Abstract
Purpose
To analyze the hepatitis C virus (HCV) burden in Lebanon and the value of comprehensive screening and treatment for different age groups and fibrosis stages.
Methods
We used a multicohort, health-state-transition model to project the number of HCV genotype 1 and 4 patients achieving a sustained virologic response 12 weeks after treatment or progressing to compensated cirrhosis (CC), decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC), or liver-related death (LrD) from 2016 to 2036. In the low/medium/high screening scenarios, the proportion of patients screened for HCV was projected to increase to 60%/85%/99%, respectively, by 2036. We analyzed four treatment strategies: 1) no treatment, 2) all-oral direct-acting antivirals (DAAs) given to F3–F4 (CC) patients only, 3) all-oral DAAs to F2–F3–F4 (CC) patients, and 4) all-oral DAAs to all fibrosis patients.
Results
Low, medium, and high HCV screening scenarios projected that 3,838, 5,665, and 7,669 individuals will be diagnosed with HCV infection, respectively, from 2016 to 2036, or 40% of those aged 18–39 years, and 60% of those aged 40–80 years. With no treatment, the projected number of patients reaching CC, DCC, HCC, or LrD in 2036 was 899, 147, 131, and 147, respectively, for the 18–39 years age group. For the 40–80 years age group, these projections were substantially greater: 2,828 CC, 736 DCC, 668 HCC, and 958 LrD. The overall economic burden without treatment reached 150 million EUR. However, introducing DAAs for F0–F4 patients was projected to increase the proportion of remaining life-years spent in sustained virologic response 12 weeks after treatment by 43% and 62% compared to DAAs given at F2–F4 or F3–F4 only, respectively.
Conclusion
An enhanced screening policy combined with broader access to DAAs can diminish the future clinical and economic burden of HCV in the Lebanese population and, for the middle-aged and elderly, provide the greatest health benefit with net cost savings.
Introduction
Hepatitis C virus (HCV) infections are a major health problem. Prevalence is 2.3% and 1.5% in World Health Organization (WHO) Eastern Mediterranean and European Regions, respectively, and there are 71 million people with chronic HCV infections worldwide.Citation1 Approximately 75%–85%, 60%–70%, and 5%–20% of people infected with HCV will develop chronic hepatitis, hepatic steatosis or fibrosis, and cirrhosis, respectively. Also, 1%–5% of people acutely infected with HCV will incur life-threatening complications and hepatocellular carcinoma (HCC) within 20 years.Citation2
Lebanon is a low endemic region for hepatitis C, with a prevalence of 0.2% in the general population.Citation3 The viremic rate is 75%.Citation3 People over 40 years have the highest prevalence and are at the most advanced fibrosis stage.Citation4 Genotype 1 is the most prevalent genotype (80% is genotype 1b), followed by genotype 4, representing together >80% of the total infected population.Citation5
Eradicating HCV is the most effective way to reduce the incidence of liver decompensation and HCC.Citation6 In addition, chronic hepatitis C (CHC) is a systemic condition associated with extrahepatic manifestations such as diabetes mellitus, cardiovascular disease, psychiatric disorders, renal dysfunction, and rheumatologic conditions.Citation7
The treatment of HCV infection has undergone profound and rapid innovation. For more than two decades, administering interferon (IFN) alpha in combination with ribavirin was the basis of all HCV therapies, to which 30%–90% of patients responded.Citation8 By 2017, the Food and Drug Administration and the European Medical Agency had approved eight all-oral, interferon-free direct-acting antiviral (IFN-free DAA) regimens to treat different genotypes. The sustained virologic response (SVR) rates in pivotal Phase II and III trials were between 92% and 100%, even in pretreated HCV genotype 1-infected patients.Citation9 For most patients, treatment is now possible with highly effective, safe, and tolerable combinations of oral DAAs.
The eradication of hepatitis C is currently considered as within reach, but pricing and funding remain important barriers to treatment access. The prices of DAAs have caused public discussion in most countries and the commitment to fund comprehensive elimination campaigns remains low.Citation10 Nonetheless, recent studies have indicated that for genotype 1 the current all-oral DAA-based regimens are cost-effective.Citation11 And if we consider, in addition to liver-related clinical outcomes, extrahepatic complications, HCV therapy could be even more cost-effective.Citation12
HCV infections have been shown to impose a high economic burden on the US health care system, individuals, and society.Citation13 Recent simulation studies have estimated that the clinical and economic burdens of HCV will continue to increase over the next two decades.Citation14–Citation16 Despite this burden, the vast majority of HCV-infected patients remain undiagnosed and, therefore, not appropriately managed.Citation17 This is troubling given the availability of highly effective therapy, as described above. Given the heavy economic burden of HCV, it is important to understand the direct costs attributable to HCV infection, complications, and treatment to better clarify the value and cost-effectiveness of new treatments.
