Abstract
Access to antiretroviral therapy (ART) is improving worldwide. Immune reconstitution inflammatory syndrome (IRIS) is a common complication of ART initiation. In this review, we provide an overview of clinical and epidemiological features of HIV-associated IRIS, current understanding of pathophysiological mechanisms, available therapy, and preventive strategies. The spectrum of HIV-associated IRIS is described, with a particular focus on three important pathogen-associated forms: tuberculosis-associated IRIS, cryptococcal IRIS, and Kaposi’s sarcoma IRIS. While the clinical features and epidemiology are well described, there are major gaps in our understanding of pathophysiology and as a result therapeutic and preventative strategies are suboptimal. Timing of ART initiation is critical to reduce IRIS-associated morbidity. Improved understanding of the pathophysiology of IRIS will hopefully enable improved diagnostic modalities and better targeted treatments to be developed.
Introduction
Antiretroviral therapy (ART) has dramatically reduced HIV-associated mortality, which decreased from a peak of 2.3 million in 2005 to 1.6 million in 2012.Citation1–Citation3 This reflects improvement in access to ART in the last decade, especially for HIV-infected patients in low- and middle-income countries, where the number of patients receiving ART has increased more than 30-fold (from 300,000 in 2002 to 9.7 million in 2012) and life expectancy is increasing.Citation3,Citation4 It is now recognized that commencement of ART earlier in HIV infection improves outcomes, and international guidelines have been updated to reflect this.Citation5 Tuberculosis (TB) is now considered to be an indication for ART irrespective of CD4 count, as ART reduces mortality in TB patients.Citation6
However, ART initiation is not without risk of complications, particularly in the first 6 months.Citation7,Citation8 HIV-associated immune reconstitution inflammatory syndrome (IRIS) has emerged as an important early complication of ART initiation, associated with considerable morbidity and mortality, particularly in patients who commence ART with advanced immunosuppression.Citation9 In this condition, immune recovery following ART initiation associates with a pathological inflammatory response, usually directed toward microbial antigens. Although there is considerable clinical and pathophysiological heterogeneity, key features include clinical deterioration in the first weeks to months of ART, with evidence of localized tissue inflammation with or without a systemic inflammatory response.
In this review, we provide an overview of clinical and epidemiological features of HIV-associated IRIS, current understanding of pathophysiological mechanisms, available therapy, and preventive strategies, with a particular focus on three important pathogen-associated forms: TB-associated IRIS (TB-IRIS), cryptococcal IRIS (C-IRIS), and Kaposi’s sarcoma (KS) IRIS. A key message is that while IRIS- associated morbidity may be considerable, ART is key to survival in HIV and timing of initiation of ART is critical. Rarely should ART be interrupted or discontinued because of IRIS.
Our discussion is limited to HIV-associated forms of IRIS. However, IRIS has been described following reversal of other forms of immunosuppression, including after reversal of iatrogenic immunosuppression in transplant recipients, following bone marrow recovery after chemotherapy for hematological malignancies, and following discontinuation of anti-tumor necrosis factor-α (TNF-α) therapy for rheumatoid arthritis or treatment with the monoclonal antibody natalizumab for multiple sclerosis.Citation10–Citation13
Historical perspective and case definitions
Among the first accounts of IRIS-type phenomena were reports of zidovudine-induced fever associated with lymphadenitis in patients with nontuberculous mycobacterial infections.Citation14,Citation15 French et al reported a case series of unusually localized Mycobacterium avium-intracellulare (MAI) infections presenting with fevers and lymphadenitis without mycobacteraemia, which developed soon after commencement of zidovudine monotherapy.Citation16 These presentations were accompanied by the emergence of delayed-type hypersensitivity reactivity to purified protein derivative (PPD) in patients who had previously been PPD unresponsive.
IRIS has emerged as a highly heterogeneous condition, with features differing according to the associated pathogen. However, two distinct temporal patterns of disease were commonly described and are now recognized as “paradoxical IRIS” and “unmasking IRIS” (see ).Citation17–Citation19 In paradoxical forms of IRIS, symptoms and signs associated with a known opportunistic infection (OI), for which treatment is under way, recur or become acutely worse, despite an earlier favorable response to therapy prior to ART. In unmasking IRIS, a new OI presents with a pronounced inflammatory component following ART initiation. Responding to clinical and research needs, the International Network for the Study of HIV-associated IRIS (INSHI) published consensus case definitions for C-IRIS and TB-IRIS.Citation18,Citation19 Such definitions are suitable for use in low-resource settings, as CD4 count and HIV viral load responses are not included as criteria. Consensus case definitions for other forms of IRIS are lacking although much needed.Citation20 The main challenge with all proposed definitions of paradoxical IRIS is the requirement to adequately exclude other causes of clinical deterioration. There is no definitive diagnostic test for IRIS. In resource-constrained settings, clinicians may find themselves treating multiple conditions concurrently in a sick patient, being uncertain of the definitive diagnosis due to limited laboratory support, with IRIS as a diagnosis of exclusion following unsuccessful treatment of other conditions.
Figure 1 Schematic demonstrating sequence of key events in paradoxical immune reconstitution inflammatory syndrome (IRIS) (A) and unmasking IRIS (B).
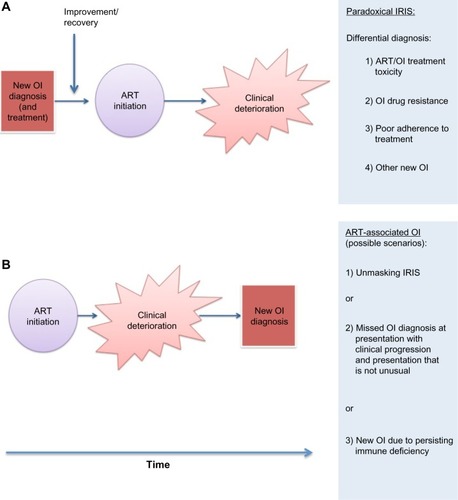
The concept of unmasking IRIS is less well defined than that of paradoxical IRIS. The broader term of an “ART-associated OI” is proposed to encompass all OI diagnosed during early ART, as unmasking IRIS may be difficult to differentiate from development of an OI in a patient who is still immunocompromised during early ART and which progresses along a typical clinical course (see ). Some recent reports have defined all new OI in the first 6 months of ART as cases of unmasking IRIS.Citation21,Citation22 This approach may reduce comparability with earlier studies and increase heterogeneity of IRIS cases, complicating efforts to precisely define IRIS immunopathology.Citation23
Epidemiology and risk factors
Numerous infective and noninfective conditions are associated with IRIS in HIV infection (see ).Citation15,Citation24–Citation34 A meta-analysis of 54 studies (published between 1998 and 2009) of 13,103 HIV-infected patents starting ART, reported 1,699 (13%) cases of IRIS.Citation9 The incidence was slightly higher (16.1%) in studies of unselected HIV-infected patients, and reported incidence varied widely depending on study design, population, and associated pathogen. For example, 37.7% of patients with a diagnosis of cytomegalovirus (CMV) retinitis prior to ART initiation developed IRIS, compared to 6.4% patients with a diagnosis of KS.Citation9
Table 1 Pathogens and key clinical features of associated IRIS
