Abstract
Introduction
Human immunodeficiency virus (HIV) impairs immune function leading to oral mucosal lesions. While highly active antiretroviral therapy (HAART) has reduced the incidence of HIV-associated oral lesions (HIV-OLs), these lesions can still manifest across all HIV stages due to various patient-related factors.
Purpose
To evaluate the occurrence of HIV-OLs and clinical characteristics across all HIV stages in people living with HIV (PLWH).
Patients and Methods
Five patients aged 7 to 60 with canker sores visited the Oral Medicine Clinic. One newly diagnosed patient with stage II HIV had not yet started ART, while others in stages I, III, and IV were already receiving ART. Diagnosed oral lesions included recurrent intraoral herpes (RIH) in patients with stages I, II, and III; linear gingival erythema (LGE) in stages I; acute pseudomembranous candidiasis (APC) and oral hairy leukoplakia (OHL) in stages II; traumatic ulcers in stages III; erythema multiforme (EM) and angular cheilitis (AC) in stages IV. Potential risk factors for these oral lesions included poor oral hygiene, low CD4+ T-cell counts, detectable viral load, non-adherence to ART, smoking, medication use for systemic diseases, nutritional deficiency, and comorbidities.
Results
Treatment included antiviral for RIH; antifungal for APC and AC; topical corticosteroid and antiseptic mouthwash for oral ulcers and improving oral hygiene; application of normal saline-soaked gauze dressing followed by topical steroid for EM; petroleum jelly for dry lips; and multivitamins. Lesions improved over 5–15 days. Addressing risk factors involved improving oral hygiene, treating comorbidities, promoting weight gain, smoking cessation, and starting ART for those untreated.
Conclusion
Oral lesions are prevalent throughout the stages of HIV and are influenced by immune status, medication adherence, and overall health, underscoring the need for holistic care to enhance the quality of life, potentially alter HIV progression, and reduce morbidity through integrated oral health assessments in routine care.
Introduction
The HIV infection/acquired immunodeficiency syndrome (AIDS) epidemic remains a significant public health issue in Southeast Asia. HIV infection harms the mucous epithelium and immunity, particularly in the oral cavity, leading to various mucosal lesions.Citation1 Oral lesions appeared in up to 50% of HIV-infected patients and up to 80% of AIDS patients.Citation2 While highly active antiretroviral therapy (HAART) has markedly improved the survival rate of people living with HIV (PLWH), the presence of oral lesions can increase morbidity and affect patients’ quality of life.Citation3,Citation4 Oral lesions in PLWH have a substantial impact on both physical and mental health making them crucial for diagnosing infection, predicting disease progression, and staging AIDS.Citation5 Oral lesions can occur or manifest at all clinical stages of HIV. Given the oral cavity’s accessibility, oral manifestations are a key indicator of HIV infection and are used in staging, classification, and treatment planning.Citation6–8
The classification and diagnostic criteria for HIV-OLs were developed by EC-Clearinghouse in 1993, dividing them into three major groups: (1) lesions strongly associated with HIV infection, (2) lesions less commonly associated with HIV infection, and (3) lesions seen in HIV infection.Citation2 However, the type of HIV-OLs may differ from this classification due to several influencing risk factors, including age, sex, antiretroviral therapy (ART), immunocompromised states (infections, immune-mediated disorders, and other diseases), wasting or low body mass index (BMI), CD4+ cell count level, viral load level, oral hygiene, medication use, also smoking and alcohol consumption.Citation5,Citation7,Citation9–11
This report aims to evaluate the occurrence of HIV-related oral lesions (HIV-OLs) and clinical characteristics across different HIV stages in people living with HIV (PLWH), both on antiretroviral therapy (ART) and those who have not yet started ART. A thorough understanding of the development of HIV-OLs and patient-related factors at all stages of HIV is crucial for devising effective strategies to reduce oral lesion-related morbidity and slow the progression of HIV disease.
