiation of ART, stratified into categories (,50, 50–99, 100–149, 150–249, 250+ cells/μL). We constructed Kaplan–Meier curves to assess the differences in survivorship for patients left-censored at 1 year and 2 years after treatment initiation. We used Cox proportional hazards regression to model the associations between CD4 nadir and mortality. We adjusted mortality for loss-to-follow-up.
Results
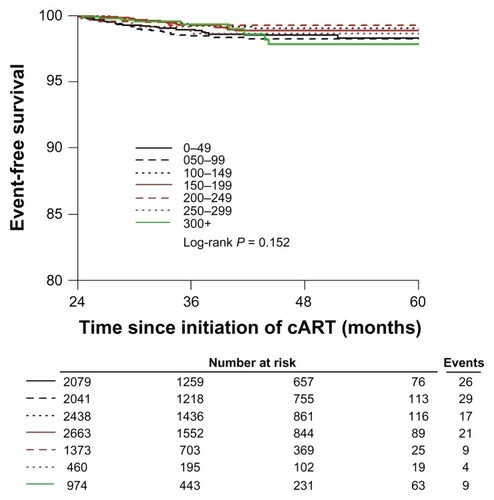
Of 22,315 patients, 20,129 patients had greater than 1 year of treatment follow-up. Among these patients, 327 (1.6%) died and 444 (2.2%) were lost to follow-up. After left-censoring at one year, relative to lowest CD4 strata, patients with higher CD4 counts had significantly lower rates of mortality (CD4 150–249, hazard ratio [HR] 0.60, 95% confidence interval [CI]: 0.45–0.82, P = 0.001; 250+, HR 0.66, 95% CI, 0.44–1.00, P = −0.05). Male sex, older age, and duration of time on ART were independently associated with mortality. When left-censoring at 2 years, CD4 nadir was no longer statistically significantly associated with mortality.
Conclusion
After surviving for 1 year on ART, a CD4 nadir was strongly predictive of longer-term mortality among patients in Uganda. This should argue for efforts to increase engagement with patients to ensure a higher CD4 nadir at initiation of treatment.
Introduction
CD4 T-cell status is a strong prognostic indicator of mortality and disease progression among individuals infected with HIV.Citation1–Citation3 CD4 cell status strongly correlates with World Health Organization (WHO) disease staging.Citation4 Ascertaining CD4 status is now recommended as guidance for determining when to begin patients on antiretroviral therapy (ART).Citation5 In 2010, WHO issued guidance to resource-constrained settings to expand the eligibility of the treated population, by recommending initiation of ART when a patient’s CD4 T-cell count reached 350/μL or less, or was clinically necessitated.Citation6 Most countries in resource-limited settings aim to deliver care at ≤350 cells, yet the average CD4 cell count that patients actually initiate therapy at, is typically well below 200 cells/μL.Citation7
Although patients who survive the initial period of most severe disease and possible immune reconstitution inflammatory syndrome are expected to have a nearly normal life after initiation of ART,Citation8–Citation10 progressive damage to a patient’s immune system that occurred prior to accessing ART may have an important long-term effect on mortality. Many clinicians recognize the risk of increased mortality among patients with low CD4 status in the first several months of ART, but whether a low CD4 nadir (the lowest point a CD4 count achieves) is predictive of mortality after stabilization on ART is less clear. In 1999, an early EUROSida study demonstrated the importance of CD4 nadir in predicting longer-term negative outcomes.Citation11 However, a larger study from North America and Europe using the Antiretroviral Cohort Collaboration (ART–CC) showed that mortality among patients with differing CD4 nadirs made no significant difference once a patient stabilized.Citation10 A study we previously conducted in Uganda found that after 6 months on ART, patients with lower baseline CD4 counts had significantly worse outcomes than patients whose baseline count was higher.Citation12 We aimed to examine mortality after longer periods of ART treatment and examined differences between patients after 1 and 2 years on treatment.