The epidemiology of IRIS reflects the epidemiological distribution of HIV-associated OI and the prevalence of various key risk factors in a given population. reports the proportion of IRIS attributable to different OI/inflammatory conditions in recently published studies of unselected patients commencing ART, demonstrating that reported incidence varies according to geographic region and by study design.Citation7,Citation21,Citation35–Citation38 Risk factors for IRIS include an advanced state of immunosuppression (low CD4 count) and high infective antigen burden/disseminated OI at ART initiation (see ). These characterize a substantial proportion of patients with newly diagnosed HIV in developing countries where suboptimal access to HIV care and health services in general, and stigma associated with HIV, contribute to late presentation.Citation39 While advanced immunosuppression and late presentation are also encountered in patients presenting to ART services in higher-resource settings, in low-resource settings IRIS incidence and associated mortality appear to be higher.Citation8,Citation40
Table 2 Recently published studies reporting IRIS incidence rates in unselected cohorts
Table 3 Risk factors for HIV-associated IRIS
Overall mortality in IRIS is reported to be between 0% and 15%, with variability attributed to geography, associated OI, baseline morbidity, and degree of immunosuppression.Citation9,Citation40,Citation41 IRIS affecting the central nervous system (CNS) confers a particularly high mortality. In patients with cryptococcal meningitis (CM)-associated IRIS, mortality is reported at 20.8% and CNS TB-IRIS mortality rates are up to 75%.Citation9,Citation42,Citation43 Where space is limited around a critical organ, such as the brain, excess inflammation with associated cerebral edema has severe effects. Establishing an accurate cause of death and correctly attributing it to IRIS is difficult in many circumstances and reported mortality rates may therefore be under- or overestimates. High rates of mortality occur in the first 6 months of ART in resource-limited settings, even in patients without an IRIS diagnosis. There is difficulty determining from available data sources what the exact contribution of IRIS to these deaths is.Citation8,Citation38,Citation44
Pathophysiology
In both C-IRIS and TB-IRIS, at the time of IRIS onset, elevated concentrations of proinflammatory mediators, including C-reactive protein (CRP) and cytokines (eg, interleukin [IL]-6, IL-12, TNF-α) are detectable in serum and may also be elevated in cerebrospinal fluid (CSF) in CM-IRIS and TB meningitis (TBM) IRIS.Citation45–Citation49 A proinflammatory cytokine cascade may be a final common pathway by which IRIS inflammation occurs.Citation45,Citation50
The increased incidence of IRIS in patients with lower pre-ART CD4 counts and disseminated OI suggests that more advanced immunodeficiency prior to ART initiation may lead to a higher pathogen load, resulting in excessive inflammation, once the immune system starts to recover. For example, individuals who develop C-IRIS have significantly reduced CSF inflammation during the initial episode of CM compared to non-IRIS patients (lower CSF white cell count, interferon-γ [IFN-γ], IL-6, IL-8, and TNF-α) and higher pre-ART serum cryptococcal antigen titers.Citation46,Citation49 In TBM-IRIS, CSF culture positivity for Mycobacterium tuberculosis (M.tb) at TBM diagnosis confers a ninefold greater risk of IRIS, compared to those with culture-negative TBM, also suggesting that antigen load at OI diagnosis is important. However, in TBM, prior to ART initiation, higher TNF-α levels and raised CSF neutrophil counts were observed in IRIS patients compared to those that did not develop IRIS, suggesting that a pro- rather than anti-inflammatory milieu precedes TBM IRIS onset.Citation51
As ART initiation leads to a rapid increase in peripheral blood CD4 T lymphocyte count in most patients, the recovery of pathogen-specific cell-mediated immune responses has been studied in TB and other OI, in HIV-infected patients following ART initiation. In HIV-infected patients, increased CD4 Th1 responses to mycobacterial antigens have been reported following ART initiation.Citation16,Citation52–Citation55 Studies indicate these increased responses associate with IRIS.Citation16,Citation52,Citation53,Citation55 However, a detailed longitudinal study of CD4 T-cell responses to a range of M.tb recombinant antigens found that highly dynamic IFN-γ responses occurred in both TB-IRIS patients and patients who did not develop IRIS and did not clearly differentiate the two groups.Citation54 This finding has been supported by the results of two further studies of Th1 responses to mycobacterial antigens in TB-IRIS, which also call into question a causal link.Citation56,Citation57
While a disturbance of regulatory T-cell number or function could explain excessive inflammation, this has not been convincingly demonstrated. A few studies have demonstrated an increased rather than decreased number of regulatory CD4 T-cells in mycobacterial and C-IRIS.Citation54,Citation58,Citation59 Reduced IL-10 has been associated with IRIS in some studies, suggesting that regulatory function may be impaired.Citation58,Citation60 However, a recent comparison of 20 TB-IRIS patients and 20 non-IRIS control patients found increased IL-10 concentrations in serum of TB-IRIS patients, and increased IL-10 transcript in peripheral blood mononuclear cells of TB-IRIS patients compared to controls, after restimulation with M.tb.Citation61 One study demonstrated reduced numbers of inhibitory natural killer (NK) receptors on mycobacteria-specific Vδ2 TCRγδ T-cells.Citation53 Further studies of regulatory cell types and function in IRIS are required.
Barber et al (studying a murine model of MAI-IRIS) argue that because IRIS is not specific to CD4 T-cell depletion in HIV (and occurs following reversal of HIV-unrelated immunosuppression, eg, post-TNF-α treatment), it is unlikely that CD4 T-cell responses are the central contributory factor.Citation62 Rather, they propose that an uncoupling of innate and adaptive immunity is responsible. They hypothesize that in HIV infection, CD4 deficiency and thus deficiency of CD4 co-stimulation impairs full activation of innate immune cells, particularly macrophages in TB and MAI infection. The resultant antigen accumulation and excessive priming of innate immune cells lead to an excessive inflammatory response, once activation does occur following immune restoration.Citation62
Given their importance in antigen processing and pathogen trafficking, cells of the innate immune system such as monocytes, macrophages, and neutrophils are of increasing interest in IRIS pathophysiology. Favoring a role for innate immunity is the formation of organized tissue granulomas in IRIS (such as granulomatous hepatitis in TB-IRIS). Granulomatous inflammation, which is notably absent in untreated advanced HIV, suggests enhanced macrophage activity during immune reconstitution.Citation63 A fatal case of unmasking TB-IRIS associated with a pronounced macrophage-dominated pulmonary infiltrate on postmortem has been reported.Citation64 Suppurative inflammation may develop in IRIS, which suggests heightened neutrophil activity.Citation60,Citation65 Several studies have reported increased inflammatory cytokines and chemokines of myeloid origin present at, and prior to, IRIS development, evident in serum and on restimulation of cells ex vivo with antigen.Citation45,Citation66,Citation67 A cross-sectional study of peripheral blood mononuclear cell responses reported elevated matrix metalloproteinase (MMP) transcripts in TB-IRIS patients compared to controls, accompanied by elevated serum concentrations of MMP-7.Citation68 MMPs are host enzymes that are upregulated in response to TB infection, mainly myeloid and epithelial cell derived and activated by proinflammatory cytokines.Citation69–Citation72 They are capable of breaking down and remodeling extracellular matrix, and may contribute to tissue damage in IRIS.