Cases
Case 1
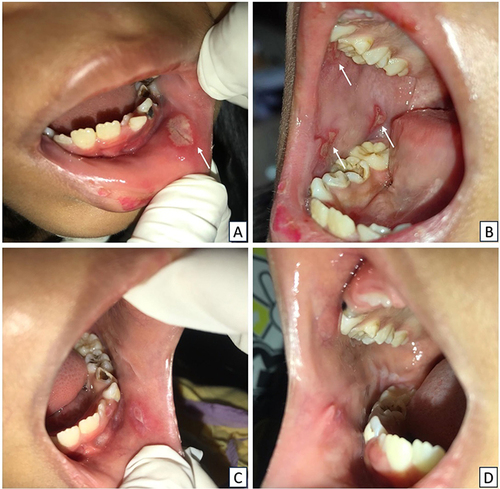
A 26-year-old man with stage I HIV, who had intermittently adhered to ART, presented to the Oral Medicine Clinic with painful sores on the left side of his tongue that had persisted for two days. He had been living with HIV for 6 years and was on antiretroviral therapy, although he experienced a lapse in medication adherence from 2020 to 2021. No smoking, alcohol, or drug use was reported. Extraoral examination revealed dry and exfoliating lips. Intraoral examination revealed multiple oval-shaped ulcers on the left lateral border of the tongue and floor of the mouth, as well as erythematous lesions on the gingival margins (). Oral hygiene was fair (OHI-S score 2.2). The viral load was detected, and reactive IgG anti HSV-1 was 65.8 U/M. Hematological analysis revealed elevated erythrocyte sedimentation rate (ESR) (77 mm/hour), high immunoglobulin E (IgE) (248.8 IU/mL), and low hemoglobin (Hb), hematocrit (Ht), and mean corpuscular hemoglobin concentration (MCHC). The lesions were diagnosed as RIH and LGE. He was treated with acyclovir, chlorhexidine gluconate mouthwash 0.2%, multivitamin, and petroleum jelly. The lesions improved a week later ().
Figure 1 Clinical characteristics of Case 1. (A and B) On the first visit, the patient had several multiple yellowish-white ulcers on the lateral border of the tongue and floor of the mouth. (C) An elongated band-shaped erythematous lesion on the upper and lower anterior gingiva. (D–F) The lesions notably improved within a week.
Case 2
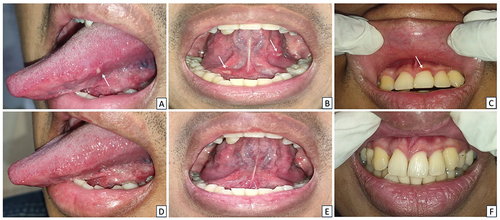
A 60-year-old man, with type II diabetes mellitus and nutritional deficiency, who was recently diagnosed with stage II HIV, was referred to the Oral Medicine Specialist for a recurring sore mouth that had persisted for a year. He had been taking glibenclamide for the past year but had not started ART. The patient had a history of smoking but did not drink or use drugs. The extraoral examination revealed anemic conjunctiva, icteric sclera, dry lips, and no exfoliating lips. An intraoral examination revealed erythematous lesions on the lower labial mucosa, a yellowish-white plaque with erythematic areas on various oral surfaces, and a bilateral corrugated white plaque on the lateral tongue (). The patient had poor oral hygiene, with an OHI-S score of 5.4. Treponema pallidum hemagglutination assay (TPHA) and venereal disease research laboratory (VDRL) test were positive, and serological examination revealed reactive IgG anti-HSV-1. Hematological examination revealed low levels of Hb, Ht, erythrocytes, leucocytes, and MCHC, with a high monocyte count. Candida albicans culture was positive. The diagnoses included recurrent intraoral herpes (RIH), oral hairy leukoplakia (OHL), and acute pseudomembranous candidiasis (APC). The patient was treated with acyclovir, hyaluronic acid mouthwash 0.025%, nystatin oral suspension, multivitamin, and petroleum jelly. Follow-up visits after 14 days revealed that the oral lesions had completely healed ().
Figure 2 Intraoral appearances of the patient in Case 2 (A–C) On the first examination, there was a single erythematous lesion, yellowish-white plaque that could not be scrapped, reddish-yellow single ulcer, and corrugated white plaque that could not be scrapped (D–F) The lesions resolved after 14 days.
