Abstract
Background
Nonalcoholic steatohepatitis (NASH) is a common liver disease that can progress to cirrhosis. Oxidative stress is one of the central mechanisms causing hepatocellular injury in the disease. In this study, antioxidant therapy using both vitamins C and E was conducted in patients with NASH.
Methods
Vitamin E 300 mg/day and vitamin C 300 mg/day were administered orally to 23 patients with NASH for 12 months. Body mass index was measured during therapy. Serum levels of alanine aminotransferase, thioredoxin (an oxidative stress marker), and high-sensitivity C-reactive protein were measured before treatment and after 12 months in all patients. Ten of the 23 patients underwent liver biopsy before and after treatment.
Results
Body mass index remained unchanged during treatment with vitamins C and E. Serum alanine aminotransferase, thioredoxin, and high-sensitivity C-reactive protein levels were decreased significantly at 12 months compared with pretreatment. Liver biopsies showed improved necroinflammatory activity in eight cases and improved fibrosis staging in 4.
Conclusion
Serum alanine aminotransferase, thioredoxin, and high-sensitivity C-reactive protein levels, and liver histology were clearly improved with vitamin C and E therapy. These findings suggest that combination therapy using these vitamins may be useful in patients with NASH to minimize damage from oxidative stress and slow the processes leading to cirrhosis.
Introduction
Nonalcoholic steatohepatitis (NASH) is a very common chronic liver disease that resembles alcoholic liver disease clinically and histologically, but occurs in individuals in the absence of a history of significant alcohol consumption. NASH is frequently associated with clinical conditions such as obesity, type 2 diabetes mellitus, hyperlipidemia, and hypertension. These background characteristics indicate that NASH may be part of the spectrum of “metabolic syndrome”. More recently, NASH has been proposed as a possible cause of cryptogenic cirrhosis.Citation1 The pathogenesis of NASH is multifactorial, involving abnormal lipid metabolism, production of reactive oxygen species, increased hepatic lipid peroxidation, activated stellate cells, and abnormal patterns of cytokine production. Oxidative stress appears to be a key factor in the progression from steatosis to NASH and potentially to cirrhosis.Citation2–Citation5
No universally effective treatment for NASH has been identified. Vitamin E refers to a group of naturally occurring compounds with antioxidant properties. A pilot trial of vitamin E aimed at decreasing oxidative stress in pediatric patients with presumed NASH showed that serum alanine aminotransferase and aspartate aminotransferase levels normalized during this treatment.Citation6 We have previously evaluated the efficacy of vitamin E therapy in patients with NASH.Citation7 Significant improvements in serum alanine aminotransferase and gamma glutamyl transferase were observed during treatment. At the same time, levels of the oxidative stress markers, thioredoxin and thiobarbituric acid, were significantly decreased. Furthermore, Harrison et al recently reported that treatment with vitamins C and E improves liver fibrosis in patients with NASH.Citation8 In the present study, vitamins C and E were administered for 12 months at lower doses than those used by Harrison et al, and changes in levels of alanine aminotransferase, oxidative stress markers, and high-sensitivity C-reactive protein were measured before and after treatment to clarify the efficacy and mechanisms of action underlying vitamin C and E therapy. Thioredoxin is a stress-inducible thiol-containing protein that has been shown to be an indicator of oxidative stress in a variety of diseases. Weight reduction is reported to improve liver enzyme abnormalities and liver histology in obese patients with NASH.Citation9,Citation10 However, many patients find it virtually impossible to maintain body weight loss. Therefore, this study evaluated the effects of vitamin C and E on serum alanine aminotransferase, thioredoxin, high-sensitivity C-reactive protein, and liver histology in patients with NASH in association with changes in body mass index during treatment.
Materials and methods
Patients
Twenty-three patients with NASH (ten men and 13 women, Matteoni classification 3 or 4, mean age 53.1 ± 14.9 years) were included in this study. Twenty patients had a body mass index > 25 kg/m2. Nineteen patients had hypertension and 18 patients had hyperinsulinemia and dyslipidemia (). Before pretreatment, all patients had been diagnosed as having NASH by liver biopsy.