Two studies have examined NK cell function in TB-IRIS. In a study of unmasking IRIS, these cells were found to express increased activation markersCitation73 In a longitudinal study, NK cells isolated from paradoxical TB-IRIS patients had higher expression of CD107a, a degranulation marker, than non-IRIS controls, prior to IRIS onset.Citation74 The authors hypothesized that increased NK cell-mediated lysis of M.tb-infected cells may increase antigen load.Citation74
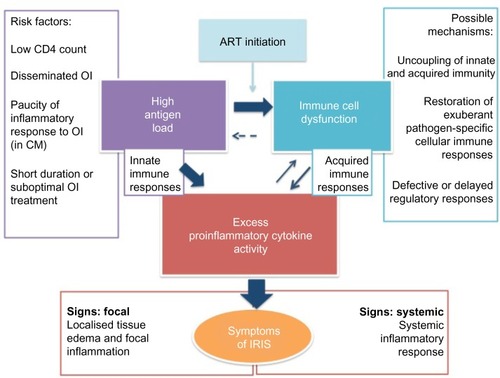
In summary, recent evidence suggests that innate immune dysfunction in the context of high antigen load plays a role in driving pathological proinflammatory responses in IRIS. The role of pathogen-specific cell-mediated immunity and regulatory mechanisms are less clear (see ). Studies of patients are frequently limited by small sample size and examination of peripheral blood rather than tissue immune responses. Development of animal models of IRIS may better allow dissection of precise pathophysiological mechanisms, which may differ for paradoxical and unmasking IRIS, and for different forms of pathogen-associated IRIS.Citation75
Figure 2 A conceptual model of immune reconstitution inflammatory syndrome (IRIS) pathophysiology with three key features represented in central rectangles.
Clinical manifestations
The time of onset of IRIS symptoms is variable, but is typically from a few days to 6 months after ART initiation. Although presentation varies by associated pathogen, a common feature is that onset is usually acute and there are features of inflammation, which may be generalized (eg, fever, tachycardia) or localized (eg, lymphadenitis). In paradoxical IRIS, symptoms of the previously diagnosed OI may recur or worsen, but a clear improvement is usually reported after the start of OI treatment prior to starting ART (see ). The original descriptions of MAI-IRIS reported that mycobacteraemia, which was typical of MAI in advanced HIV pre-ART, was not typical of MAI-IRIS, which was characterized by focal lymphadenitis and paucity of bacteria. In severe forms of IRIS (eg, TB-IRIS, CMV immune restoration uveitis, and C-IRIS), paucity of viable pathogen is characteristic at the time of IRIS, despite severe inflammation. Clinical features associated with different forms of IRIS are summarized in and described in more detail in subsequent sections.
Management and prevention of IRIS
As IRIS is antigen-driven, optimization of treatment of the underlying OI is an important aspect of treatment in many forms of IRIS (see following sections on KS-IRIS and C-IRIS), in order to quickly reduce pathogen load.Citation76 Supportive management may be required, including intravenous fluids and oxygen.Citation77 ART is key to eventual immune recovery and we recommend that ART should not be interrupted unless there is concern about concurrent drug toxicity, in which case ART substitution is preferable. There has been no trial of ART cessation in management of IRIS. ART interruption may also be considered in severe, life-threatening cases of CNS IRIS, in patients with a depressed level of consciousness. However, the undesirable effects of stopping ART include a risk of further OI and the emergence of ART resistance.
Various anti-inflammatory agents have been used in treatment of paradoxical and unmasking IRIS, including corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs). A randomized controlled trial of oral prednisone for paradoxical TB-IRIS showed benefit, and this is discussed in more detail in the section on TB-IRIS. Use of corticosteroids in other forms of IRIS is based solely on expert opinion. Systemic corticosteroid use is associated with a number of potential adverse effects in HIV, including infective complications, such as reactivation of herpes virus infections, KS progression, and mucocutaneous candidiasis.Citation76,Citation78,Citation79 Additionally, noninfective conditions are associated with chronic oral corticosteroid use, including hyperglycemia, hypertension, osteoporosis, and gastrointestinal ulceration. Therefore, aside from cases of TB-IRIS, systemic corticosteroids are recommended for more severe forms of IRIS inflammation, in the absence of contraindications, and more commonly for mycobacterial and fungal-associated IRIS than for viral-associated IRIS. Periocular or intravitreal corticosteroids have been used to treat immune restoration uveitis.Citation80 NSAIDs are used in milder forms of IRIS, and are not associated with reactivation of other infections, but their efficacy has not been tested by clinical trials. Gastrointestinal irritation and nephrotoxicity are a concern in chronic NSAID usage.
Adjunctive oral corticosteroids are routinely prescribed with TB treatment for certain forms of TB (pericardial and CNS TB). TBM-IRIS may develop despite corticosteroid therapy.Citation51 The principles of IRIS prevention include optimal prophylaxis of OI in advanced HIV (eg, cotrimoxazole to prevent Pneumocystis jirovecii pneumonia [PCP]), optimal screening for subclinical OI prior to ART initiation (eg, for serum cryptococcal antigen in patients with CD4 <100), reduction of risk factors for IRIS where possible (see ), and optimal timing of ART initiation informed by clinical trial data for that pathogen (see discussion on TB-IRIS and C-IRIS in following sections).
In the next section, we examine in more detail three common and clinically important forms of IRIS, highlighting clinical features, pathophysiology, and management issues. We then briefly discuss other common forms of IRIS. Reviews of pulmonary and CNS manifestations of IRIS and IRIS management have recently been published.Citation25,Citation76,Citation81,Citation82 The clinical characteristics of TB-IRIS and C-IRIS are summarized in .
Table 4 Clinical characteristics of TB-IRIS and C-IRIS
TB-IRIS
TB-IRIS is among the commonest forms of IRIS given the global distribution of TB infection (). Paradoxical TB-IRIS was reported to occur in 15.7% (95% credibility interval 9.7%–24.5%) of TB patients starting ART in the previously described meta-analysis by Müller et al,Citation9 which reported on 16 studies of TB-IRIS, although higher rates are reported in some settings.Citation9,Citation83 For example, in a recent Indian study, an incidence of 54.2% was reported in patients with culture-confirmed pulmonary TB and a South African study reported 47% incidence of paradoxical TB-IRIS in patients with TBM.Citation51,Citation84 In South Africa, where more than 60% of TB patients are HIV coinfected, this translates into a considerable disease burden.Citation85
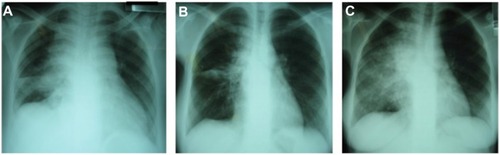
Both paradoxical and unmasking forms of TB-IRIS are now widely reported, although paradoxical IRIS has been more extensively studied.Citation28,Citation83,Citation86 Paradoxical TB-IRIS typically presents in pulmonary TB cases as a recurrence or worsening of respiratory symptoms (cough or shortness of breath), associated with a recurrence or worsening of constitutional symptoms (weight loss, night sweats, fever), and not uncommonly new or expanding infiltrates on chest radiograph, as shown in .Citation87 Paradoxical IRIS may occur at any disease site, including lymph nodes, which typically manifests with rapid enlargement followed by suppuration.Citation76 CNS TB-IRIS typically presents with new or worsening meningitis and/or features of raised intracranial pressure, due to enlarging cerebral tuberculomas or intracranial abscesses, with a high mortality.Citation42,Citation82,Citation88 It may also present with epidural abscesses, spondylitis, and radiculomyelopathy.Citation51,Citation88 Hepatosplenic (typically granulomatous disease leading to hepatitis and/or appearance of microabscesses), abdominal (eg, lymphadenopathy, peritonitis), and musculoskeletal (eg, mono- or polyarthritis) features are not infrequent.Citation83 Extrapulmonary TB-IRIS manifestations may occur in patients who originally presented with pulmonary TB, and vice versa. Accompanying laboratory features usually include a raised CRP, and may include worsening anemia.
Figure 3 This series of three chest radiographs demonstrates features of paradoxical tuberculosis (TB) immune reconstitution inflammatory syndrome in a 21-year-old antiretroviral therapy (ART)-naïve patient, with CD4 count 34 cells/mm3, who was diagnosed with drug-sensitive pulmonary TB on sputum culture.
