Abstract
Thrombocytopenia is the most common hematological abnormality encountered in patients with chronic liver disease (CLD). In addition to being an indicator of advanced disease and poor prognosis, it frequently prevents crucial interventions. Historically, thrombocytopenia has been attributed to hypersplenism, which is the increased pooling of platelets in a spleen enlarged by congestive splenomegaly secondary to portal hypertension. Over the past decade, however, there have been significant advances in the understanding of thrombopoiesis, which, in turn, has led to an improved understanding of thrombocytopenia in cirrhosis. Multiple factors contribute to the development of thrombocytopenia and these can broadly be divided into those that cause decreased production, splenic sequestration, and increased destruction. Depressed thrombopoietin levels in CLD, together with direct bone marrow suppression, result in a reduced rate of platelet production. Thrombopoietin regulates both platelet production and maturation and is impaired in CLD. Bone marrow suppression can be caused by viruses, alcohol, iron overload, and medications. Splenic sequestration results from hypersplenism. The increased rate of platelet destruction in cirrhosis also occurs through a number of pathways: increased shear stress, increased fibrinolysis, bacterial translocation, and infection result in an increased rate of platelet aggregation, while autoimmune disease and raised titers of antiplatelet immunoglobulin result in the immunologic destruction of platelets. An in-depth understanding of the complex pathophysiology of the thrombocytopenia of CLD is crucial when considering treatment strategies. This review outlines the recent advances in our understanding of thrombocytopenia in cirrhosis and CLD.
Keywords:
Introduction
Thrombocytopenia is the most common hematological abnormality encountered in patients with chronic liver disease (CLD),Citation1 occurring in 64%–84% of patients with cirrhosis or fibrosis.Citation2 Among patients undergoing bone marrow biopsies for thrombocytopenia of unknown etiology, the prevalence of cirrhosis is as high as 35%.Citation3 In addition to being an indicator of advanced disease,Citation4 thrombocytopenia is associated with a poorer prognosis,Citation1 and it frequently prevents patients from receiving crucial interventions such as medications, as well as invasive diagnostic or therapeutic procedures.Citation5
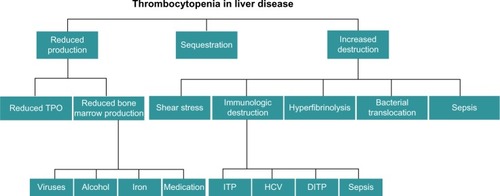
Historically, thrombocytopenia has been attributed to hypersplenism, namely, the increased pooling of platelets in a spleen enlarged by congestive splenomegaly secondary to portal hypertension.Citation6 Over the past decade, however, there have been significant advances in the understanding of thrombopoiesis, which, in turn, has led to an improved understanding of thrombocytopenia in cirrhosis. Multiple factors contribute to the development of thrombocytopenia in the cirrhotic patient and these can broadly be divided into those leading to decreased production, splenic sequestration, and increased destruction (). This review outlines the recent advances in our understanding of the pathophysiology of thrombocytopenia in cirrhosis.
Figure 1 Factors that contribute to the development of thrombocytopenia in patients with cirrhosis.
Abbreviations: DITP, drug-induced thrombocytopenia; HCV, hepatitis C virus; ITP, idiopathic thrombocytopenia purpura; TPO, thrombopoietin.
Decreased platelet production
Platelet production can be decreased due to depressed thrombopoietin (TPO) levels and direct bone marrow suppression.
Thrombopoietin
Hepatic production of TPO plays a pivotal role in thrombopoiesis (). In 1990, the oncogene v-mpl was identified from the murine myeloproliferative leukemia virus, which was capable of immortalizing bone marrow hematopoietic cells from different lineages.Citation7 In 1992, the human homologue, c-mpl, was cloned, and sequence data revealed that it encoded a protein that was homologous to members of the hematopoietic receptor superfamily.Citation8 Antisense oligodeoxynucleotides of c-mpl were shown to selectively inhibit megakaryocyte colony formation, demonstrating that c-mpl regulated thrombopoiesis.Citation9
Figure 2 Normal thrombopoiesis.