The aim of this study was to estimate the HCV burden in Lebanon and the potential benefits of different screening and treatment strategies that leverage novel antiviral agents compared to older IFN-based treatments.
Methods
We have analyzed the HCV data from the published literature on HCV in Lebanon, including prevalence, genotypic distribution, degree of liver fibrosis, progression of CHC, treatment efficacy and cost, as well as cost and complications of liver disease. We constructed a mathematical model of CHC progression to examine the effectiveness of different screening and treatment policies over the next 20 years, on the progression of fibrosis gradually develops stage-by-stage in those with chronic infection than cirrhosis that progresses to decompensated cirrhosis (DCC) stage over time.
Target population
The Lebanese population was selected and divided into two age groups: 18–39 and 40–80 years. The age distribution of the population was retrieved from the Lebanese Ministry of Public Health statistics bulletin of 2013. Using the national growth rate and an open sequential multicohort model, projections were conducted from 2016 to 2036 for the two groups.
Model structure
The multicohort model was designed using Microsoft Excel®. It forecasts population health outcomes for different treatment strategies adjusted by the defined HCV screening scenario. The CHC natural history used in the model is shown in .
Figure 1 Model schematic of the natural history of chronic hepatitis C.
Abbreviations: CC, compensated cirrhosis (Metavir fibrosis score F4); D, all-cause death; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LrD, liver-related death (ie, death from DCC, HCC, or LT); LT, liver transplant; SVR, sustained virologic response.
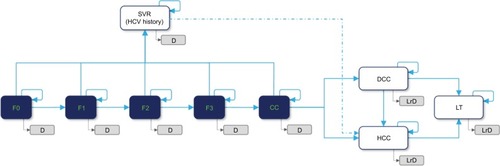
The natural history of CHC was modeled through a health-state-transition process. A Markov model simulating chronic HCV progression was used to estimate disease treatment costs and hepatic outcomes over patients’ lifetimes. At the time of screening, the identified CHC subjects were allocated to the diagnosed fibrosis stages F0–F4/compensated cirrhosis (CC). The subjects then progressed annually from fibrosis stage F0 to F4/CC and to DCC and/or HCC. Subjects could also die from background mortality from any health state. It was assumed that death from DCC, HCC, or liver transplant (LT) constituted a liver-related death (LrD).
For simplicity, the model assumed an overall rate of screening that takes into account the different screening and diagnosis steps. For instance, a rate of 10% means that the HCV status (genotype and fibrosis stage) is known for 10% of the individuals who tested positive.
Each year, a new cohort of patients diagnosed with GT1–4 CHC was added to the previous cohort as the overall population became increasingly screened over the 2016–2036 period. Each cohort evolved through the CHC health-state-transition model described above (), with transition probabilities adjusted for the respective treatment effectiveness. Each year, the model computed the number of patients in each health state within the treated CHC population.
Prevalence and fibrosis distribution
A recent study showed that the prevalence of hepatitis C in the Lebanese general population was 0.2%Citation3 and the highest prevalence was in people older than 60 years (0.55%), followed by those aged 40–60 years (0.37%).Citation4 Also, fibrosis severity was recently studied in Lebanon, with differences noted between different age groups. Advanced fibrosis was seen in 6.4% of people aged <40 years, in 45% of those aged 40–60 years, and in 83.5% of those aged >60 yearsCitation4 ().
Table 1 Proportion of patients in each fibrosis stage
For simplicity, we divided our population into two groups: 18–39 years, reflecting in majority the population of drug users and 40–80 years, reflecting the baby-boomer population ().
Screening scenarios
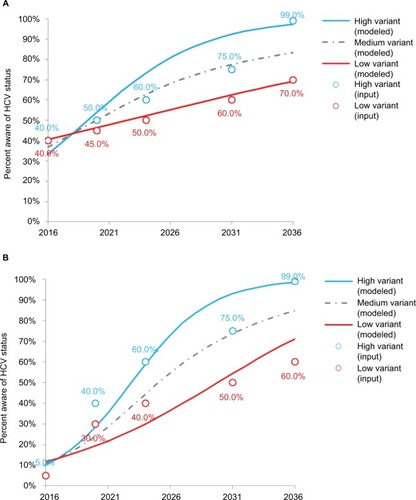
Three HCV screening scenarios were used for the following groups: low, medium, and high variants. The medium variant corresponded to the mean between the low and the high variant. The screening scenarios were defined for the following 20 years (from 2016 to 2036) by setting five-point estimates (2016, 2021, 2026, 2031, and 2036), each corresponding to a 5-year average screening rate. These estimates capture the forecasted/targeted cumulative rates of HCV awareness among the survivors of the selected population at the respective dates. As the model uses a yearly cycle, a logistic regression was fitted to these five estimates to yield yearly cumulative rates of HCV screening. shows the screenings used for the two groups.