The differential diagnosis of TB-IRIS includes other new infections (eg, pneumonia, influenza), unmasking of other OI (eg, PCP), drug reactions (eg, pyrazinamide arthropathy), and multidrug-resistant TB.Citation89 In resource-limited settings, where M.tb drug-susceptibility testing is not routinely available, the latter is difficult to exclude. Additionally, TB-IRIS may occur in cases of drug-resistant TB.Citation89 There is no laboratory test for TB-IRIS and exclusion of differential diagnoses with certainty can be very challenging in practice.
The only randomized placebo-controlled trial of treatment of IRIS was conducted in patients with paradoxical TB-IRIS, in South Africa.Citation90 One hundred and ten patients were enrolled with a median CD4 count of 116 cells/mm3 and paradoxical TB-IRIS diagnosed according to INSHI criteriaCitation19, limited to those with increasing infiltrates on chest radiograph, enlarging lymph nodes, serous effusion, or cold abscess. Patients with immediately life-threatening manifestations of TB-IRIS (respiratory failure, altered level of consciousness, new focal neurological sign/s, or compression of a vital structure) were excluded from the study. The intervention arm (n=55) consisted of prednisone 1.5 mg/kg/day for 14 days, reduced to 0.75 mg/kg/day for a further 14 days. This led to a reduction in the composite primary endpoint of days of hospitalization and outpatient therapeutic procedures (median per patient 0 versus 3 in placebo arm, P=0.04). There was also more rapid improvement in symptom scores and chest radiographs in prednisone, compared to placebo-treated, patients. There were more mild infections (eg, oral candidiasis) in the prednisone-treated arm, but no excess of severe infections. There were no significant differences in possible drug-related side effects reported in each arm. These data support use of prednisone in TB-IRIS, for moderate and severe cases. Unfortunately, TB-IRIS symptoms may recur following steroid withdrawal, requiring longer courses of treatment.Citation90,Citation91 Other immunomodulatory therapies have been considered in management of TB-IRIS, with case reports of favorable outcomes with thalidomide and montelukast, but none has been tested in randomized controlled trials (RCTs).Citation76,Citation77,Citation92,Citation93
Strategies for TB-IRIS prevention have focused on optimizing the timing of ART initiation, after it was observed that a shorter time between TB treatment initiation and ART initiation increased TB-IRIS risk. Three RCTs that studied the optimal timing of ART in TB patients have informed practice.Citation94–Citation96 These trials demonstrated that in patients with a CD4 count <50 cells/mm3, starting ART around 2 weeks after TB treatment reduced mortality or a combined endpoint of mortality and AIDS progression. Thus, while commencing ART earlier is associated with increased IRIS risk, the survival benefit in these patients with low CD4 counts overrides this. However, in patients with CD4 >50 cells/mm3, ART can be commenced between 2 and 8 weeks post-TB treatment, and delaying to 8 weeks is not associated with excess mortality, but may reduce IRIS risk. A recent placebo-controlled study of ART timing in smear-positive pulmonary TB patients with CD4 count >220 cells/mm3 demonstrated no benefit in a combined endpoint of TB treatment failure, TB recurrence, and mortality, when ART was commenced at 2 weeks post-TB treatment initiation, compared to delaying until after TB treatment was completed. IRIS rates were similar in the early and late arms (10%).Citation97 A single RCT of ART timing in TBM demonstrated no survival benefit from starting ART within 7 days of commencing TB therapy, compared to following 2 months of TB treatment, and an increased probability of serious adverse events.Citation98 As a result, it is recommended that ART initiation be delayed until 8 weeks after TB therapy is commenced in TBM patients.Citation43
In environments with a high incidence of both TB infection and HIV infection, a significant proportion of undiagnosed TB may be diagnosed by routine screening of all HIV-infected patients entering care, by sputum culture or GeneXpert (the latter being less sensitive), even those who are asymptomatic. Isoniazid preventive therapy may be indicated for those who do not have active disease. Strategies such as these are likely to reduce the prevalence of undiagnosed TB in patients starting ART and thus may reduce risk of unmasking TB-IRIS.Citation65 This could be performed alongside ART counseling, but should not unnecessarily delay commencement of ART in immunosuppressed patients. There are considerable logistic and financial challenges to implementing such a strategy in programmatic settings. The World Health Organization currently recommends an approach based on symptom screening, to identify active TB in HIV-infected individuals, in resource-constrained settings.Citation85 However, the performance of this strategy varies across different clinical settings.Citation99
There have been no successful trials of preventive strategies for paradoxical TB-IRIS, although two randomized placebo-controlled trials for paradoxical TB-IRIS prevention are currently under way in South Africa. A randomized placebo-controlled trial of prednisone for TB-IRIS prevention in high-risk patients is recruiting patients in Cape Town (NCT01924286). The TB-IRIS NSAID Cox-2 Inhibitor Prevention Trial is investigating meloxicam for TB-IRIS prevention (NCT02060006).
Cryptococcal IRIS
Paradoxical C-IRIS is reported to occur in 13%–45% of HIV-infected persons who start ART after treatment for CM. It occurs a median of 4–9 weeks following ART initiation but delayed cases have been reported up to a year after initiation of ART.Citation46,Citation100–Citation102 The usual presentation is a recurrence of meningitis symptoms (headache, nuchal rigidity, visual disturbance, and vomiting) along with other CNS signs, such as raised intracranial pressure, impaired consciousness, seizures, and focal neurology. Non-neurological presentations are less commonly described but include lymphadenitis, pneumonitis, and eye and soft tissue disease.Citation18
As with other neurological forms of IRIS, C-IRIS causes substantial morbidity and mortality (13%–36%) and is an independent predictor of death in CM patients starting ART.Citation46 The risk of developing C-IRIS is increased in individuals who have high CSF fungal burdens during the initial episode of CM, and in those who fail to clear the infection prior to the initiation of ART.Citation103 In one study, lower pre-ART CD4 count was also associated with increased risk of C-IRIS.Citation104
Diagnosis of paradoxical C-IRIS is based on INSHI criteria with diagnostic workup targeted at excluding other causes.Citation18 In patients presenting with recurrent meningitis symptoms, a lumbar puncture should be performed and the opening pressure measured; CSF should be sent for bacterial, mycobacterial, and fungal culture to exclude relapsed CM and an alternative cause of meningitis.Citation76
As there have been no clinical trials, management of suspected paradoxical C-IRIS is based solely on expert opinion. Reduction of pathogen load is an underlying objective and cryptococcal treatment should be optimized. Raised intracranial pressure can be controlled with therapeutic CSF drainage by lumbar puncture (repeated daily if necessary). Corticosteroids can be considered in severe cases, preferably once other etiologies are excluded and CSF fungal culture result is known to be negative. In patients with life-threatening neurological deterioration, steroids should be started immediately while simultaneously treating with amphotericin B to cover the possibility of a cryptococcal relapse.Citation76