Case 3
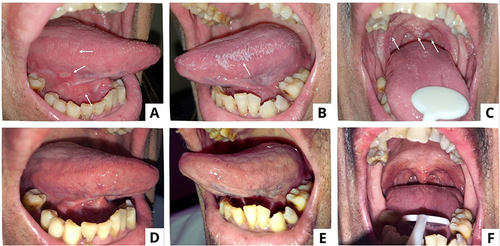
A 7-year-old girl with stage III HIV was referred to the Oral Medicine Specialist for recurrent oral sores. She had received ART. Extraoral examination revealed dry and exfoliating lips. The intraoral examination discovered multiple yellowish ulcers on both the buccal mucosa and the labial mucosa ( and ). Oral hygiene was fair, with an OHI-S score of 1.67. Viral load testing revealed an undetected virus. The mother respectfully declined to consent to an HSV-1 serological examination specifically. The diagnosis was recurrent intraoral herpes (RIH) with a traumatic ulcer on the labial mucosa. She was given povidone-iodine mouthwash 1%, triamcinolone acetonide in orabase 0.1%, a multivitamin, and petroleum jelly. Seven days later, oral ulcers had significantly improved ( and ).
Case 4
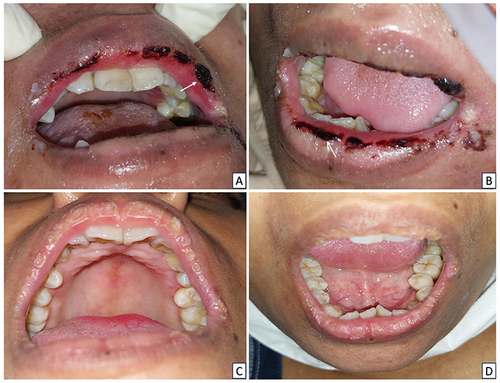
An 11-year-old girl, with lung tuberculosis and bronchopneumonia, stage IV HIV, was referred to an Oral Medicine Specialist after experiencing lip pain for a week after applying bee propolis. She is currently receiving ART and has been prescribed ethambutol, prednisone, cefotaxime, fluconazole, metronidazole, rifampicin, isoniazid, B6 vitamin, and zinc. The patient had anemic conjunctiva and a hemorrhagic crust on the lower lips that bleed and was painful (). An intraoral examination revealed no lesions. The patient had poor oral hygiene, with an OHI-S score of 3.2. Viral load testing revealed an undetected virus. Hematological analysis revealed low levels of Hb, erythrocytes, lymphocytes, and MCHC. The oral lesion was diagnosed as bee propolis-induced erythema multiforme, and treated with normal saline and hydrocortisone cream 1%. The lesions healed in five days ().
Case 5
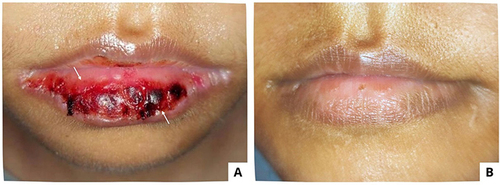
A 34-year-old woman, diagnosed with stage IV HIV, who presented with space occupying lesion (SOL), multiple cerebral infarctions, ec cerebral toxoplasmosis, and a clindamycin allergy-induced drug eruption, using a nasogastric tube, was referred to the Oral Medicine Specialist due to a chief complaint of bleeding lips and difficulty opening her mouth for the previous 2 days. She is currently on ART and has been prescribed folic acid, dexamethasone, omeprazole, cotrimoxazole, and fluconazole. The extraoral examination revealed anemic conjunctiva and a hemorrhagic crust on the upper and lower lips that tended to bleed ( and ). No ulcers were found during the intraoral examination. The patient had fair oral hygiene, with an OHI-S score of 1.4. The viral load examination revealed an undetected virus, whereas the hematological examination revealed low levels of Hb, erythrocytes, lymphocytes, and MCHC. The oral lesions were diagnosed as drug-induced erythema multiforme (DIEM) and angular cheilitis. The treatment included normal saline, triamcinolone acetonide in orabase 0.1%, chlorhexidine gluconate mouthwash 0.2%, multivitamins, and miconazole cream 0.2% medications. After 15 days, the lesions had healed ( and ).
Figure 5 The extraoral lesions in patient Case 5 (A and B) On the first visit, there was a hemorrhagic crust on the upper and lower lips (C and D) The lesions resolved 15 days later.
A brief comparison of HIV-OLs and clinical characteristics of the patients in all cases is presented in .