Disease progression
Annual transition probabilities were taken from multiple sources in the literature ().
Table 2 Annual transition probabilities in different studies
Treatments’ effectiveness
shows the different sustained virologic responses 12 weeks after treatment (SVR12s) that were assumed according to the therapy and liver fibrosis stages. All estimates were taken from randomized clinical trials.
Table 3 Different SVR12s assumed according to therapy and liver fibrosis stage
Treatments of adverse events
Only severe adverse events from the various treatment regimens were considered: severe anemia, severe depression, and severe rash. The incidence estimates of severe adverse events were taken from a recent French modeling study.Citation18
Health care costs and perspective
Only direct inpatient and outpatient medical costs were considered. They are the costs of HCV screening, therapy, adverse events, fibrosis stages, LrDs, and liver complications (DCC, HCC, and liver transplantation). The 2014 and 2015 average costs from major health insurance claims were collected. The model used the perspective of the Lebanese health care system.
Base-case analysis
Newly diagnosed patients received either all-oral, IFN-free DAAs or no treatment, depending on their fibrosis stage (ie, treatment strategy A, B, C, or D). We compared four treatment strategies for the two age groups: A, no treatment at all; B, all-oral, IFN-free DAAs given to F3–F4 patients only; C, all-oral, IFN-free DAAs given to F2–F3–F4 patients only; and D, all-oral, IFN-free DAAs given to all, ie, F0–F1–F2–F3–F4 patients.
The model forecasted and compared the number of patients reaching SVR, DCC, HCC, and LT health states for the four treatment strategies. Likewise, the number of life-years (LYs) spent in each health state and the number of LrDs were computed and compared. Population-level health outcomes were also presented as the percentage of years lived under each health state (SVR, DCC, HCC, and LT) out of the total number of years lived by these selected population.
Sensitivity analysis
One-way deterministic sensitivity analyses were carried on the costs of treatment per LY spent in SVR12 for each treatment strategy. All model parameters were varied within ±20% ranges subject to plausibility check (ie, the transition probabilities could not be <0 or >1). The distribution of fibrosis stages was varied between a “mild” scenario (F0: 96%, F1: 1%, F2: 1%, F3: 1%, and F4: 1%) and a “severe” scenario (F0: 1%, F1: 1%, F2: 1%, F3: 1%, and F4: 96%) in comparison to the baseline distribution. We also assessed outcomes in the “low” and “high” screening scenarios and varied the discount rate from 0% to 5% for future costs and LYs in SVR12. The impact of the 10 most influential parameters was plotted on tornado diagrams, as appropriate.
Results
Size of the GT1- and GT4-diagnosed populations initiated on treatment
shows the predicted number of newly diagnosed HCV patients over the 2016–2036 time periods according to the three screening variants and two age groups. The low, medium, and high HCV screening scenarios showed, respectively, that 1,621, 2,526, and 3,432 individuals aged 18–39 years in 2016 would be diagnosed with HCV infections between 2016 and 2036 compared to 2,217, 3,139, and 4,237 individuals aged 40–80 years.
Table 4 Newly diagnosed HCV patients between 2016 and 2036 in the 18–39 and 40–80 age groups
Burden of illness and public health impact in Lebanon
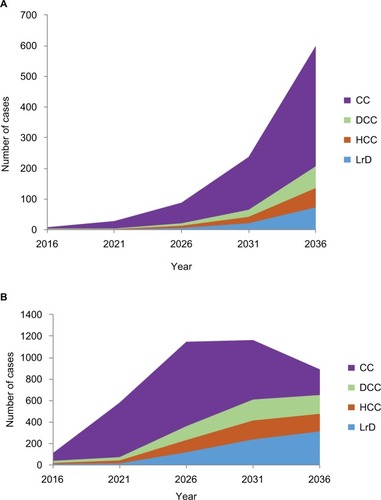
In the high screening scenario with no treatment given, the number of patients reaching CC, DCC, HCC, and LrD in 2036 are summarized in in the two age groups.