Abbreviation: TPO, thrombopoietin.
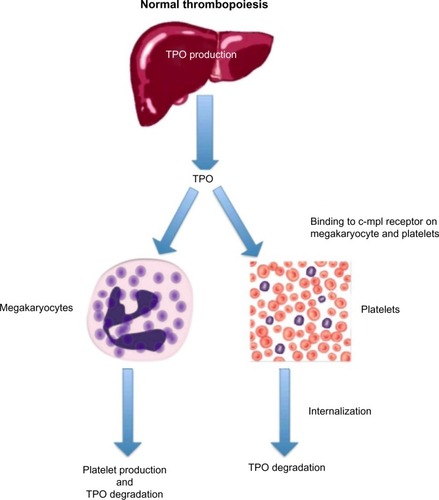
The ligand for c-mpl, TPO, was cloned in 1994. It has a 353-amino acid transmembrane domain with two extracellular cytokine receptor domains and two intracellular cytokine receptor box motifs.Citation10 TPO is the major regulator of megakaryocytopoiesis, and it regulates both platelet production and maturation. It is a glycoprotein (GP) that shares significant amino acid sequence homology with erythropoietin (EPO).Citation11 TPO is primarily made in the liver by both parenchymal cells and sinusoidal endothelial cells and is secreted into the circulation at a constant rate.Citation12
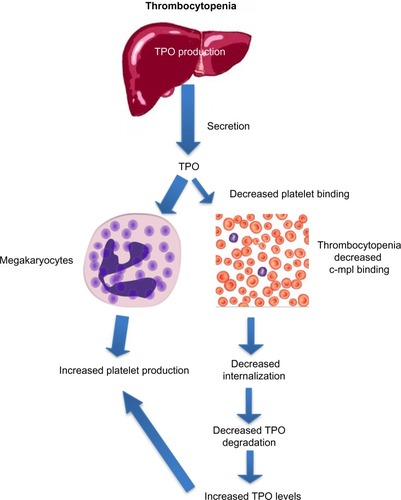
After binding to the surface of platelets and megakaryocytes through the c-mpl receptor,Citation13 TPO is internalized and destroyed, thereby reducing further platelet and megakaryocyte exposure to the hormone.Citation14 Stimulation of the TPO receptor results in activation of a number of signaling pathways via Janus kinase type 2 (JAK2) and tyrosine kinase 2 (TYK2).Citation15 Mitogen-activated protein kinase activation subsequently leads to changes in gene expression, promoting progression of stem cells along the megakaryocytic pathway, megakaryocyte maturation, and subsequent release of normally functioning platelets into the peripheral circulation.Citation16 Because the circulating level of TPO is inversely correlated to the platelet mass, low platelet counts lead to higher TPO levels due to decreased degradation (). The increased exposure of undifferentiated bone marrow cells to TPO leads to their differentiation into megakaryocytes and maturation. This increased platelet cell mass, in turn, binds increasing amounts of TPO, reducing its circulation level, and leading to decreased platelet production. This negative feedback mechanism is highlighted by the observation that mice genetically altered to be defective in c-mpl have low platelet and megakaryocyte numbers and elevated TPO levels.Citation17
Figure 3 Increased thrombopoietin levels in thrombocytopenia lead to increased platelet production.
Abbreviation: TPO, thrombopoietin.