Table 1 Comparison of HIV-OLs and the Patient’s Clinical Characteristics Across All HIV Stages
Discussion
The clinical stage of HIV according to the World Health Organization (WHO) in 2007 is divided into four stages: I, II, III, and IV.Citation12 This clinical stage is further classified into adults, infants, and children infected with HIV. According to WHO, oral mucosal lesions commonly occur between stages II and IV of HIV/AIDS. There are no oral lesions in clinical stage I HIV/AIDS; herpes zoster, angular cheilitis (AC), LGE, and recurrent oral ulceration (ROA) in stage II; persistent OC, OHL, and acute necrotizing ulcerative gingivitis in stage III; and HSV infection, cytomegalovirus (CMV) infection, and Kaposi’s Sarcoma (KS) in stage IV.Citation12,Citation13 In this report, oral findings were discovered in all clinical stages of PLWH. The variations in the prevalence and types of oral lesions in HIV patients across different countries can be attributed to multiple factors, such as the availability of combined antiretroviral therapy, socioeconomic status linked to ART non-adherence and health literacy, demographic conditions, and access to and utilization of healthcare facilities. These factors significantly influence the spectrum of HIV-OLs.Citation14
The 2018 World AIDS Day celebration’s slogan is “Know your status”. Oral manifestations can be extremely helpful in terms of diagnosis and prognosis, especially in clinical settings with limited resources.Citation5 HIV-OLs fall into two categories. The first is based on HIV-OLs etiology, which includes viral, fungal, and bacterial infections, as well as neoplastic lesions and other conditions. Second, according to the EC-Clearinghouse on Oral Problems Related to HIV Infection and the WHO Collaborating Centre on Oral Manifestations of the HIV Infection.Citation2 In this case report, we found some lesions belonging to Group I including OHL, OC, AC, and LGE; Group II including HSV infection; and Group III including erythema multiforme.Citation13
The treatment of oral lesions involves several approaches. RIH was treated with antiviral acyclovir, APC was managed with nystatin oral suspension, and miconazole cream was used for AC. Chlorhexidine (CHX) gluconate mouthwash 0.2%, was administered to patients with poor oral hygiene. Over the past 50 years, CHX has been extensively used as the gold standard mouthwash for controlling chemical dental plaque and reducing oral biofilm. CHX possesses rapid antimicrobial and antifungal properties, remaining effective even at low concentrations. It targets both aerobic and anaerobic bacteria and can destroy DNA and RNA viruses. Additionally, CHX inactivates lipophilic-enveloped viruses such as HIV, influenza A, parainfluenza, hepatitis B, herpes simplex virus, and cytomegalovirus.Citation15 In another case, hyaluronic acid (HA) mouthwash 0.025% was given to the patient with multiple ulcers. Known for its antioxidant, antibacterial, and anti-inflammatory properties, HA has recently been proposed for topical use in treating oral ulcers and other painful oral lesions. HA has multiple physiological and structural functions, including cellular and extracellular interactions, interactions with growth factors, and the regulation of osmotic pressure and tissue lubrication. HA promotes re-epithelialization through the proliferation of basal keratinocytes and helps reduce collagen deposition and scarring.Citation16 Topical corticosteroids were applied for oral ulcers. For EM cases, applying normal saline-soaked gauze followed by topical steroids proved highly effective. Additionally, petroleum jelly was used for dry lips, and multivitamins were given to the patients. Furthermore, we observed that multiple risk factors might influence the development of HIV-OLs) as shown in . Therefore, addressing these risk factors in patient management was crucial, including improving oral hygiene, treating comorbidities, promoting weight gain, encouraging smoking cessation, and initiating ART. The lesions in all cases improved over 5 to 15 days.
One of the risk factors for HIV-OLs is age. This report included 2 young individuals, 2 middle-aged individuals, and 1 elderly. While the immune system at birth has a diverse array of antigen-reactive T and B cells, it begins to experience immunosenescence from around the sixth decade of life. This age-related decline in immune function diminishes the body’s ability to defend against infections (eg, HIV, shingles) and cancer, and impairs wound healing. Consequently, older individuals are more susceptible to infections and cancer and exhibit slower wound healing compared to younger individuals.Citation10
Gender is also a risk factor for HIV-OLs. According to the Centers for Disease Control and Prevention (CDC) in 2019, women accounted for more than half of all HIV infections worldwide.Citation11 However, a study has reported that men are more frequently affected than women.Citation17 Intrinsic biological differences between men and women can have a significant impact on the progression of HIV infection. These sex-related factors influence HIV acquisition, viral replication, pathogenesis, and the type of ART used for pre-exposure prophylaxis or treatment.Citation11 Although the relationship between women’s hormonal and oral flora is unclear,Citation5 higher levels of estrogen are thought to be associated with lower HIV acquisition due to decreased migration of T cells and macrophages, as well as reduced pro-inflammatory signaling.Citation11 Conversely, lower estrogen levels may increase susceptibility to Candida albicans colonization.Citation8 Additionally, certain contraceptives have been linked to an increase in HIV target cells (CCR5 CD4+ cells) and a higher risk of HIV infection. In this report, there were 2 males and 3 females, with a higher occurrence of HIV-OLs noted in males in case 2.