Figure 3 Hepatic complications from HCV (cumulative cases) for the 18–40 (A) and 40–80 years age groups (B).
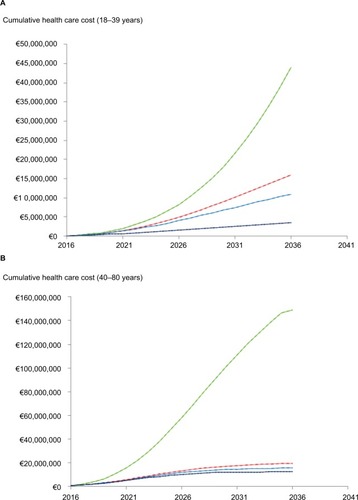
The cumulative health care cost secondary to HCV will continue to increase during the next 20 years. The cost of HCV complications is summarized in for the two age groups.
Figure 4 Cumulative cost of HCV complications in the 18–39 (A) and 40–80 years age groups (B).
Abbreviation: HCV, hepatitis C virus.
When introducing oral DAAs at different strategies, the number of life years lived with SVR12 during 2016–2036 is dramatically improved (). All-oral DAAs for F0–F4 patients aged 18–39 increased the proportion of the remaining LYs spent in SVR by 31% and 53% compared to all-oral, IFN-free DAAs given to F2–F4 or to F3–F4, respectively. Similarly, all-oral DAAs for F0–F4 patients aged 40–80 years also increased the remaining LYs spent in SVR compared to all-oral, IFN-free DAAs given to F2–F4 or to F3–F4, respectively.
Table 5 Clinical burden – number and percent of life-years spent in SVR
Budget impact and return on investment
The treatment, adverse event, and medical costs to the health care system of the four treatment strategies are shown in . The all-oral, IFN-free DAAs for the F0–F4 strategy have the greatest cost impact as they are used in a greater number of patients, with the largest impact in the 18–39 age group.
Table 6 Economic burden – health care costs
summarizes the return on investment and compares the percentage of person LYs spent in SVR and the budget impact of the four treatment strategies considered over the 2016–2036 time frame. The all-oral, IFN-free DAAs for F0–F4 increased the cost of HCV treatment but considerably increased the health benefit at the population level (ie, drastic increase in the proportion of LYs spent in SVR compared to other strategies). According to the model, adopting the high screening variant and all-oral, IFN-free DAAs for the entire 18–39 age group would cost 1,957 EUR for a life year gained in SVR12 compared to no treatment and screening. For the 40–80 age group, there would be a cost savings of −168 Euro/LY spent in SVR12 compared to no treatment strategy.
Table 7 The return on investment, the percent of person LYs spent in SVR, and the budget impact of the 4 treatment strategies considered over the 2016–2036 time frame in the 18–39 and the 40–80 years age groups
Sensitivity analysis
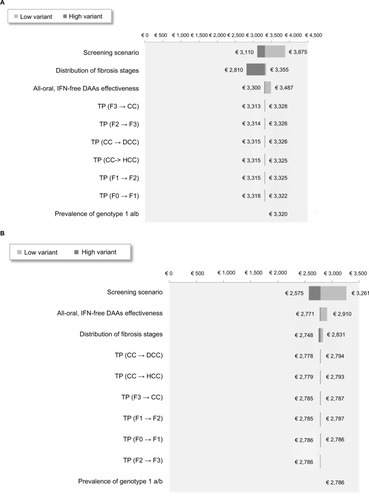
The results of the one-way deterministic sensitivity analysis on the cost of treatment/LYs in SVR12 for all-oral, IFN-free DAAs for F0–F4 are shown in . Results were most sensitive to changes in the screening scenario, fibrosis distribution, and, to a lower extent, DAA efficacy.
Figure 5 One-way deterministic sensitivity analyses on the cost of treatment/LYs in SVR12 for all-oral, IFN-free DAAs for F0–F4 in the 18–39 (A) and 40–80 years age groups (B).
Discussion
In this study, we explored the impact of providing antiviral treatment to a larger proportion of persons with HCV infection under different screening scenarios. According to the model, concerning the screening strategy, the high screening scenario will be able to detect >90% of the total population infected with HCV. According to the model, the proportion of patients with advanced HCV-related liver diseases will increase between 2016 and 2036. This phenomenon is due to the aging of the infected population and the resultant progression of liver fibrosis. The incidences of DCC, HCC, and LT are expected to peak between 2031 and 2036. Except for CC, the peak is expected in the age group above 40 years between 2026 and 2031.