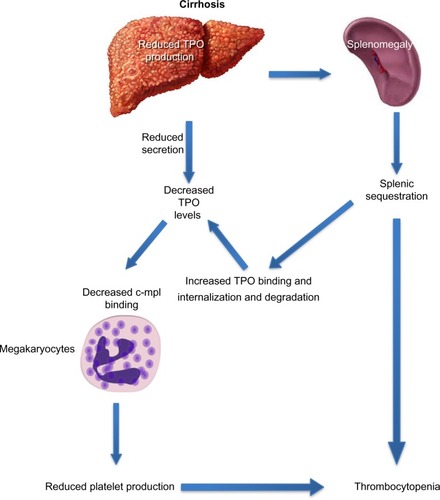
Decreased hepatic production of TPO is a critical factor in the development of thrombocytopenia in cirrhosis (). The prevalence and severity of thrombocytopenia correlate with and parallel the severity of underlying liver disease, particularly, the extent of fibrosis.Citation18 The prevalence of thrombocytopenia is higher in patients with Stages 3 and 4 fibrosis when compared to patients with Stages 0–2 fibrosis (64% vs 6%).Citation2 There is an inverse relationship between TPO levels and liver function, as assessed by tests that measure liver function, such as the indocyanine green retention and aminopyrine breath tests.Citation19 Cirrhotic patients with thrombocytopenia have lower levels of circulating TPO than those with normal platelet counts.Citation20 The key role played by TPO in thrombocytopenia of CLD is highlighted by the interaction between TPO and platelets during the perioperative period of liver transplantation: TPO levels are often undetectable in patients with cirrhosis before transplantation and rise immediately posttransplantation, which is followed by a rise in peripheral platelet count and normalization of both TPO levels and platelet count in most patients within 14 days.Citation21
Figure 4 Decreased thrombopoietin levels in cirrhosis lead to decreased platelet production.
Abbreviation: TPO, thrombopoietin.
Bone marrow suppression
Inadequate production of platelets due to bone marrow suppression in selected cases may also play a crucial role in the development of thrombocytopenia in cirrhosis.Citation22 Possible etiologies include suppression by viruses, alcohol, iron overload, and medications.
Viruses
Hepatitis A virus, hepatitis B virus, and hepatitis C virus (HCV) directly inhibit the growth and differentiation of human bone marrow progenitor cells in vitro.Citation23 Thrombocytopenia is especially common in patients infected with HCV through a variety of mechanisms, one of which is direct bone marrow suppression.Citation24 Patients with HCV without splenomegaly show depressed platelet production,Citation25 and production increases after successful treatment of the infection.Citation26
Alcohol
Thrombocytopenia occurs frequently in alcoholics through a direct effect on the bone marrow.Citation27 Alcohol reduces platelet life span and leads to ineffective megakaryopoiesis.Citation27 Following alcohol withdrawal, platelet counts rise within 5–7 days and normalize in a few weeks.Citation28
Iron
The status of iron stores is an important factor in thrombopoiesis, especially in determining the response to EPO. Thrombocytosis is a common presentation of iron deficiency anemia,Citation29 and it has been suggested that it serves as a protective mechanism by increasing the coagulation capacity in conditions with chronic bleeding.Citation30 Repletion of iron deficiency in renal failure and inflammatory bowel disease, in contrast, leads to a decrease in platelet levels, occasionally with precipitous reductions and the development of thrombocytopenia.Citation31 Experimental studies indicate that thrombocytosis in iron deficiency is due to an increased commitment of hematopoietic progenitors to the megakaryocytic lineage with accelerated differentiation that is for the most part independent of EPO and TPO.Citation30 The elevated EPO levels that are a normal physiological response to anemia,Citation32 however, do affect platelet production but in a biphasic response. An early but transient increase in platelet count followed by development of thrombocytopenia may be observed with EPO treatment due to a functional iron deficiency.Citation33
The liver serves a central role in iron storage and functions as the main site of synthesis of iron transport proteins. Hepcidin, the principal iron regulatory hormone, is produced in hepatocytesCitation34 and is secreted into circulation, wherein it binds ferroportin in macrophages and enterocytes, inducing internalization and degradation of ferroportin and inhibiting iron export.Citation35 Excess iron or inflammation triggers increased hepcidin expression,Citation36 resulting in decreased enterocyte iron absorption and reduced iron release from macrophages. Increased hepcidin expression has been implicated in anemia of inflammation,Citation37 whereas decreased hepcidin expression plays an important role in hemochromatosis.Citation38