The timing of ART initiation in patients with CM has been examined in four trials to date.Citation105,Citation107–Citation109 The first recruited 282 patients, mainly from USA, with a variety of AIDS-related OI, of which 12% had CM.Citation105 The overall trial result showed that early ART (within 14 days of OI treatment) was associated with a reduced likelihood of AIDS progression or death, compared to ART initiation after OI treatment completion, with no excess risk of IRIS. When the CM patients were analyzed separately, the point estimate showed a trend toward improved outcome in the early arm.Citation105,Citation106 A small study of CM patients in Botswana (n=27) compared early ART (within 7 days) with deferred ART (after 28 days), using intravenous amphotericin B as antifungal treatment. Similar to the US study, no difference in mortality was noted; however, early ART was associated with significantly increased IRIS risk.Citation107
An open-label randomized trial in Zimbabwe was conducted comparing ART within 72 hours of CM treatment initiation (fluconazole 800 mg per day) with ART initiation after 10 weeks.Citation108 The trial was stopped early by the data safety monitoring board after excess deaths were noted in the early treatment arm, mainly during the first 2 weeks of ART. The authors suggested IRIS to be the likely cause.Citation108
The Cryptococcal Optimal ART Timing (COAT) trial was conducted to definitively address the question of when to start ART in CM, using amphotericin B-based anticryptococcal therapy.Citation109 This was an open-label randomized trial conducted in Uganda and South Africa. ART-naïve patients with a first episode of CM were randomized to early ART initiation (7–14 days after starting amphotericin), or deferred (after 5 weeks). This trial was also stopped early by the data safety monitoring board after significantly increased mortality was noted in the early ART arm (6-month mortality 45% versus 30%, hazard ratio 1.7; 95% confidence interval [CI]: 1.1–2.8; P=0.03).Citation109 The explanation for this excess mortality was not clear; excess deaths all occurred within a month of starting ART, but reported rates of IRIS were not statistically different between the two study arms, nor was drug toxicity. Specific risk factors for death during early ART included altered mental status at time of randomization (hazard ratio 3.0; 95% CI: 1.0–8.8) and failure to mount a cellular response in the CSF (CSF white cell count <5 cells/mm3) (hazard ratio 3.3; 95% CI: 1.3–8.4).Citation109 Given that paucity of CSF inflammatory response has previously been associated with failure to sterilize the CSF and increased risk of IRIS, it seems plausible that immunopathology may underlie these excess deaths. Following these trials, guidelines for CM now suggest clinicians wait 4–6 weeks after commencing amphotericin B-based CM treatment, before ART is initiated in CM patients.Citation110
In addition to paradoxical C-IRIS, presentation with a new diagnosis of CM shortly after starting ART is also well described, occurring in up to 1% of patients starting ART and in up to 33% of those who have a cryptococcal antigenemia at time of ART initiation.Citation17,Citation111–Citation116 However, whether this occurs due to a persisting immune deficiency or an unmasking C-IRIS can be difficult to determine and has not been widely studied. INSHI provide case definitions for both ART-associated CM and unmasking C-IRIS and suggest unmasking C-IRIS should be considered if there are “unusual, exaggerated, or heightened inflammatory manifestations” (eg, CSF white cell count >50 cells/μL, persistently raised intracranial pressure refractory to therapy, rapidly expanding CNS lesion, painful or suppurating lymphadenopathy, pneumonitis, granulomatous inflammation on histology).Citation18
Persons who develop such ART-associated cryptococcosis should be managed in the same way as patients not taking ART: potent antifungal therapy to reduce antigen load seems intuitive, and therapeutic lumbar puncture to control raised intracranial pressure is frequently required for the life-threatening complication of raised intracranial pressure.Citation117
KS-related IRIS
KS is an HIV-associated malignancy that is driven by replication of human herpes virus-8 (HHV-8), occurring most commonly in regions where there is high prevalence of HHV-8. It is the commonest malignancy associated with HIV infection.Citation118 Patients typically present with localized or extensive mucocutaneous, hyperpigmented lesions, often with edema, most commonly affecting the skin but also frequently the oral mucosa. As a malignancy of lymphatic endothelium, it is capable of causing disseminated disease and may affect the lungs and gastrointestinal tract.Citation119 No specific anti-HHV-8 agent has been shown to be effective, so treatment is limited to reversal of immune suppression with ART and cytotoxic chemotherapeutic agents when disease is extensive.
Paradoxical KS-IRIS occurs when ART is initiated in 7%–31% of cases. This variation is probably related to differences in severity of KS, degree of immunosuppression, and treatment availability in different settings.Citation40,Citation120,Citation121 KS-IRIS frequently presents with inflammation or enlargement of an existing KS lesion and/or worsening edema. Alternatively, during IRIS, KS may extend or appear rapidly at new anatomical sites. Symptoms and signs vary according to the site of the KS lesion. Acute airway obstruction may occur and can be life-threatening.Citation79 Significant gastrointestinal bleeding may occur. Rapid extension of pulmonary lesions, may mimic an infective pulmonary process.Citation25 Onset is between 1 and 22 weeks, and usually in the first 12 weeks post-ART initiation.Citation40,Citation120,Citation122
Little is known about the pathogenesis of KS-IRIS. Proinflammatory and Th1 cytokines are considered to be important in KS pathogenesis.Citation123 Increased KS-IRIS risk is associated with use of ART alone as initial KS treatment, more extensive baseline KS tumor stage, baseline plasma HIV-1 RNA more than 105 copies/mL, and baseline detectable plasma HHV-8 DNA.Citation40
Treatment for KS-IRIS includes systemic chemotherapy and supportive measures, eg, radiotherapy should airway obstruction occur. Liposomal anthracyclines (eg, doxorubicin) are the preferred first-line chemotherapeutic agents for KS and may be indicated in KS-IRIS where available. Corticosteroids may be harmful as there is an association with acute progression of KS lesions, possibly due to a permissive effect on HHV-8 viral replication.Citation79 ART should be continued. Use of systemic chemotherapy for extensive disease prior to ART initiation may help prevent KS-IRIS, but this has not been systematically studied.
Mucocutaneous IRIS
Mucocutaneous conditions caused by viruses, such as herpes simplex virus causing genital ulceration, varicella zoster virus reactivation, molluscum contagiosum virus, and human papilloma virus, in addition to mucocutaneous fungal infections (eg, candida, tinea) collectively form the most common type of IRIS reported in many series (see ).Citation21,Citation36–Citation38 Other cutaneous manifestations include worsening of pruritic papular eruption and acne flares.Citation7,Citation21 Management typically involves targeting the causative organism where a treatment is available (eg, acyclovir for herpes simplex virus) and symptomatic treatment. No evidence-based guidelines are available. Although common and distressing for patients, these forms of IRIS are rarely severe.