We also demonstrated that the burden of hepatitis C and associated costs will continue to grow over the next 20 years. But, introducing DAAs is projected to improve the life years lived with SVR, especially when all stages of liver fibrosis are treated. If a high screening strategy is adopted, all oral DAAs and the treatment of all liver fibrosis stages will have the best budget impact and the highest return of investment in the different age groups.
We also found that all-oral, IFN-free DAAs for F0–F4 patients can increase by 52% the number of years that the HCV-infected Lebanese population aged 40–80 years in 2016 will live with SVR12 compared to alternative strategies that leave mild stages of the disease untreated. Our results suggest that an enhanced screening policy, especially for baby boomers, coupled with broader access to all-oral, IFN-free DAAs can diminish the future burden of HCV.
Several studies have already demonstrated the effectiveness and cost-effectiveness of HCV screening.Citation19–Citation21 To enhance screening cost-effectiveness, the screening should focus on populations with higher HCV prevalence.Citation20–Citation22 However, screening should also be accompanied by effective treatment and quality follow-on care.Citation19
We found three cost-effectiveness studies of new all-oral, IFN-free regimens for CHC.Citation18,Citation23,Citation24 Hagan et alCitation23 reported that new all-oral, IFN-free regimen was cost-effective compared to standard of care in a 50-year-old cohort of CHC patients. Deuffic-Burban et alCitation18 showed that waiting for IFN-free regimens and then treating all patients regardless of their fibrosis stage was the most effective approach, except for F4 patients, and Younossi et alCitation24 determined the cost-effectiveness of IFN-containing vs. IFN-free regimens to treat HCV GT1-infected patients. Interestingly, they assessed the impact of the treatment decision based on the liver disease stage (staging-guided approach) vs. treat-all approach on the cost-effectiveness of these two regimens. The authors showed that an all-oral, IFN-free regimen for all HCV GT1 patients might be the most cost-effective regimen in this patient population.
As the population with chronic HCV ages, the incidence of advanced liver disease complications (HCC and DCC) is expected to substantially increase, and as the HCV population ages and the costs for treatment increase, the economic burden of chronic HCV infection is expected to grow proportionally with its clinical burden over the next 10–20 years. Previous work examining the direct costs of HCV treatment revealed that HCV costs were similar to the costs of treating other chronic diseases such as asthma or rheumatoid arthritis.Citation25,Citation26 Similarly, other studies showed that the economic burden of HCV will continue to growCitation27 and that the annual per patient cost burden to payers for chronic HCV exceeds that of more common conditions such as cardiovascular disease ($18,953)Citation28 and type 2 diabetes ($9677).Citation29 Despite a decreased rate of in-hospital mortality, the hospital volume and the charges related to hepatitis C have substantially increased.Citation30 Another study demonstrated increasing trends in hospitalization rates because of HCV and advanced liver disease between 2004 and 2011, and it showed an 18%–50% increase in the average cost of hospitalizations.Citation31
For managed care organizations, chronic HCV is a costly disease. Disease-related costs in HCV exceed all-cause costs in demographically matched controls. The direct medical expenditure attributable to chronic HCV in the United States between 2010 and 2019 is expected to be $10.7 billion. During this period, HCV may lead to 720,700 years of DCC and HCC and to the loss of 1.83 million years of life in those younger than 65. Over the 10 years from 2010 through 2019, the societal cost of premature mortality for those younger than 65 was projected to be $54.2 billion, and the cost of morbidity from disability related to DCC and HCC was projected to be $21.3 billion.Citation32
It was found that increased efforts in HCV screening and early treatment, particularly before progression to liver cirrhosis, may lead to long-term cost savings in HCV management for managed care systems.Citation27 The majority of the models’ structure considers patients with SVR to be cured and, therefore, free of progression independent of the level of fibrosis in which such response is reached. A recent study found that achieving SVR can increase survival, reduce liver and extrahepatic morbidity, and lower long-term costs. Even unsuccessful CHC treatments appear to have a protective effect against mortality and liver morbidity. Treatment with DAAs in early fibrosis stages is generally cost-effective compared to older regimens or late treatment.Citation33 Compared to no treatment, all treatments reduce the proportion of patients who progress to cirrhosis, HCC, LT, or death.Citation34–Citation36 Therefore, there is compelling clinical and economic evidence that treating patients in early and all fibrosis stages improves survival, reduces liver-related and extrahepatic morbidity, and is cost-effective.