Table 1 Demographic feature of patients at baseline
Diagnostic criteria
NASH was diagnosed based on the following criteria: negative results for hepatitis B surface antigen and hepatitis C virus RNA, and exclusion of autoimmune liver disease, drug-induced hepatic disorder, or metabolic liver disease (eg, Wilson’s disease, hemochromatosis); alcohol intake ≤ 30 g/week; and presence of steatosis (>30%) or steatohepatitis. The pathological classification proposed by Matteoni et alCitation11 was used to diagnose histological types 1 and 2 as simple steatosis and types 3 and 4 as NASH. Fibrosis was graded from stage 0 to stage 4 in accordance with the staging system, and inflammatory activity in the liver was graded from grade 0 to grade 3, also according to the grading system proposed by Brunt et al.Citation12 Steatosis was defined according to the percent of hepatocytes affected, and divided into four grades: grade 0, 0%; grade 1, >0% but <33%; grade 2, 33%–66%; and grade 3, >66%. Some pathologists read the liver biopsy blinded. Diabetes mellitus was diagnosed based on the following criteria proposed by the Japan Diabetic Society: fasting blood glucose ≥ 126 mg/dL; two-hour post-75 g oral glucose tolerance test result ≥ 200 mg/dL; casual blood glucose ≥ 200 mg/dL; and glycosylated hemoglobin ≥ 6.1 or treatment with one or more antidiabetic agents. Dyslipidemia was defined as triglyceride > 150 mg/dL and/or a low-density lipoprotein cholesterol level > 140 mg/dL or treatment with one or more lipid-lowering drugs. Hypertension was defined as systolic/diastolic blood pressure ≥ 140/90 mmHg or treatment with one or more antihypertensives.
Changes in body mass index were observed monthly in all patients during vitamin C and E therapy. Serum thioredoxinCitation13 and high-sensitivity C-reactive proteinCitation14 levels were determined using enzyme-linked immunosorbent assays (Mitsubishi Kagaku Yatoron, Tokyo, Japan). Serum thioredoxin levels were also estimated using an enzyme-linked immunosorbent assay kit (Ledox BioScience, Kyoto, Japan). Vitamin E (α-tocopherol, Eisai, Tokyo, Japan) and vitamin C (ascorbic acid, Sionogi, Tokyo, Japan) were each administered orally at 300 mg/day for 12 months.
During therapy, the lifestyle of all patients remained unchanged. Body mass index and serum alanine aminotransferase, thioredoxin, and high-sensitivity C-reactive protein levels were measured before and after treatment. Liver biopsy was performed for all patients before treatment, and ten patients from whom consent was able to be obtained underwent liver biopsy after treatment. Liver histology was evaluated by staging of fibrosis and grading of necroinflammatory activity and steatosis. Immunostaining for 8-hydroxydeoxyguanosine, an oxidative stress marker, was also done before and after treatment.
The study was approved by the ethics committee at our institution and complied with the Declaration of Helsinki and internationally recognized guidelines. Informed consent was obtained.
Statistical analysis
The analysis was performed using JMP version 9.0.1 software (SAS Institute Japan, Tokyo, Japan). Univariate and multivariate analyses of predictors of survival were assessed using the Cox proportional hazards model. A P value less than 0.05 was considered to be statistically significant.
Results
During the 12 months of treatment with vitamins C and E, body mass index remained unchanged (26.6 ± 3.1 kg/m2 at baseline and 26.8 ± 3.1 kg/m2 after treatment). Serum alanine aminotransferase and high-sensitivity C-reactive protein levels decreased significantly during the 12 months, respectively, from 96.5 ± 45.9 IU/L to 40.3 ± 17.6 IU/L (P < 0.0001, ) and from 133.5 ± 59.8 mg/L to 67.5 ± 47.5 mg/L (P < 0.005, ). Before therapy, serum thioredoxin levels were 43.5 ± 17.2 ng/mL, but remained below 35.7 ± 11.5 ng/mL by 12 months following vitamin C and E therapy (P = 0.08, ). In the liver, staging of fibrosis, grading of necroinflammatory activity, and steatosis were compared before and after treatment. Staging of fibrosis improved in four of 10 cases, grade of necroinflammatory activity decreased in eight of 10 cases, and grade of steatosis decreased in six of 10 cases. In four patients, the grade of steatosis remained unchanged, but the grade of necroinflammatory activity improved in all cases, and staging of fibrosis also improved in two cases (). compares histological data for case 3 following treatment with vitamins C and E. Fibrosis stage improved from F3 to F1, while the grading of necroinflammatory activity decreased from A3 to A1. However, no change in fatty deposition was evident. Treatment with vitamins C and E also resulted in a decrease in 8-hydroxydeoxyguanosine levels ().