Methods
Setting
Our study used data collected by The AIDS Support Organization (TASO). TASO provides clinical care, psychosocial support, and antiretroviral therapy to individuals with HIV at 11 major clinical sites and 35 smaller clinics throughout Uganda, involving both urban and rural populations. TASO began providing widespread combination antiretroviral therapy in 2004 with resource support from the US President’s Emergency Plan for AIDS Relief, and more than 24,000 patients are currently receiving this treatment. Criteria for initiation of antiretroviral therapy include a diagnosis of WHO stage 3 or 4, or a CD4 cell count < 350 cells/μL.Citation13 Patients initiating antiretroviral therapy typically receive a nonnucleoside reverse transcriptase inhibitor with first-line treatment comprising nevirapine, lamivudine, and stavudine; second-line therapy is comprised of boosted lopinavir, didanosine and zidovudine.Citation14,Citation15
Cohort characteristics
The cohort has been described in detail previously.Citation16 Briefly, detailed demographic information, clinical characteristics, and treatment information are routinely collected on standardized forms at each patient visit. These data are entered into a centralized clinical database at each clinic. Upon enrolment at TASO, each patient is provided with a unique coded identification number. For this study, we included all patients ≥ 14 years of age who initiated antiretroviral therapy at TASO clinics in Uganda between January 1, 2000 and February 1, 2010. Patients were followed until either time of confirmed death or end of the study period (February 1, 2010). The following pertinent patient information was recorded: age at the start of antiretroviral therapy, sex, CD4 count history, WHO clinical disease stage, loss from follow-up (a 3-month absence from a clinic), date of last visit, and date of death (where applicable).
Analysis
We used parametric testing to assess differences between patient clinical status and demographics among patients receiving less than or more than, 1 year of treatment. We considered patients’ lowest monitored CD4 count to be their nadir. We stratified patients’ CD4 nadirs according to the following categories <50, 50–99, 100–149, 150–249, 250+ cells/μL. We then calculated survival probabilities based on the CD4 nadir and date of death using a Kaplan–Meier plot for both the 1 year and 2 year periods postinitiation and compared these using the log-rank test. Patients who were lost to follow-up were censored at the date they were last seen at the clinic, and a weighted analysis was applied, whereby 30% of patients lost to follow-up were assumed dead, weighted by lower CD4, age, and male sex.Citation17,Citation18 Survival times were expressed in months. We used unadjusted and adjusted Cox proportional hazards regressions to express the magnitude of association between CD4 nadir and the probability of survival after 1 year of ART, while adjusting for age, sex, and WHO clinical disease stage.Citation19 Hazard proportionality was assessed by analysis of scaled Schoenfeld residuals. To compensate for missing baseline CD4 nadirs, we conducted analyses using the multiple imputation method.Citation20 All significance tests were two-sided with a P-value of <0.05. All analyses were conducted using SAS software (version 8; SAS Institute, Cary, NC).
Institutional review
Approval to conduct this study was received from the administrative headquarters ethics board of TASO Uganda, and the Research Ethics Boards of the University of Ottawa and the University of British Columbia in Canada.
Results
Patient demographics
Of the 22,315 patients ≥ 14 years of age in the TASO program between 2000 and 2010, 20,129 (90.2%) patients had 1 or more years of follow-up and were included in this study. Their characteristics are summarized in . Patients were followed for a median period of 33 months (Interquartile range [IQR], 23–47) and the majority, 70.3%, were female. The median patient age was 37 years (IQR, 31–43) and the median CD4 cell count was 147 cells/μL (IQR, 77–209) with 71.4% of patients having CD4 cell counts below 200 cells/μL at the initiation of treatment. Most patients, 56.3% and 33.3% were classified into WHO disease stage II or III, respectively.
Table 1 Characteristics of included patients
Mortality
The majority of deaths (78%) occurred in the first year of treatment and were therefore excluded from our survival analyses. Of patients with >1 year of follow up, 327 of patients died (1.6%) and 444 patients (2.2%) were lost to follow-up. shows a Kaplan-Meier graph that projects the survival of patients on ART with >1 year of follow-up, with different baseline CD4 cell count ranges. Baseline CD4 counts of 200–249 cells/μL proved to be the best initial count for a higher probability of survival (HR 0.60, 95% CI: 0.45–0.82, P = 0.001). This was no longer statistically significant after left-censoring for 2 years of treatment ().
Regression analysis
displays the unadjusted and adjusted Cox proportional hazard models for patients with 1 or more years of follow-up. The adjusted model indicates that relative to the lowest CD4 strata, CD4 cell count nadir is independently associated with mortality. Male sex and older age are also important predictors of mortality.
Table 2 Unadjusted and adjusted Cox proportional hazard models of patients with 1+ years follow up (with assumption that 30% of lost to follow-up were deaths)
Discussion
Our study examined the prognostic value of CD4 nadir after 1 and 2 years of ART in HIV-infected patients in Uganda. Our study demonstrates that after 1 year on ART, a CD4 nadir continues to strongly predict mortality. Among patients who have survived 2 years on ART, CD4 nadir was no longer statistically significant. The reasons for death after stabilization on ART are poorly understood in resource-limited settings, but are not limited to occurrence of AIDS and may include early presentation of chronic diseases, exacerbated by HIV infection.