The use of antiretroviral therapy (ART) has significantly improved the longevity and quality of life for HIV-positive patients while reducing the incidence of opportunistic infections and mortality. The reduction of certain oral manifestations, such as oral candidiasis, oral hairy leukoplakia, and Kaposi’s sarcoma, serves as an important marker of ART efficacy.Citation8 HIV-OLs are more frequently observed in individuals who have not been diagnosed with HIV or who have not received antiretroviral therapy,Citation18 as shown in case 2. OC occurred in up to 90% of patients before the introduction of ART, as shown in case 2. The prevalence of OC among patients receiving ART is 50% lower than among those who do not receive ART,Citation2 as seen in case 5. Among Candida spp., the most common pathogen is Candida albicans. Erythematous candidiasis, pseudomembranous candidiasis, and angular cheilitis are the three most common manifestations of OC.Citation2 EM is more likely to occur in HIV-positive individuals, as evidenced by cases 4 and 5, and has been reported in ART-related patients.Citation19 EM can be triggered by several factors, including food additives and chemicals such as those found in bee propolis products.Citation20 Comparable mechanisms could amplify EM in the context of both local and systemic immunoinflammatory reactions targeting HIV-infected epithelial cells. Although ART rarely causes life-threatening toxicity, it may still be discontinued in some cases.Citation19 OC and OHL were inversely and significantly associated with the type of ART used. This finding contradicts previous research, which found that these therapeutic agents can effectively protect against oral lesions.Citation5 In this report, 4 patients have received ART (cases 1,3,4,5), while 1 patient has not received ART (case 2).
Without sufficient energy nutrient intake, the body will catabolize its tissues for fuel, resulting in weight loss.Citation21 HIV-infected people frequently waste energy (low BMI) and lose weight. Low BMI, as observed in case 2, and weight loss, are significant risk factors for HIV development and mortality, regardless of CD4+ lymphocyte count or other immune system performance markers.Citation21 In contrast, Jana et al found no significant correlation between oral lesion presence and BMI,Citation5 as seen in cases 1,3,4,5.
HIV patients’ oral symptoms are linked to CD4+ lymphocyte countsCitation8 and viral loads.Citation7,Citation9 The previous study showed that CD4+ < 200 cells/μL are at risk for recurrent aphthous ulcer, xerostomia periodontal diseases; as well as CD4+ <100 cells/μL for oral herpes virus and oral hyperpigmentation.Citation8 The prevalence of HIV-OLs was higher in individuals with CD4+ cell counts below 200 cells/mm³ compared to those with counts above 200 cells/μL.Citation22 Viral load is classified as low (167 to 10,000 copies/μL), medium (10,000 to 100,000 copies/μL), and high (more than 100,000 copies/μL).Citation22 Individuals with high viral load (VL) levels or detectable viral load in HIV-positive patients,Citation5 as in case 1, may experience complete depletion of mucosal Langerhans cells, reducing mucosal immunologic protection. This cellular event is linked to fungal and viral colonization, particularly Epstein-Barr, in the affected area. Local inflammatory conditions can cause salivary levels of HIV-RNA, to rise above plasma levels. This increase in viral load leads to a higher incidence of oral symptoms.Citation18 OHL is a benign epithelial hyperplasia on the tongue’s lateral borders that affects more men than women. OHL, which is almost exclusively found in patients with untreated advanced HIV disease and other immunocompromised individuals,Citation23 is caused by latent Epstein-Barr virus (EBV) reactivation.Citation2 OHL is associated with increased exposure to EBV, lower CD4+ count, and higher HIV viral load.Citation8
HIV suspicion can arise from the common lesions that frequently occur in immunocompromised states because oral lesions can be detected early in HIV infection,Citation18 as seen in cases 2, 4, and 5. Moderate to severe immunodeficiency was found to increase the risk of developing oral lesions. Immunocompromised patients are unable to respond normally to infections because their immune systems are impaired or weakened. This inability to fight infection can be caused by several factors including underlying disease (malignancy, organ or stem cell transplantation, systemic vasculitis, connective tissue diseases, etc.) associated conditions (diabetes, malnutrition, etc.) and drug-induced immune suppression.Citation24 Individuals with HIV typically have a weakened immune system, making them more susceptible to opportunistic infections such as oral candidiasis.Citation13 Oral candidiasis has become more common among HIV-infected persons, with estimates ranging from 25% to 90%.Citation25 T helper (Th)17 T cells are important for the immune response to Candida spp. at the mucosal surface. During the early stages, HIV-associated T cell decline results in a disproportionate depletion of this T cell subgroup, which compromises host surveillance and increases pathogenicity. HIV infection is highly permissive of Candida-specific Th17 T cells.