Most notable is the evidence from a review of 38 studies (n=73,861) showing significant mortality benefit of SVR in patients at all fibrosis stages.Citation33 A systematic review has shown that SVR reduces liver-related mortality (3.3- to 25-fold), HCC incidence (1.7- to 4.2-fold), and hepatic decompensation (2.7- to 17.4-fold).Citation37 Another meta-analysis showed that SVR is associated with lower HCV-related morbidity and mortality.Citation38 Modeling studiesCitation39 have confirmed these results by showing that delaying treatment could significantly increase mortality, morbidity, and medical costs. Additionally, there are >200 studies showing that chronic HCV infection is associated with several serious extrahepatic manifestations, some of which are associated with high mortality.Citation40,Citation41 The incidence of end-stage liver diseases and LRDs can be reduced by focusing treatment on patients with high fibrosis (F3 or F4) but a significant reduction in the total number of infections cannot be achieved unless treatment is expanded to patients in the early stages of fibrosis. The WHO currently recommends that all patients with chronic HCV should be assessed for treatment, but the challenge is clearly in how to provide treatment at a total cost that health systems and patients can afford.Citation42
Our results demonstrate the benefits of treating early stages of fibrosis, which is consistent with evidence published in other patient populations like USA,Citation43 Switzerland,Citation44 and France.Citation24 Another study showed that treatment with sofosbuvir and ledispavir not only reduces HCV-related complications but also is cost-effective for most patients.Citation45 Considering the higher acquisition costs, second-generation DAAs are generally cost-effective in treating CHC patients in many countries.Citation34–Citation36,Citation46,Citation47
A systematic review of the economic evaluations of DAAs found that DAAs were either cost-saving or cost-effective in 88% of analyses.Citation48 When other costs (such as productivity losses) are also considered, IFN+RBV-free second-generation DAA regimens were found to be even more cost-effective.Citation34 New-generation DAAs offer 90%–100% cure rates, have a significantly better safety profiles, and are treatment options to patients in all stages of liver disease.Citation49 Untreated HCV infection was found to be associated with substantial economic costs to society through the loss of productivity and increased use of health care resources, with decreased patient well-being.Citation50
Key drivers in the CHC economic models are the progression to and the costs associated with advanced liver disease, including HCC. But, two recent studies have reported higher rates of recurrence of HCC in DAA-treated patients compared to what the authors expected in untreated or IFN-treated patients.Citation51,Citation52 Nevertheless, a third study (reviewing patients from three French cohorts) showed no increased risk of HCC recurrence.Citation53
Conclusion
The present study sought to contribute to better understanding of the burden associated with HCV infection in Lebanon by estimating the prevalence and the number of deaths that can be attributed to HCV infection and by assessing the health care and societal costs associated with HCV infection under different scenarios of diagnosis and treatment rates. We project that improvements in HCV screening combined with broader access to DAAs can diminish the future clinical and economic burden of HCV in the Lebanese population and, for the middle-aged and elderly, provide the greatest health benefit with net cost savings.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors wish to thank Rani Geha, PhD, for providing medical writing and editing services in developing the manuscript. Financial support for these services was provided by AbbVie. The study was a collaboration between AbbVie and all authors. AbbVie had no influence on the conclusion of the study. None of the authors have received financial compensation for writing or authoring this manuscript.
Disclosure
AbbVie authors may own AbbVie stocks or options. Olivier Ethgen owns SERFAN innovation and is a consultant for AbbVie. The authors report no other conflicts of interest in this work.