CNS IRIS
CNS IRIS contributes the bulk of IRIS mortality.Citation81 In addition to C-IRIS and TBM-IRIS (discussed above), progressive multifocal leukoencephalopathy (PML) IRIS and, less commonly, IRIS-associated with cerebral toxoplasmosis, have been described.Citation24,Citation26 PML-IRIS has been reviewed in detail recently by Post et al, who highlight a role for neuroimaging in PML-IRIS diagnosis, with contrast enhancement of lesions and mass effect due to interstitial edema in PML-IRIS, not typically found in PML.Citation124 Neuroimaging is not available in many settings and autopsy studies suggest that PML is underdiagnosed, so it is probable that PML-IRIS is also underdiagnosed.Citation125 Corticosteroids are used in cases of PML-IRIS, although there are no RCT data to support this. A recently published case report described use of maraviroc, a CCR5 antagonist, in an HIV-uninfected patient with PML-IRIS, with a favorable outcome, but efficacy has not yet been assessed in a clinical trial.Citation126
CNS IRIS has also been described in cases with no evidence of an OI, where it is hypothesized that the reconstituting immune response targets CNS HIV proteins or alternatively host antigens, the latter an autoimmune process.Citation127,Citation128 A CD8 (rather than a CD4) lymphocyte infiltration in the perivascular spaces characterizes this condition. Significant mortality is reported. Corticosteroids have been used in some cases, with favorable outcome.Citation129
HIV-associated IRIS – other infectious causes
A wide variety of infectious pathogens have been linked to HIV-associated IRIS (). CMV reactivation is associated with advanced immune suppression (CD4 count below 50 cells/mm3), most commonly causing retinitis, which may lead to permanent visual impairment. Immune restoration uveitis may occur following ART initiation in such patients and can be sight-threatening. In HIV–hepatitis B virus coinfected patients, an acute hepatitis flare (with potentially fulminant course) may occur post-ART initiation, and may be difficult to distinguish from ART drug toxicity.Citation130 P. jirovecii is a common fungal cause of IRIS. Calligaro et al have reviewed PCP-IRIS and other pulmonary IRIS manifestations in more detail.Citation25 Lawn has reviewed 24 cases of IRIS associated with parasitic infections, including Schistosoma mansoni, Strongyloides stercoralis, Leishmania spp., and Toxoplasma gondii.Citation26
Summary and conclusion
Access to ART is improving worldwide, but because many patients still commence ART with low CD4 counts, IRIS remains a common complication. IRIS is a heterogeneous condition with a number of case definitions in use. Clinical manifestations and epidemiology are well described for some forms of IRIS, but specific diagnostic tests and evidence-based treatment strategies are lacking. CNS IRIS is associated with a high mortality and requires more effective interventions. While ART initiation causes IRIS, it is key to recovery of immune function and improved health outcomes, therefore delay or discontinuation of ART due to IRIS is not usually recommended. A notable exception is in patients with CM, in whom ART should be delayed until 4–6 weeks after CM treatment initiation. Prevention strategies include: 1) treatment of HIV before advanced immunosuppression develops; 2) OI prevention in advanced HIV; 3) screening for and treatment of OI prior to ART initiation; and 4) optimal timing of ART initiation (this varies according to pathogen and CD4 count and takes into account mortality and IRIS risk). Treatment of IRIS involves optimal treatment of the underlying pathogen to reduce antigen load; supportive measures; and, in some cases, immunosuppression with corticosteroids.
Key knowledge gaps in the diagnosis and treatment of IRIS exist. There are no standardized clinical case definitions for many forms of IRIS (TB-IRIS and C-IRIS being the exceptions). Confirmatory diagnostic tests are lacking for all forms of IRIS. Most forms of IRIS lack evidence-based management strategies, the use of prednisone in TB-IRIS being the exception. For all forms of IRIS, other immunomodulatory therapies have not been systematically studied. The specific cell phenotypes and inflammatory and regulatory pathways that are central in the development of IRIS, in the context of an abundance of foreign antigen, need to be more clearly defined. Improved understanding of the pathophysiology of IRIS will hopefully enable improved diagnostic modalities and better targeted treatments to be developed.
Acknowledgments
This work was supported by the Medical Research Council (U1175.02.002.00014.01), Wellcome Trust (references 084323, 088316, 094000, 094013, and 098316), and the European Union (PIRSES-GA-2011-295214, and FP7 HEALTH-F3-2012-305578). Graeme Meintjes was supported in part by the National Research Foundation (NRF) of South Africa [UID: 85858]. The grant holders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by the NRF-supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard.
Disclosure
The authors report no conflicts of interest in this work.
References
- PalellaFJJrDelaneyKMMoormanACDeclining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study InvestigatorsN Engl J Med1998338138538609516219
- JacobsonMAFrenchMAltered natural history of AIDS-related opportunistic infections in the era of potent combination antiretroviral therapyAIDS199812Suppl AS157S1639632998
- World Health OrganizationGlobal update on HIV treatment 2013: Results, impact and opportunitiesGenevaWorld Health Organization2013 Available from: http://www.who.int/hiv/pub/progressreports/update2013/en/Accessed December 3, 2013
- BorJHerbstAJNewellMLBärnighausenTIncreases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatmentScience2013339612296196523430655
- World Health OrganizationMarch 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infectionGenevaWorld Health Organization2014 Available from: http://who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_march2014/en/Accessed February 28, 2014
- World Health OrganizationTreatment of tuberculosis: guidelines for national programmesGenevaWorld Health Organization2010 Available from: http://www.who.int/tb/features_archive/new_treatment_guidelines_may2010/en/Accessed February 22, 2014
- HaddowLJMoosaMYMosamAMoodleyPParboosingREasterbrookPJIncidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South AfricaPLoS One2012711e4062323152745
- BraitsteinPBrinkhofMWDabisFAntiretroviral Therapy in Lower Income Countries (ART-LINC) CollaborationART Cohort Collaboration (ART-CC) groupsMortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countriesLancet2006367951381782416530575
- MüllerMWandelSColebundersRAttiaSFurrerHEggerMIeDEA Southern and Central AfricaImmune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysisLancet Infect Dis201010425126120334848
- SunHYSinghNOpportunistic infection-associated immune reconstitution syndrome in transplant recipientsClin Infect Dis201153216817621690625
- FineAJSorbelloAKortepeterCScarazziniLProgressive multifocal leukoencephalopathy after natalizumab discontinuationAnn Neurol201475110811524242357
- CadenaJThompsonGR3rdHoTTMedinaEHughesDWPattersonTFImmune reconstitution inflammatory syndrome after cessation of the tumor necrosis factor alpha blocker adalimumab in cryptococcal pneumoniaDiagn Microbiol Infect Dis200964332733019501793
- MiceliMHMaertensJBuvéKImmune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: proof of principle, description, and clinical and research implicationsCancer2007110111212017525971
- JacobsonMAMcGrathMSJosephPMolaghanJBTadepalliSQuinnRZidovudine-induced feverJ Acquir Immune Defic Syndr1989243823882666640
- FrenchMAPricePStoneSFImmune restoration disease after anti-retroviral therapyAIDS200418121615162715280772
- FrenchMAMallalSADawkinsRLZidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patientsAIDS1992611129312971472334
- HaddowLJEasterbrookPJMosamADefining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohortClin Infect Dis20094991424143219788360
- HaddowLJColebundersRMeintjesGInternational Network for the Study of HIV-associated IRIS (INSHI)Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitionsLancet Infect Dis2010101179180221029993
- MeintjesGLawnSDScanoFInternational Network for the Study of HIV-associated IRISTuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settingsLancet Infect Dis20088851652318652998