Injectable drug abuse and tuberculosis are two clinical risk factors for oropharyngeal candidiasis in HIV-positive people, in addition to immunosuppression. Other risk factors include diabetes mellitus, topical or systemic corticosteroids, and poorly managed gastroesophageal reflux disease (GERD), as Candida spp. thrive in acidic environments.Citation26 Herpesviruses reactivate and spread when an individual has HIV infection, including HSV-1 in the oral mucosa, as seen in cases 1, 2, and 3. HIV-associated HSV-1 depletes CD4+ T cells in peripheral blood, lymphoid organs, and mucosal tissues, causing CD8+ T cell dysfunction and immune system attenuation. Herpesviruses can become active due to HIV-mediated depletion and malfunction of CD4+ /CD8+ T immune cells impairing the function of the oral mucosa barrier across the epithelium.Citation1 HIV tat and gp120 proteins, found in saliva and salivary tat, when penetrating mucosal epithelium, contribute significantly to the mucosal barrier’s impairment by disrupting epithelial tight junctions.Citation1 Atypical ulceration, including traumatic ulcer, occurs in 3–13% of HIV-infected patients, as seen in case 3. Several conditions, including trauma, infection, immune-mediated diseases, systemic disease, or neoplasia, can cause oral ulcers. Due to the high prevalence of infections in HIV patients, ulcer etiology may include infections.Citation2
Another risk factor associated with HIV/AIDS is the presence of multispecies biofilms caused by poor oral hygiene. “Oral biofilm” is the new term for dental plaque. Oral biofilm is a community of microorganisms that live on the tooth surface or within the sulcus (periodontal pocket) and are embedded in a matrix of polymers derived from the host and bacteria. Oral biofilm bacteria can regulate numerous processes using quorum sensing, a cell-to-cell communication mechanism that synchronizes gene expression in the biofilm in response to the density of the cell population. This includes secreting specific enzymes that either activate or deactivate the genes of other bacteria. These bacterial byproducts elicit an immune response from the host, attracting white blood cells (WBC) to the site to kill the invading bacteria. The bacteria can confuse the defending WBC chemotactically by releasing chemicals into the surrounding environment, rendering the immune response ineffective.Citation27 Oral pathogens can spread to distant body organs via the local, oral blood circulation, through the gastrointestinal tract, and into the systemic circulation. When oral pathogens reach an organ, they alter the immune response and cause the release of inflammatory mediators, resulting in systemic disease.Citation24,Citation27,Citation28 Oral hygiene status was evaluated using the simplified oral hygiene index (OHI-S). OHI-S scores are classified as good (0–1.2), fair (1.3–3.0), and poor (3.1–6.0).Citation29 In this report, there were 3 cases of fair oral hygiene (case 1,3,5) and 2 cases of poor oral hygiene (case 2.4).
The significant predictors of the occurrence of oral lesions conducted in the Jana et al study were tobacco and alcohol use.Citation5 Smoking and alcohol consumption increased susceptibility to the development of HIV-OLs.Citation8 Patients who smoked were more likely to develop OHL regardless of their CD4+ counts or VL levels.Citation18 Case 2 involved a smoker who developed oral lesions of the RIH, OHL, and OC pseudomembranous type. Cigarette smoke’s constituents have the potential to cause chronic inflammation of the oral mucosa, harm the body’s natural defenses against infections, and inhibit cell growth via apoptosis mechanisms. These effects of smoking reduce the production of salivary enzymes, immunoglobulin, and lymphocytes, resulting in an imbalance of the oral microflora. These changes are likely to increase EBV infectivity, which increases the risk of OHL.Citation18 There were no reports of alcohol consumption in this study. Candida albicans oxidizes salivary ethanol, resulting in high levels of acetaldehyde production. This product affects the oral mucosa by increasing its permeability, resulting in atrophic areas on the surface of the epithelial that have become depleted in extracellular lipids as a result of alcohol consumption.Citation18
The patients and a patient’s relative provided their written and informed consent for the publication of all photos and case details in this report. This set of cases adhered to the Helsinki Declaration. The institution has also approved the publication of this case report.