References
- World Health Organization [webpage on the Internet]Hepatitis C Fact Sheet2017 Available from: http://www.who.int/mediacentre/factsheets/fs164/en/Accessed July 2017
- AnsaldiFOrsiASticchiLBruzzoneBIcardiGHepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapyWorld J Gastroenterol201420299633965225110404
- Abou RachedAAbou KheirSSabaJAmmarWEpidemiology of hepatitis B and hepatitis C in LebanonArab J Gastroenterol2016171293327032492
- Abou RachedAAbou KheirSSabaJYaghiCAmmarWPrevalence of hepatitis C and fibrosis stage per age group in Lebanese populationGastroenterology Insights2017812327
- Abou RachedAYaghiCKhalilLSabaJAmmarWPrevalence of hepatitis C virus genotypes and subtypes in Lebanese population and major high risk groupsArab J Gastroenterol201718211411728579342
- van der MeerAJVeldtBJFeldJJAssociation between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosisJAMA2012308242584259323268517
- ReauNVekemanFWuEBaoYGonzalezYSPrevalence and economic burden of extrahepatic manifestations of hepatitis C virus are underestimated but can be improved with therapyHepatol Commun2017143945229404471
- European Association for the Study of the LiverEASL Clinical Practice Guidelines: management of hepatitis C virus infectionJ Hepatol201155224526421371579
- LiangTJGhanyMGCurrent and future therapies for hepatitis C virus infectionN Engl J Med2013368201907191723675659
- ReauNSJensenDMSticker shock and the price of new therapies for hepatitis C: is it worth it?Hepatology20145941246124924493069
- NajafzadehMAnderssonKShrankWHCost-effectiveness of novel regimens for the treatment of hepatitis C virusAnn Intern Med2015162640741925775313
- SimonTGChungRTThe new hepatitis C virus bottleneck: can delaying therapy be justified?Hepatology201562366666726058922
- El KhouryACKlimackWKWallaceCRazaviHEconomic burden of hepatitis C-associated diseases in the United StatesJ Viral Hepat201219315316022329369
- DavisGLAlterMJEl-SeragHPoynardTJenningsLWAging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progressionGastroenterology20101382513521521 e511e51619861128
- GordonSCPockrosPJTerraultNAImpact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infectionHepatology20125651651166022610658
- PyensonBFitchKIwasakiKConsequences of Hepatitis C Virus (HCV): Costs of a Baby Boomer Epidemic of Liver DiseaseNew York, NYMilliman, Inc2009
- ColvinHMMitchellAEInstitute of Medicine (US) Committee on the Prevention and Control of Viral Hepatitis InfectionHepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and CWashington (DC)National Academies Press(US)2010
- Deuffic-BurbanSSchwarzingerMObachDShould we await IFN-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis (ANRS 95141)J Hepatol201461171424650691
- LiuSCiprianoLEHolodniyMGoldhaber-FiebertJDCost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United StatesPLoS One201383e5897523533595
- ReinDBSmithBDWittenbornJSThe cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settingsAnn Intern Med2012156426327022056542
- SroczynskiGEstebanEConrads-FrankALong-term effectiveness and cost-effectiveness of screening for hepatitis C virus infectionEur J Public Health200919324525319196737
- TramarinAGennaroNCompostellaFAGalloCWendelaar BongaLJPostmaMJHCV screening to enable early treatment of hepatitis C: a mathematical model to analyse costs and outcomes in two populationsCurr Pharm Des200814171655166018673188
- HaganLMYangZEhteshamiMSchinaziRFAll-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analysesJ Viral Hepat2013201284785724304454
- YounossiZMSingerMEMirHMHenryLHuntSImpact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patientsJ Hepatol201460353053724269472
- LeighJPBowlusCLLeistikowBNSchenkerMCosts of hepatitis CArch Intern Med2001161182231223711575980
- ShahBBWongJBThe economics of hepatitis C virusClin Liver Dis200610471773417164114
- DavisKLMitraDMedjedovicJBeamCRustgiVDirect economic burden of chronic hepatitis C virus in a United States managed care populationJ Clin Gastroenterol2011452e17e2420628308
- NicholsGABellTJPedulaKLO’Keeffe-RosettiMMedical care costs among patients with established cardiovascular diseaseAm J Manag Care2010163e86e9320205493
- DallTMZhangYChenYJQuickWWYangWGFogliJThe economic burden of diabetesHealth Aff (Millwood)201029229730320075080
- LuoRGreenbergAStoneCDIncreasing volume but decreasing mortality of hospitalized hepatitis C patients in the United States, 2005 to 2011J Clin Gastroenterol201549762062725203363
- XuFTongXLeidnerAJHospitalizations and costs associated with hepatitis C and advanced liver disease continue to increaseHealth Aff (Millwood)201433101728173525288416
- WongJBMcQuillanGMMcHutchisonJGPoynardTEstimating future hepatitis C morbidity, mortality, and costs in the United StatesAm J Public Health200090101562156911029989