Figure 1 Body mass index remained unchanged, but changes were seen in serum alanine aminotransferase, thioredoxin, and high-sensitivity C-reactive protein levels in patients with nonalcoholic steatohepatitis before and 12 months following treatment with vitamins C and E.
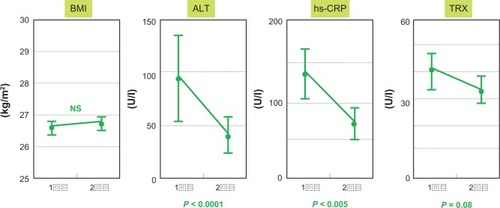
Table 2 Steatosis, grading and fibrosis score in NASH patients before and after 12 months of vitamin E and C (n = 10)
Table 3 Histological interpretation of the effect of vitamin E and C on NASH (A) pretreatment (B) post treatment
Table 4 Change of 8OHdG expression of the effect of vitamin E and C on NASH (A) pretreatment (B) post treatment
Discussion
The pathogenesis of NASH remains poorly understood. Obesity, type 2 diabetes mellitus, hyperinsulinemia, increased triglyceride levels, toxins, and medical conditions can lead to increased serum fatty acid levels, which are then presented to the liver. The multihit theory suggests that the first hit involves accumulation of excess fat in the hepatic parenchyma. The second hit involves oxidative stress resulting from an imbalance between pro-oxidant and antioxidant processes in the liver, which may result from induction of microsomal cytochrome P450 2E1, mitochondrial release of reactive oxygen species, release of hydrogen peroxide from peroxisomal-beta oxidation of fatty acids, release of cytokines from activated inflammatory cells, and insulin resistance.Citation1–Citation5
Optimal therapies for NASH have yet to be established. Maintenance of weight loss results in significant clinical and histological improvement.Citation9,Citation10 However, adverse effects on liver histology, such as progression of fibrosis, have also been noted.Citation15 Furthermore, most obese patients find it virtually impossible to maintain weight loss. Other treatments include modification of the clinical conditions associated with NASH, in particular type 2 diabetes mellitus, hyperlipidemia, and obesity.
Medications that minimize oxidative stress may prove useful in the treatment of NASH. Vitamin E is a well known and potent antioxidant. A pilot study of vitamin E therapy has been done in a small, open-label study of 11 pediatric patients with presumed NASH.Citation6 These children were given 400–1200 IU of vitamin E for 4–10 months. The vitamin E was well tolerated and resulted in normalization of liver function tests during treatment. However, biochemical improvement was not sustained when vitamin E was discontinued. Hasegawa et alCitation16 did a study in patients with NASH receiving vitamin E 300 mg/day for one year. Serum alanine aminotransferase levels decreased, accompanied by decreases in plasma levels of transforming growth factor β1. We have also reported a pilot study of vitamin E treatment in NASH.Citation7 In this study, significant improvements were observed in both serum thioredoxin and thiobarbituric acid-reactive substance levels, which are believed to be clinically useful indicators of oxidative stress, along with a decrease in alanine aminotransferase. We have previously reported that liver damage induced by oxidative stress is reduced by vitamin E in patients with chronic hepatitis C.Citation17 A report investigating the effects of aerobic exercise in patients with NASH revealed no differences according to administration or nonadministration of vitamin E,Citation18 but this lack of apparent effect was probably due to the efficacy of treatment with diet and exercise alone. In a recent report, Sanyal reported that vitamin E (n = 84) and pioglitazone (n = 80) in patients with NASH significantly improved serum alanine aminotransferase levels, hepatic steatosis, and lobular inflammation, as compared with placebo, but with no improvement in fibrosis.Citation19
Furthermore, significant improvements in liver fibrosis were reported in patients with NASH treated using a combination of vitamins C and E. When administered together with vitamin C, vitamin E can actively combat oxidative stress. Harrison et al reported that 21 patients with NASH were given vitamin E 1000 IU and vitamin C 1000 mg or placebo daily for six months, yielding a significant improvement in fibrosis score among treated patients.Citation8 In Japan, the approved daily doses of vitamin E and vitamin C (300 mg/day and 600 mg/day, respectively) are lower than those used by Harrison et al. Therefore, the present study investigated the utility of 300 mg vitamin E and 600 mg vitamin C for the treatment of NASH in Japanese patients. Furthermore, because previous studies of treatment with vitamin E for NASH have not evaluated the antioxidant potential of vitamin E, we also conducted immunostaining for the oxidative stress markers, thioredoxin and 8-hydroxydeoxyguanosine, in serum and hepatic tissue, respectively.