Strengths of our analysis include our nationally representative sample that comprised a diverse population of patients likely to be found in other parts of Africa, such as adolescents and elderly patients, patients suffering from conflict and food insecurity, and patients who switched treatment after initiation. Our loss of patients from follow-up was low compared with most AIDS service organizations in Africa, where loss to follow-up can exceed 50%Citation21,Citation22 due to our use of default tracers. Nevertheless, we recognized that there may have been misclassification of deaths among those lost to follow-up and we attempted to correct for this bias by applying an assumption that 30% of these patients were deceased, based on findings from our own previous tracking study, and a similar analysis at a relevant local Ugandan setting.Citation18,Citation23 We further weighted this assumption, to reflect older patients and those with lower last CD4 counts.Citation24 The fact that our analysis includes a relatively small number of patients initiating therapy at high CD4 levels (.350 cells/μL) will likely have reduced our estimates at the very highest CD4 baseline status. About 10% of individuals did not have baseline CD4 evaluations. This is common in programs across Africa, where patients may be initiated due to clinical circumstances or poor laboratory infrastructure. We explored the impact of these individuals on our overall analysis and did not find a different effect. We imputed their probable CD4 nadirs. TASO does not conduct routine viral load assessments and therefore we cannot make inferences about risks of nadir on viral load status. Finally, as with any observational study, our mortality rate may be subject to residual confounding beyond those confounders adjusted for in multivariate analysis.
Our findings are an extension of a previous analysis where we examined mortality in patients who survived the first 6 months on treatment. That study indicated that baseline CD4 was predictive of mortality even after survival for 6 months.Citation12 Our current study displayed that the CD4 nadir was predictive of mortality among those with at least 1 year of treatment but not more than 2 years of treatment. This finding is not surprising as we would expect that patients who have survived greater than 2 years would have a better long-term expected survival rate than those at the early stages of treatment, as these patients have survived the period where most death occurs (ie, the first few months of therapy)Citation25 and are more likely to have adjusted to treatment in terms of adherence patterns and involvement with treatment supporters.Citation26,Citation27
Consistent with previous studies from Africa, we found that mortality varied according to sex. This finding builds on an emerging body of literature displaying consistent shortcomings in treatment programs involving men. Men are less likely to access antiretroviral treatment, usually will start treatment with more advanced disease, have higher rates of early mortality, and are more likely to be lost to follow-up.Citation28–Citation30
Unfortunately, accessing and treating patients at early stages can be a challenge for both health infrastructure, and identifying patients. In Uganda, the ministry of health guidelines recommend treatment of patients with ART when a CD4 nadir reaches below < 350 cells/μLCitation13 and yet the median CD4 of patients initiating treatment within our cohort in 2009 was far lower, at 156 cells/μL. There is a clear need to increase the identification of infected individuals while their CD4 status is high so that they can be engaged in care and treatment initiated as early as possible. Strategies to engage patients at an early stage include home-based testing campaigns and campaigns targeting specific groups, including men.Citation31,Citation32 Residual benefits of increasing early access to ART include economic benefits, increased life expectancy, and a decreased likelihood of transmission of the virus to sexual partners.Citation33,Citation34
In conclusion, our study demonstrates that in Uganda, a patient’s CD4 nadir is strongly predictive of longer-term mortality even after 1 year of treatment. Efforts to increase access to care for patients before their immune status becomes depleted continue to represent an important challenge that has long-term repercussions for patients.
Acknowledgments
The Canadian Institutes of Health Research (CIHR) funded this study. TASO receives core funding from the US Presidents Emergency Plan for AIDS Relief.
Disclosure
The authors declare no conflicts of interest in this work.