Conclusion
This case series underscores that oral lesions are prevalent throughout the stages of HIV and are influenced by a range of factors including immune status, medication adherence, and overall health. This highlights the need for a holistic approach to patient care. By addressing these multifaceted influences on oral health, we can better manage lesions, improve patient quality of life, and potentially alter the course of HIV progression. Additionally, integrating oral health assessments into routine HIV care is crucial for reducing the morbidity associated with oral lesions and improving patient outcomes.
Abbreviation
AC, Angular cheilitis; APC, Acute pseudomembranous candidiasis; ART, Antiretroviral therapy; BMI, Body mass index; DIEM, Drug-induced erythema multiforme; EBV, Epstein-Barr virus; HIV, Human immunodeficiency virus; HSV, Human simplex virus; LGE, Linear gingival erythema; OC, Oral candidiasis; OHL, Oral hairy leukoplakia; RIH, Recurrent intraoral herpes; VL, Viral load; WBC, White blood cells; WHO, World Health Organization.
Informed Consent
All patients and a patient’s relative have provided their informed consent for the publication of their case details and any accompanying images. The institution has also approved the publication of this report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation. They took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this article.
Acknowledgments
The authors are grateful to the patients who agreed to participate in this case report. Furthermore, the authors appreciate the assistance provided by the staff of Teratai Clinic, Dr. Hasan Sadikin General Hospital. The authors would like to thank Universitas Padjadjaran for funding the publication of this report.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sufiawati I, Tugizov SM. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS One. 2014;9(2):e88803. doi:10.1371/journal.pone.0088803
- Aškinytė D, Matulionytė R, Rimkevičius A. Oral manifestations of HIV disease: a review. Stomatologija. 2015;17(1):21–28.
- Mary EO, Abiola OA, Titilola G, Mojirayo OO, Sulaimon AA. Prevalence of HIV related oral lesions in people living with HIV and on combined antiretroviral therapy: a Nigerian experience. Pan Afr Med J. 2018;31:180. doi:10.11604/pamj.2018.31.180.13574
- Sufiawati I, Amalia T, Dewi TS, Wisaksana R. The association between oral mucosal lesions and oral health-related quality of life using the validated Indonesian version of OHIP-14 among people living with HIV/AIDS. HIV AIDS. 2024;16:9–16.
- Jana PK, Sahu SK, Sivaranjini K, Hamide A, Roy G. Prevalence of oral lesions and its associated risk factors among PLHIV availing anti‑retroviral therapy from a selected tertiary care hospital, Puducherry ‑ A cross sectional analytical study. Indian J Community Med. 2022;47(2):235–239. doi:10.4103/ijcm.ijcm_850_21
- Pakfetrat A, Falaki F, Delavarian Z, Dalirsani Z, Sanatkhani M, Marani MZ. Oral manifestations of human immunodeficiency virus-infected patients. Iran J Otorhinolaryngol. 2015;27(78):43–54.
- Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. 2015;1(1):15035. doi:10.1038/nrdp.2015.35
- Lomelí-Martínez SM, González-Hernández LA, Ruiz-Anaya A de J, et al. Oral manifestations associated with HIV/AIDS patients. Medicina. 2022;58(9):1214. doi:10.3390/medicina58091214
- Berberi A, Aoun G. Oral lesions associated with human immunodeficiency virus in 75 adult patients: a clinical study. J Korean Assoc Oral Maxillofac Surg. 2017;43(6):388–394. doi:10.5125/jkaoms.2017.43.6.388
- Weyand CM, Goronzy JJ. Aging of the immune system: mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Suppl 5):S422–S428. doi:10.1513/AnnalsATS.201602-095AW
- Moran JA, Turner SR, Marsden MD. Contribution of sex differences to HIV immunology, pathogenesis, and cure approaches. Front Immunol. 2022;13:905773. doi:10.3389/fimmu.2022.905773
- World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. World Health Organization; 2007.