In the present study, serum alanine aminotransferase levels improved, accompanied by decreases in serum thioredoxin, high-sensitivity C-reactive protein, and 8-hydroxydeoxyguanosine levels, on combination therapy using vitamins C and E. In both arteriosclerosis and metabolic syndrome, high-sensitivity C-reactive protein offers a sensitive marker of chronic low-grade inflammation. Targher et al reported that the severity of liver histology in patients with NASH was strongly associated with increasing plasma levels of high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, and fibrinogen, and decreasing plasma adiponectin concentrations. In addition, NASH and visceral adiposity predicted cardiovascular risk biomarkers independent of potential confounders, and NASH can predict a more atherogenic risk profile in a manner that is partly independent of the contribution of visceral adiposity in adult men.Citation20
Our histological study found that grade of necroinflammatory activity improved in eight of ten treated patients, and fibrosis stage in four treated patients. The grade of steatosis improved in five of ten cases, indicating that the effects of combination therapy are not associated with weight loss. Although the mechanism is not clear, it may be that if liver inflammation is improved for a long time, liver fibrosis may also be improved.
Based on our experience in this pilot study, we conclude that treatment of NASH for 12 months with vitamins C and E results in significant improvement in alanine aminotransferase, thioredoxin, and high-sensitivity C-reactive protein levels, as well as in liver cell damage and inflammation. Given that no proven treatments for NASH are known, the possible benefits of vitamins C and E should be investigated further in a randomized controlled trial.
Disclosure
The authors report no conflicts of interest in this work.
References
- Neuschwander-TetriBACaldwellSHNonalcholic steatohepatitis. Summary of an AASLD single topic conferenceHepatology2003371202121912717402
- DayCJamesOSteatohepatitis: a tale of two hits?Gastroenterology19981148428459547102
- ChitturisSFarrellGCEtiopathogenesis of non alcoholic steatohepatitisSemin Liver Dis200121274111296694
- ReidAENonalcoholic steatohepatitisGastroenterology200112171072311522755
- TilqHDiehlAMCytokines in alcoholic and nonalcoholic steatohepatitisN Engl J Med20003431467147611078773
- LavinJEVitamin E treatment of nonalcoholic steatohepatitis in children: a pilot studyJ Pediatr200013673473810839868
- KawanakaMMahmoodSNiiyamaGControl of oxidative stress and reduction in biochemical markers by vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot studyHepatol Res200429394115135345
- HarrisonSATorgersonSHayashiPWardJSchenkerSVitamin E and vitamin C treatment improved fibrosis in patients with nonalcoholic steatohepatitisAm J Gastroenterol2003112485249014638353
- OkitaMHayashiMSasagawaTEffect of moderately energy restricted diet on obese patients with fatty liverNutrition20011754254711448571
- HuangMAGreensonJKChaoCOne-year intense nutritional counseling result in histological improvement in patients with nonalcoholic steatohepatitis: a pilot studyAm J Gastroenterol20051001072108115842581
- MatteoniCAYounossiZAGramlichTNonalcoholic fatty liver disease: a spectrum of clinical and pathological severityGastroenterology19991161413141910348825
- BruntEMJanneyCGDi BisceglieAMNonalcoholic steatohepatitis: a proposal for grading and staging the histological lesionsAm J Gastroenterol1999942467247410484010
- NakamuraHDe RossaSRoedererMElevation of plasma thioredoxin levels in HIV-infected individualsInt Immunol199686036118671648
- DaneshJMuirJWongYKRisk factor for coronary heart disease and acute-phase proteins. A population-based studyEur Heart J19992095495810361047
- AndersenTGluudCFranzmannMBChristoffersenPHepatic effects of dietary weight loss in morbidly obese subjectsJ Hepatol1991122242292051001
- HasegawaTYonedaMNakamuraKMakinoITeranoAPlasma transforming growth factor-β1 level and efficacy of α-tocopherol in patients with non alcoholic steatohepatitis: a pilot studyAliment Pharmacol Ther2001151667167211564008
- MahmoodSYamadaGNiiyamaGEffect of vitamin E on serum aminotransferase and thioredoxin levels in patients with viral hepatitis CFree Radic Res20033778178512911275
- KugelmasMHillDBVivianBCytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin EHepatology20033841341912883485
- SanyalAChalasaniNKoedleyKVPioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitisN Engl J Med20103621675168520427778
- TargherGBertoliniLRodellaSNASH predicts plasma inflammatory biomarkers independently of visceral fat in menObesity2008161394139918369343