Iron overload associated with spur cell hemolytic anemia is common in advanced cirrhosis, occurring through several mechanisms.Citation39 Prohepcidin expression is reduced in proportion to the severity of liver disease,Citation40 leading to decreased hepcidin levels and increased iron absorption. Spur cell hemolytic anemia is caused by a combination of altered red blood cell (RBC) membrane composition, oxidative damage, decreased RBC membrane fluidity that leads to decreased RBC survival, and hemolytic anemiaCitation41 and further contributes to increased iron absorption and hepatic iron loading.Citation42 Experimentally, the iron status is a major determinant of the platelet response to EPO. Compared to animals with depletion of iron stores, animals with iron overloading show a more pronounced degree of thrombocytopenia due to competition between erythroid and megakaryocytic development pathways of stem cells in the absence of the protection afforded by iron deficiency.Citation33
Medications
Cirrhotic patients are exposed to a plethora of drugs that have the potential to cause drug-induced thrombocytopenia (DITP) through multiple mechanisms that include both direct bone marrow suppression and immunological platelet destruction. Examples of medications commonly prescribed to the cirrhotic patient and that are associated with impaired thrombopoiesis include azathioprine, antibiotics, and interferon (IFN).
Azathioprine is a purine antimetabolite used as an immunosuppressive agent to treat a range of autoimmune disorders, including chronic autoimmune hepatitis. Its mechanism of action in blocking purine synthesis hinders proliferation of several cell lines, with its most pronounced effect being on lymphocytes. The most common, but often serious, side effect of this agent is bone marrow suppression, an effect that is dose dependent. Although only 5% of patients taking azathioprine show bone marrow toxicity, which can include thrombocytopenia in up to 2%,Citation43 effective therapy is frequently not possible in cirrhotic patients with baseline thrombocytopenia. Beta-lactam antibiotics and fluoroquinolones have also been proposed as potential causes of thrombocytopenia, acting through bone marrow suppression.Citation44
IFN-based therapies were until recently the standard of care for patients with chronic hepatitis C. Because dose-dependent thrombocytopenia is a frequent side effect of IFN, baseline thrombocytopenia in cirrhotic patients frequently prevented them from receiving effective therapy. IFN-induced bone marrow toxicity and resultant cytopenias were a common reason for treatment discontinuation or cessation.Citation45 The mechanism for the development of IFN-induced thrombocytopenia is multifactorial and includes direct impairment of late-stage megakaryocytopoiesisCitation46 and altered TPO levels. IFN inhibits the expression of transcription factors regulating late-stage megakaryocytopoiesis and impairs thrombopoiesis by preventing cytoplasmic maturation of megakaryocytes and preventing platelet production.Citation46 It is also associated with both a blunted TPO response to thrombocytopenia and a direct reduction in TPO levels.Citation47 Patients with advanced liver disease especially lack an appropriate compensatory increase in TPO in response to thrombocytopenia.Citation47
Splenic sequestration
Historically, thrombocytopenia in cirrhosis was attributed to increased pooling of platelets in an enlarged spleen.Citation6 The term hypersplenism was first used in 1909 to describe the presence of splenomegaly in patients with hemolytic anemia. The concept subsequently evolved to describe a distinct clinical syndrome of splenic hyperactivity associated with splenomegaly, a reduction in one or more peripheral cell types, an appropriately proliferative bone marrow response, and potential for reversal with splenectomy.Citation48 Congestive splenomegaly develops as a result of portal hypertension and is characterized by a redistribution of blood flow and platelets from the circulating pool to the splenic pool.Citation49 As a result, splenomegaly leads to thrombocytopenia by sequestration, and there is an inverse relationship between spleen size and platelet count.Citation18 Because the sequestrated platelets are still capable of removing TPO from the circulation, they further contribute to the development of thrombocytopenia by lowering TPO levels.Citation50
Increased platelet destruction
Increased platelet destruction occurs in cirrhosis through increased shear stress leading to an increased rate of platelet aggregation, immunologic destruction, increased fibrinolysis, bacterial translocation, and infection.