- LetangENanicheDBowerMMiroJMKaposi sarcoma-associated immune reconstitution inflammatory syndrome: in need of a specific case definitionClin Infect Dis201255115715822491336
- NovakRMRichardsonJTBuchaczKHIV Outpatient Study (HOPS) InvestigatorsImmune reconstitution inflammatory syndrome: incidence and implications for mortalityAIDS201226672173022233655
- HuruyKMuluAMengistuGImmune reconstitution inflammatory syndrome among HIV/AIDS patients during highly active antiretroviral therapy in Addis Ababa, EthiopiaJpn J Infect Dis200861320520918503170
- MeintjesGBoulleAImmune reconstitution inflammatory syndrome in a large multicenter cohort study: case definition and comparabilityExpert Rev Anti Infect Ther201210773774122943397
- Huis in’t VeldDSunHYHungCCColebundersRThe immune reconstitution inflammatory syndrome related to HIV co-infections: a reviewEur J Clin Microbiol Infect Dis201231691992721964588
- CalligaroGMeintjesGMendelsonMPulmonary manifestations of the immune reconstitution inflammatory syndromeCurr Opin Pulm Med201117318018821346572
- LawnSDImmune reconstitution disease associated with parasitic infections following initiation of antiretroviral therapyCurr Opin Infect Dis200720548248817762781
- DhasmanaDJDhedaKRavnPWilkinsonRJMeintjesGImmune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and managementDrugs200868219120818197725
- BalkhairAAhamedSSankhlaDUnmasking immune reconstitution inflammatory syndrome (IRIS): a report of five cases and review of the literatureSultan Qaboos Univ Med J20111119510321509214
- PassosLTalhariCSantosMRibeiro-RodriguesRFerreiraLCTalhariSHistoplasmosis-associated immune reconstitution inflammatory syndromeAnn Bras Dermatol2011864 Suppl 1S168S172 English, Portuguese
- LeeCHTzaoCChangTHCase of pulmonary cryptococcosis mimicking hematogeneous metastases in an immunocompetent patient: value of absent 18F-fluorodeoxyglucose uptake on positron emission tomography/CT scanKorean J Radiol201314354054323690726
- DepsPDLockwoodDNLeprosy occurring as immune reconstitution syndromeTrans R Soc Trop Med Hyg20081021096696818639911
- FukunagaAIwamotoYInanoSImmune reconstitution inflammatory syndrome mimics a relapse of AIDS-related Burkitt lymphomaIntern Med201352192265226924088764
- LinRJSongJAn unusual cause of chest pain: Mycobacterium avium complex and the immune reconstitution inflammatory syndromeJ Hosp Med20116530931120652963
- IntalapapornPPoovorawanYSuankratayCImmune reconstitution syndrome associated with parvovirus B19-induced pure red cell aplasia during highly active antiretroviral therapyJ Infect2006532e79e8216313965
- AchenbachCJHarringtonRDDhanireddySCraneHMCasperCKitahataMMParadoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infectionClin Infect Dis201254342443322095568
- Hoyo-UlloaIBelaunzarán-ZamudioPFCrabtree-RamirezBGalindo-FragaAPérez-AguinagaMESierra-MaderoJGImpact of the immune reconstitution inflammatory syndrome (IRIS) on mortality and morbidity in HIV-infected patients in MexicoInt J Infect Dis2011156e408e41421493116
- KumarSRGopalanNPatrawallaPMenonPMayerKSwaminathanSImmune reconstitution inflammatory syndrome in HIV-infected patients with and without prior tuberculosisInt J STD AIDS201223641942322807536
- LetangEMiróJMNhampossaTIncidence and predictors of immune reconstitution inflammatory syndrome in a rural area of MozambiquePLoS One201162e1694621386993
- Abdool KarimSSStigma impedes AIDS preventionNature20114747349293121637237
- LetangELewisJJBowerMImmune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UKAIDS201327101603161323462220
- Ablanedo-TerrazasYAlvarado-De La BarreraCReyes-TeránGTowards a better understanding of Kaposi sarcoma-associated immune reconstitution inflammatory syndromeAIDS201327101667166924047765
- AgarwalUKumarABeheraDFrenchMAPricePTuberculosis associated immune reconstitution inflammatory syndrome in patients infected with HIV: meningitis a potentially life threatening manifestationAIDS Res Ther2012911722620862
- TörökMEFarrarJJWhen to start antiretroviral therapy in HIV-associated tuberculosisN Engl J Med2011365161538154022010921
- PepperDJMaraisSWilkinsonRJBhaijeeFDe AzevedoVMeintjesGBarriers to initiation of antiretrovirals during antituberculosis therapy in AfricaPLoS One201165e1948421589868
- TadokeraRMeintjesGSkolimowskaKHHypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndromeEur Respir J20113751248125920817712
- BoulwareDRMeyaDBBergemannTLClinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort studyPLoS Med2010712e100038421253011
- Conesa-BotellaAMeintjesGCoussensAKCorticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndromeClin Infect Dis20125571004101122715179
- BarberDLAndradeBBMcBerryCSeretiISherARole of IL-6 in Mycobacterium avium – associated immune reconstitution inflammatory syndromeJ Immunol2014192267668224337386
- BoulwareDRBonhamSCMeyaDBPaucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndromeJ Infect Dis2010202696297020677939
- RuhwaldMRavnPImmune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm?AIDS200721788288417415049
- MaraisSMeintjesGPepperDJFrequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndromeClin Infect Dis201356345046023097584
- BourgaritACarcelainGMartinezVExplosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patientsAIDS2006202F1F716511406
- BourgaritACarcelainGSamriATuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cellsJ Immunol200918363915392319726768
- MeintjesGWilkinsonKARangakaMXType 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndromeAm J Respir Crit Care Med2008178101083108918755923
- MahnkeYDGreenwaldJHDerSimonianRSelective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndromeBlood2012119133105311222219223
- ElliottJHVohithKSaramonySImmunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapyJ Infect Dis2009200111736174519874177
- TieuHVAnanworanichJAvihingsanonAImmunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in ThailandAIDS Res Hum Retroviruses200925111083108919886838
- SeddikiNSassonSCSantner-NananBProliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration diseaseEur J Immunol200939239140319180462
- TanDBYongYKTanHYImmunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitisHIV Med20089530731618400078
- LimAD’OrsognaLPricePFrenchMAImbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration diseaseAIDS Res Ther20085918442415
- TadokeraRWilkinsonKAMeintjesGARole of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosisJ Infect Dis201320771148115623303806
- BarberDLAndradeBBSeretiISherAImmune reconstitution inflammatory syndrome: the trouble with immunity when you had noneNat Rev Microbiol201210215015622230950
- WalkerNFMeintjesGWilkinsonRJHIV-1 and the immune response to tuberculosisFuture Virol201381578023653664
- LawnSDWainwrightHOrrellCFatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophagesAIDS200923114314519050399
- LawnSDMeintjesGPathogenesis and prevention of immune reconstitution disease during antiretroviral therapyExpert Rev Anti Infect Ther20119441543021504399
- OliverBGElliottJHPricePMediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapyJ Infect Dis2010202111728173720977362
- BoulwareDRHullsiekKHPuronenCEINSIGHT Study GroupHigher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or deathJ Infect Dis2011203111637164621592994
- TadokeraRMeintjesGAWilkinsonKAMatrix metalloproteinases and tissue damage in HIV-tuberculosis immune reconstitution inflammatory syndromeEur J Immunol201444112713624136296
- WalkerNFClarkSOOniTDoxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinasesAm J Respir Crit Care Med2012185998999722345579
- ElkingtonPTUgarte-GilCAFriedlandJSMatrix metalloproteinases in tuberculosisEur Respir J201138245646421659415
- ElkingtonPTGreenJAFriedlandJSAnalysis of matrix metalloproteinase secretion by macrophagesMethods Mol Biol200953125326519347322
- VolkmanHEPozosTCZhengJDavisJMRawlsJFRamakrishnanLTuberculous granuloma induction via interaction of a bacterial secreted protein with host epitheliumScience2010327596446646920007864
- ConradieFFoulkesASIvePNatural killer cell activation distinguishes Mycobacterium tuberculosis-mediated immune reconstitution syndrome from chronic HIV and HIV/MTB coinfectionJ Acquir Immune Defic Syndr201158330931821826013
- PeanPNerrienetEMadecYCambodian Early versus Late Introduction of Antiretroviral Drugs (CAMELIA) study teamNatural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosisBlood2012119143315332022343919
- LaiRPNakiwalaJKMeintjesGWilkinsonRJThe immunopathogenesis of the HIV tuberculosis immune reconstitution inflammatory syndromeEur J Immunol20134381995200223928963