- Nuno SolinisRArratibel UgartePRojoASanchez GonzalezYValue of treating all stages of chronic hepatitis C: a comprehensive review of clinical and economic evidenceInfect Dis Ther20165449150827783223
- IgarashiATangWGuerraIMarieLCureSLoprestiMCost-utility analysis of ledipasvir/sofosbuvir for the treatment of genotype 1 chronic hepatitis C in JapanCurr Med Res Opin2017331112127609424
- McEwanPWardTWebsterSEstimating the cost-effectiveness of daclatasvir plus asunaprevir in difficult to treat Japanese patients chronically infected with hepatitis C genotype 1bHepatol Res201646542343326440999
- YounossiZMParkHSaabSAhmedADieterichDGordonSCCost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infectionAliment Pharmacol Ther201541654456325619871
- NgVSaabSEffects of a sustained virologic response on outcomes of patients with chronic hepatitis CClin Gastroenterol Hepatol201191192393021699815
- SingalAGVolkMLJensenDDi BisceglieAMSchoenfeldPSA sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virusClin Gastroenterol Hepatol201083280288288 e28119948249
- LinthicumMTGonzalezYSMulliganKValue of expanding HCV screening and treatment policies in the United StatesAm J Manag Care2016226 Spec NoSP227SP23527266953
- CacoubPGragnaniLComarmondCZignegoALExtrahepatic manifestations of chronic hepatitis C virus infectionDig Liver Dis201446Suppl 5S165S17325458776
- NegroFFortonDCraxiASulkowskiMSFeldJJMannsMPExtrahepatic morbidity and mortality of chronic hepatitis CGastroenterology201514961345136026319013
- World Health OrganizationGuidelines for the Screening, Care and Treatment of Persons with Hepatitis C InfectionGeneva, Switzerland2014
- MisurskiDAP0816: reduction in annual medical costs with early treatment of HCV using AbbVie 3D (ABT-450/ritonavir/ombitasvir and dasabuvir) +/− ribavirin in the United StatesJ Hepatol2015621S642
- SemelaDBlachSBihlFKBruggmannPLavanchyDNegroFThe impact of time on a scenario to minimize complications of hepatitis C infectionHepatology201460912A
- ChhatwalJKanwalFRobertsMSDunnMACost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United StatesAnn Intern Med2015162639740625775312
- IgarashiATangWCureSCost-utility analysis of sofosbuvir for the treatment of genotype 2 chronic hepatitis C in JapanCurr Med Res Opin2017331110
- SampJCPerryRPiercyJWoodRBaranRWPatient health utility, work productivity, and lifestyle impairment in chronic hepatitis C patients in FranceClin Res Hepatol Gastroenterol201539330731425511922
- ChhatwalJHeTLopez-OlivoMASystematic review of modelling approaches for the cost effectiveness of hepatitis C treatment with direct-acting antiviralsPharmacoeconomics201634655156726748919
- Gonzalez-GrandeRJimenez-PerezMGonzalez ArjonaCMostazo TorresJNew approaches in the treatment of hepatitis CWorld J Gastroenterol20162241421143226819511
- El KhouryACVietriJPrajapatiGThe burden of untreated hepatitis C virus infection: a US patients’ perspectiveDig Dis Sci201257112995300322674399
- ContiFBuonfiglioliFScuteriAEarly occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antiviralsJ Hepatol201665472773327349488
- ReigMMarinoZPerelloCUnexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapyJ Hepatol201665471972627084592
- ANRS Collaborative Study Group on Hepatocellular CarcinomaLack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts)J Hepatol201665473474027288051
- TheinHHYiQDoreGJKrahnMDEstimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regressionHepatology200848241843118563841
- LiuSCiprianoLEHolodniyMOwensDKGoldhaber-FiebertJDNew protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysisAnn Intern Med2012156427929022351713
- AlazawiWCunninghamMDeardenJFosterGRSystematic review: outcome of compensated cirrhosis due to chronic hepatitis C infectionAliment Pharmacol Ther201032334435520497143
- RazaviHElkhouryACElbashaEChronic hepatitis C virus (HCV) disease burden and cost in the United StatesHepatology20135762164217023280550
- SingalAKSinghAJaganmohanSAntiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosisClin Gastroenterol Hepatol20108219219919879972
- ThuluvathPJGuidingerMKFungJJJohnsonLBRayhillSCPelletierSJLiver transplantation in the United States, 1999–2008Am J Transplant2010104 Pt 21003101920420649
- JacobsonIMMcHutchisonJGDusheikoGADVANCE Study TeamTelaprevir for previously untreated chronic hepatitis C virus infectionN Engl J Med2011364252405241621696307
- PoordadFMcConeJJrBaconBRBoceprevir for untreated chronic HCV genotype 1 infectionN Engl J Med2011364131195120621449783
- LawitzEMangiaAWylesDSofosbuvir for previously untreated chronic hepatitis C infectionN Engl J Med2013368201878188723607594
- FerenciPBernsteinDLalezariJABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCVN Engl J Med2014370211983199224795200
- FeldJJKowdleyKVCoakleyETreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirinN Engl J Med2014370171594160324720703
- PoordadFHezodeCTrinhRABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosisN Engl J Med2014370211973198224725237