Shear stress
Shear stress, or the level of fluid stress applied to platelets and plasma components within the vasculature, provokes platelet aggregation. Under conditions of excessive high fluid shear stress, ultralarge von Willebrand factor (ULVWF) undergoes a conformational transition from a globular state to an extended chain conformation that is more adhesive to platelets.Citation51,Citation52 This leads to aggregated complexes within the vasculature and thrombus formation. ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 motif 13) is a shear-dependent metalloproteinase produced by hepatic stellate cells, which cleaves unusually large vWF.Citation53,Citation54 High levels of shear stress both enhance vWF–platelet aggregation and promote cleavage of vWF by ADAMTS13.Citation55
The levels and activity of ADAMTS13 are reduced in patients with advanced cirrhosisCitation56 due to enhanced consumption of ADAMTS13, presence of inflammatory cytokines, and the presence of an ADAMTS13 plasma inhibitor.Citation57 Decreased levels of ADAMTS13 result in the accumulation of UL-VWFM, which, in turn, enhances high shear-stress-induced platelet aggregation. Low platelet counts in cirrhosis parallel-depressed levels of ADAMTS13 activity.Citation56 Finally, TPO can also prime platelet aggregation in conditions of high shear stress such as portal hypertension and congestive splenomegaly.Citation58
Immunologic destruction
Immune-mediated destruction involving antiplatelet antibodies is frequently present in cirrhosis. Among patients with CLD of diverse etiologies, up to 64% have platelet-associated anti-GP antibodies that are primarily directed against the GP IIb–IX complex.Citation59 Lower platelet counts in patients with cirrhosis are correlated with both larger spleen volumes and higher levels of platelet-associated immunoglobulin G (PAIgG).Citation60 In a study utilizing kinetic radio-labeled platelet techniques, platelet levels were directly correlated with platelet survival times and inversely correlated with PAIgG levels and splenic volumes.Citation61 Specific situations in which immune-mediated thrombocytopenia are encountered include idiopathic thrombocytopenia purpura (ITP), chronic hepatitis C, infection, and medications.
Idiopathic thrombocytopenia purpura
ITP, also known as immune or autoimmune thrombocytopenia purpura, is an autoimmune disease characterized by thrombocytopenia caused by the interaction of PAIg with platelet antigens.Citation62 In “classic” ITP, autoantibodies are predominantly, but not exclusively, produced against platelet GP IIb/IIIa and Ib/IX.Citation63 Antibodycoated platelets are recog-nized by macrophages in the spleen and liver and removed from the circulation.Citation64 Splenomegaly is typically not present, and patients usually respond well to immunosuppression.Citation65
Autoimmune liver diseases (autoimmune hepatitis and primary biliary cirrhosis [PBC]) are frequently associated with other autoimmune conditions. Approximately 50% of patients with PBC are affected by at least one additional autoimmune disease, which may include ITP.Citation66 Up to 40% of patients with PBC have raised levels of PAIgG,Citation66 and there are case reports of patients with autoimmune-related liver disease with ITP.Citation67
Viral infections have been associated with ITP, especially HCV. Up to 30% of patients with ITP without evidence of advanced liver disease are seropositive for HCV.Citation68 The rate of ITP among patients infected with HCV is 30.2 per 100,000 person-years compared to 18.5 per 100,000 person-years for non-HCV-infected individuals.Citation69 HCV-related ITP thrombocytopenia may be severe, usually affecting women, and usually has a good response to corticosteroids.Citation70 Finally, ITP has also been described in association with several other viral infections, including cytomegalovirus and Epstein–Barr Virus.Citation71
Chronic hepatitis C