- MeintjesGScrivenJMaraisSManagement of the immune reconstitution inflammatory syndromeCurr HIV/AIDS Rep20129323825022752438
- MaraisSWilkinsonRJPepperDJMeintjesGManagement of patients with the immune reconstitution inflammatory syndromeCurr HIV/AIDS Rep20096316217119589302
- LeshoEEvidence base for using corticosteroids to treat HIV- associated immune reconstitution syndromeExpert Rev Anti Infect Ther20064346947816771623
- VolkowPFCornejoPZinserJWOrmsbyCEReyes-TeránGLife-threatening exacerbation of Kaposi’s sarcoma after prednisone treatment for immune reconstitution inflammatory syndromeAIDS200822566366518317012
- StewartMWOptimal management of cytomegalovirus retinitis in patients with AIDSClin Ophthalmol2010428529920463796
- MateenFJNathACentral nervous system-immune reconstitution inflammatory syndrome in resource-limited settings: current burden and future needsAIDS201226151851185522781220
- BahrNBoulwareDRMaraisSScrivenJWilkinsonRJMeintjesGCentral nervous system immune reconstitution inflammatory syndromeCurr Infect Dis Rep201315658359324173584
- MeintjesGRabieHWilkinsonRJCottonMFTuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapyClin Chest Med200930479781019925968
- NarendranGAndradeBBPorterBOParadoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in predictionPLoS One201385e6354123691062
- World Health OrganizationGlobal Tuberculosis Report 2013GenevaWorld Health Organization2013 Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1Accessed August 29, 2014
- WilkinsonKAMeintjesGSeldonRGoliathRWilkinsonRJImmunological characterisation of an unmasking TB-IRIS caseS Afr Med J2012102651251722668952
- LuetkemeyerAFKendallMANyirendaMAdult AIDS Clinical Trials Group A5221 Study TeamTuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programsJ Acquir Immune Defic Syndr201465442342824226057
- PepperDJMaraisSMaartensGNeurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case seriesClin Infect Dis20094811e96e10719405867
- MeintjesGRangakaMXMaartensGNovel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistanceClin Infect Dis200948566767619191655
- MeintjesGWilkinsonRJMorroniCRandomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndromeAIDS201024152381239020808204
- BreenRASmithCJBettinsonHParadoxical reactions during tuberculosis treatment in patients with and without HIV co-infectionThorax200459870470715282393
- BrunelASReynesJTuaillonEThalidomide for steroid-dependent immune reconstitution inflammatory syndromes during AIDSAIDS201226162110211222874513
- HardwickCWhiteDMorrisEMonteiroEFBreenRALipmanMMontelukast in the treatment of HIV associated immune reconstitution diseaseSex Transm Infect200682651351417151039
- Abdool KarimSSNaidooKGroblerATiming of initiation of antiretroviral drugs during tuberculosis therapyN Engl J Med2010362869770620181971
- HavlirDVKendallMAIvePAIDS Clinical Trials Group Study A5221Timing of antiretroviral therapy for HIV-1 infection and tuberculosisN Eng J Med20113651614821491
- BlancFXSokTLaureillardDCAMELIA (ANRS 1295–CIPRA KH001) Study TeamEarlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosisN Eng J Med20113651614711481
- MfinangaSGKirengaBJChandaDMEarly versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trialLancet Infect Dis201414756357124810491
- TörökMEYenNTChauTTTiming of initiation of antiretroviral therapy in human immunodeficiency virus (HIV) – associated tuberculous meningitisClin Infect Dis201152111374138321596680
- GetahunHKittikraisakWHeiligCMDevelopment of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studiesPLoS Med201181e100039121267059
- BicanicTMeintjesGRebeKImmune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective studyJ Acquir Immune Defic Syndr200951213013419365271
- ShelburneSAVisnegarwalaFDarcourtJIncidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapyAIDS200519439940615750393
- SungkanuparphSFillerSGChetchotisakdPCryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter studyClin Infect Dis200949693193419681708
- ChangCCDorasamyAAGosnellBIClinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndromeAIDS201327132089209923525034
- ChangCCLimAOmarjeeSCryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapyJ Infect Dis2013208689890623766525
- ZolopaAAndersenJPowderlyWEarly antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trialPLoS One200945e557519440326
- GrantPMKomarowLAndersenJRisk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs deferred ART during an opportunistic infectionPLoS One201057e1141620617176
- BissonGPMolefiMBellamySEarly versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitisClin Infect Dis20135681165117323362285
- MakadzangeATNdhlovuCETakarindaKEarly versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan AfricaClin Infect Dis201050111532153820415574
- BoulwareDRMeyaDBMuzooraCTiming of antiretroviral therapy after diagnosis of cryptococcal meningitisN Engl J Med2014370262487249824963568
- GovenderNPMeintjesGBicanicTGuideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 updateSouth Afr J HIV Med20131427686
- LawnSDBekkerLGMyerLOrrellCWoodRCryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programmeAIDS200519172050205216260920
- MurdochDMVenterWDFeldmanCVan RieAIncidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective studyAIDS200822560161018317001
- MeyaDBManabeYCCastelnuovoBCost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settingsClin Infect Dis201051444845520597693
- Parkes-RatanshiRWakehamKLevinJCryptococcal Trial TeamPrimary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: a double-blind, randomised, placebo-controlled trialLancet Infect Dis2011111293394121982529
- PongsaiPAtamasirikulKSungkanuparphSThe role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell countsJ Infect201060647447720347868
- JarvisJNLawnSDVogtMBanganiNWoodRHarrisonTSScreening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South AfricaClin Infect Dis200948785686219222372
- PerfectJRDismukesWEDromerFClinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of americaClin Infect Dis201050329132220047480
- Dal MasoLSerrainoDFranceschiSEpidemiology of HIV-associated malignanciesCancer Treat Res200110411811191124
- Di LorenzoGKonstantinopoulosPAPantanowitzLDi TrolioRDe PlacidoSDezubeBJManagement of AIDS-related Kaposi’s sarcomaLancet Oncol20078216717617267331
- BowerMNelsonMYoungAMImmune reconstitution inflammatory syndrome associated with Kaposi’s sarcomaJ Clin Oncol200523225224522816051964
- LetangEAlmeidaJMMiróJMPredictors of immune reconstitution inflammatory syndrome-associated with kaposi sarcoma in mozambique: a prospective studyJ Acquir Immune Defic Syndr201053558959719801945
- LeidnerRSAboulafiaDMRecrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndromeAIDS Patient Care STDS2005191063564416232048
- MesriEATargeting AIDS-Kaposi’s sarcomaNat Med19995773873910395315
- PostMJThurnherMMCliffordDBCNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 1: overview and discussion of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome and cryptococcal-immune reconstitution inflammatory syndromeAJNR Am J Neuroradiol20133471297130722790246
- ShankarSKSatishchandraPMahadevanALow prevalence of progressive multifocal leukoencephalopathy in India and Africa: is there a biological explanation?J Neurovirol20039Suppl 1596712709874
- GiacominiPSRozenbergAMetzIAraujoDArbourNBar-OrAMaraviroc in Multiple Sclerosis–Associated PML–IRIS (MIMSAPI) GroupMaraviroc and JC virus-associated immune reconstitution inflammatory syndromeN Engl J Med2014370548648824476450
- PostMJThurnherMMCliffordDBCNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 2: discussion of neuro-immune reconstitution inflammatory syndrome with and without other pathogensAJNR Am J Neuroradiol20133471308131822790252
- JohnsonTNathAImmune reconstitution inflammatory syndrome and the central nervous systemCurr Opin Neurol201124328429021499099
- RingelsteinAOelschlaegerCSalehASevere aseptic leucoencephalopathy as immune reconstitution inflammatory syndrome in Caucasian and African patientsAIDS200923111435143719424053
- AndersonAMMosunjacMBPalmoreMPOsbornMKMuirAJDevelopment of fatal acute liver failure in HIV-HBV coinfected patientsWorld J Gastroenterol201016324107411120731028
- World Health OrganizationImproving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settingsGenevaWorld Health Organization2006 Available from: http://www.who.int/tb/publications/2006/tbhiv_recommendations.pdfAccessed September 01, 2014