- Rosa DE, Sufiawati I. Case series of HIV-associated oral lesions among antiretroviral-naive patients during the COVID-19 pandemic. Int Med Case Rep J. 2023;16:73–82. doi:10.2147/IMCRJ.S398736
- da Rocha G de CT, de Souza Fonseca RR, Oliveira-Filho AB, et al. Evaluation of sociodemographic factors and prevalence of oral lesions in people living with HIV from Cacoal, Rondônia, Amazon Region of Brazil. Int J Environ Res Public Health. 2022;19(5):2614. doi:10.3390/ijerph19052614
- Poppolo Deus F, Ouanounou A. Chlorhexidine in dentistry: pharmacology, uses, and adverse effects. Int Dent J. 2022;72(3):269–277. doi:10.1016/j.identj.2022.01.005
- Casale M, Moffa A, Vella P, et al. Systematic review: the efficacy of topical hyaluronic acid on oral ulcers. J Biol Regul Homeost Agents. 2017;31(4 Suppl 2):63–69.
- Sufiawati I, Harmiyati R, Nur’aeny N, et al. Detection of human herpesviruses in sera and saliva of asymptomatic HIV-infected individuals using multiplex RT-PCR DNA microarray. Pathogens. 2023;12(8):993. doi:10.3390/pathogens12080993
- Caputo V, Libera M, Sisti S, Giuliani B, Diotti RA, Criscuolo E. The initial interplay between HIV and mucosal innate immunity. Front Immunol. 2023;14:1104423. doi:10.3389/fimmu.2023.1104423
- Manenzhe SC, Khammissa RAG, Shangase SL, Beetge MM. Exploring the association between erythema multiforme and HIV infection: some mechanisms and implications. AIDS Res Ther. 2024;21(1):24. doi:10.1186/s12981-024-00607-6
- Chiang ML, Jin YT, Chang JYF, Chiang CP. Bee propolis-induced erythema multiforme. J Formos Med Assoc. 2021;120(8):1652–1654. doi:10.1016/j.jfma.2021.03.006
- Cordeiro SA, Lopes TCP, Boechat AL, Gonçalves RL. Weight loss and mortality in people living with HIV: a systematic review and meta-analysis. BMC Infect Dis. 2024;24(1):34. doi:10.1186/s12879-023-08889-3
- Yang Y, Yu F, Fei Y, Dong G, Cao P, Liu Y. Immune indices and oral health in patients infected with the human immunodeficiency virus. BMC Oral Health. 2023;23(1):1009. doi:10.1186/s12903-023-03752-y
- Greenspan JS, Greenspan D, Webster-Cyriaque J. Hairy leukoplakia; lessons learned: 30-plus years. Oral Dis. 2016;22(Suppl 1):120–127. doi:10.1111/odi.12393
- Azoulay E, Soares M, Benoit D. Focus on immunocompromised patients. Intensive Care Med. 2016;42(3):463–465. doi:10.1007/s00134-016-4224-8
- Novianti Y, Sufiawati I. Clinical assessment and management in improving the quality of life of HIV/AIDS patients with oral candidiasis: a case series. HIV AIDS. 2023;15:683–696.
- Infectious R, Unit D, Infectious R, Unit D, Centre MM. British HIV association/British infection association guidelines on the management of opportunistic infection in people living with HIV: the clinical management of Candidiasis 2019. HIV Med. 2019;20(Suppl 8):2–24. doi:10.1111/hiv.12806
- Kurtzman GM, Horowitz RA, Johnson R, Prestiano RA, Klein BI. The systemic oral health connection: biofilms. Medicine. 2022;101(46):e30517. doi:10.1097/MD.0000000000030517
- Woelber JP, Al-Ahmad A, Alt KW. On the pathogenicity of the oral biofilm: a critical review from a biological, evolutionary, and nutritional point of view. Nutrients. 2022;14(10):2174. doi:10.3390/nu14102174
- Baishya B, Satpathy A, Nayak R, Mohanty R. Oral hygiene status, oral hygiene practices and periodontal health of brick kiln workers of Odisha. J Indian Soc Periodontol. 2019;23(2):163–167. doi:10.4103/jisp.jisp_383_18