References
- HoggRSYipBChanKJRates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapyJAMA2001286202568257711722271
- SterneJAMayMCostagliolaDfor When To Start ConsortiumTiming of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studiesLancet200937396721352136319361855
- KitahataMMGangeSJAbrahamAGfor NA-ACCORD InvestigatorsEffect of early versus deferred antiretroviral therapy for HIV on survivalN Eng J Med20093601818151826
- World Health OrganizationWHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children2007 Available from: http://www.who.int/hiv/pub/vct/hivstaging/en/index.htmlAccessed June 10, 2012
- ThompsonMAAbergJACahnPfor International AIDS Society-USAAntiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panelJAMA2010304332133320639566
- WHOAntiretroviral therapy for HIV infection in adults and adolescentsRecommendations for a public health approach: 2010 revision Available at http://www.who.int/hiv/pub/arv/adult2010/en/2010Accessed June 14, 2012
- KeiserOTweyaHBraitsteinPfor ART-LINC of IeDEA Study GroupMortality after failure of antiretroviral therapy in sub-Saharan AfricaTrop Med Int Health201015225125820003034
- MillsEBakandaCBirungiJLife expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from UgandaAnn Intern Med2011155420921621768555
- JaffeHWDe StavolaBLCarpenterLMPorterKCoxDRfor CASCADE CollaborationImmune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adultsAids201125111395140321572307
- ChêneGSterneJAMayMfor Antiretroviral Therapy Cohort CollaborationPrognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studiesLancet2003362938567968612957089
- MillerVMocroftAReissPRelations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA studyAnn Intern Med1999130757057710189326
- MillsEJBakandaCBirungiJYayaSFordNfor the TASO-CAN Writing GroupThe prognostic value of baseline CD4 cell count beyond 6 months of antiretroviral therapy in HIV-positive patients in a resource-limited settingAids201226111425142922526520
- Ministry of HealthNational Antiretroviral Treatment and Care Guidelines for Adults and ChildrenKampala, UgandaEarnest Publishers2010
- MillsEJBakandaCBirungiJYayaSFordNThe prognostic value of baseline CD4 cell count beyond 6 months of antiretroviral therapy in a resource-limited settingAids201226111425142922526520
- KibonekaANyatiaRNabiryoCCombination antiretroviral therapy in population affected by conflict: outcomes from large cohort in northern UgandaBMJ2009338b20119223338
- BakandaCBirungiJNkoyooyoACohort Profile: The TASO-CAN Cohort CollaborationInt J Epidemiol372011 [Epub ahead of print.]
- GengEHEmenyonuNBwanaMBGliddenDVMartinJNSampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in AfricaJAMA2008300550650718677022
- AmuronBNamaraGBirungiJMortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in UgandaBMC Public Health2009929019671185
- AllisonPDSurvival Analysis Using SAS: A Practical GuideCary, NCSAS Institute Inc1995
- YuanYCMultiple Imputation for Missing Data: Concepts and New Development (Version 9.0) Available from http://analysis3.com/Multiple-Imputation-for-Missing-Data-Concepts-and-New-Development-pdf-e4976.pdfAccessed July 15, 2012
- RosenSFoxMPGillCJPatient retention in antiretorviral therapy programs in sub-Sarahan Africa: a systematic reviewPLoS Med2007410e29817941716
- BrinkhofMWPujades-RodriguezMEggerMMortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysisPLoS One2009446e579019495419
- GengEHEmenyonuNBwanaMBGliddenDVMartinJNSampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in AfricaJAMA2008300550650718677022
- EggerMSpycherBSidleJCorrecting mortality for loss to follow up: a graphical approach applied to art programmes in resource-limited settings5th IAS Conference on HIV Pathogenesis, Treatment and preventionJuly 19–22, 2009Cape Town, South Africa Poster #WEPED173
- BraitsteinPBrinkhofMWDabisFfor Anteretroviral Therapy in Lower Income Countries (ART-LINC Collaboration; ART Cohort Collaboration (ART-CC) groupsMortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countriesLancet2006367951381782416530575
- FordNDarderMSpelmanTMacleanEMillsEBoulleAEarly adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow up of an observational cohortPLoS One201055e1046020485480
- ChangLWKagaayiJNakigoziGEffect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trialPLoS One201056e1092320532194
- BraitsteinPBoulleANashDfor Antiretroviral Therapy in Lower Income Countries (ART-LINC) study groupGender and the use of antiretroviral treatment in resource-constrained settings: findings from a multcenter collaborationJ Womens Health (Larchmt)2008171475518240981
- MuulaASNgulubeTJSiziyaSGender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic reviewBMC Public Health200776317459154
- MillsEJFordNMugyenyiPExpanding HIV care in Africa: making men matterLancet2009374968627527619632481
- WachiraJKimaiyoSNdegeSMamlinJBraitsteinPWhat is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya?Clin Infect Dis201254227528122156847
- BraitsteinPBoulleANashDfor Antiretroviral Therapy in Lower Income Countries (ART-LINC) study groupGender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaborationJ Womens Health (Larchmt)2008171475518240981
- CohenMSChenYQMcCauleyMfor HPTN 052 Study TeamPrevention of HIV-1 infection with early antiretroviral therapyN Engl J Med2011365649350521767103
- LarsonBAFoxMPRosenSDo the socioeconomic impacts of antiretroviral therapy vary by gender? A longitudinal study of Kenyan agricultural worker employment outcomesBMC Public Health2009924019604381