Chronic infection with HCV can lead to thrombocytopenia through multiple mechanisms, as summarized by Weksler.Citation72 Chronic HCV infection is associated with a plethora of autoimmune disorders. Approximately 38% of patients with HCV infection exhibit at least one immune-mediated, extrahepatic manifestation during the course of their disease.Citation73 Patients with CLD due to HCV develop a thrombocytopenia that parallels the severity of their disease and is mirrored by increasing titers of PAIg.Citation25,Citation74 HCV can interact directly with platelets to bind platelet membranes through multiple cell surface receptors.Citation75 Anti-HCV antibodies then coat the surface-associated HCV, ultimately leading to phagocytosis of antibody-coated platelets and accelerated platelet destruction by the reticuloendothelial system.Citation75 The binding of HCV to platelets may also induce neoantigens on the platelet surface or drive alterations in the platelet membrane GPs, contributing to autoantibody formation against platelet membrane GPs, such as GPIIb/IIIa, and subsequent development of ITP.Citation76 Finally, HCV is intimately related to cryoglobulinemia, and cryoglobulins might play a role in immune complex formation and accelerated platelet clearance.Citation70
Immune-mediated drug-induced thrombocytopenia
Due to the multiple medications that cirrhotic patients receive, DITP is commonly encountered. In DITP, drug-dependent antibodies bind to specific platelet GPs in the presence of the offending drug. Platelet counts decrease within 5–7 days after exposure to a causative agent and rise within 10 days of cessation.Citation77 Causes of DITP include antibiotics (eg, cephalosporins, linezolid, and octreotide).Citation20–Citation24 The hallmark of immune-related DITP is severe thrombocytopenia (platelet count: <30×109/L), often accompanied by petechiae and mucocutaneous bleeding.Citation78
IFN therapy can rarely induce autoimmune ITP. IFN-induced autoimmune ITP has been reported to develop after 4 weeks to 12 months of therapy,Citation79 and even after the completion of therapy.Citation80 In contrast to the dose-dependent thrombocytopenia caused by IFN-induced bone marrow suppression, IFN-induced autoimmune ITP can cause precipitous decreases in platelet levels; it usually responds to immunosuppression.Citation79
Heparin-induced thrombocytopenia (HIT) is one of the most commonly encountered causes of DITP. Following exposure to heparin, platelet factor 4 (PF4) forms complexes with the negatively charged heparin molecules.Citation81 These complexes are highly immunogenic and result in the formation of HIT antibodies and subsequent aggregation of platelets with PF4/heparin complexes.Citation82 HIT is more common in patients treated with unfractionated heparin and occurs with a frequency of 3%–6% after 7 days.Citation83 In contrast to the aforementioned “classic” drug-induced immune thrombocytopenia, HIT is characterized usually by a moderate degree of thrombocytopenia (median platelet count nadir, ∼50×109/L), and a high risk of venous and/or arterial thrombosis, rather than bleeding.Citation84
Increased fibrinolysis
The liver plays a pivotal role in the fibrinolytic system and is responsible for sustaining a balance between bleeding and thrombosis to maintain homeostasis. The liver is important in both the production of multiple factors involved in the process and clearance of breakdown products. Under normal circumstances, deposition of fibrin within the vascular system triggers the conversion of plasminogen into the active enzyme plasmin, which then degrades fibrin and liberates fibrin and fibrinogen degradation products into the circulation.Citation85 This plasminogen-to-plasmin conversion is driven by tissue plasminogen activator (t-PA) and opposed by plasminogen activator inhibitor (PAI).Citation86 Alpha-2-antiplasmin is among the major inhibitors of plasmin and fibrinolysis.Citation87 Thrombin-activatable fibrinolysis inhibitor (TAFI) inhibits recruitment of plasminogen to thrombi, slowing fibrinolysis.Citation88
Fibrinolysis is increased in cirrhosis. There is a reduced production of clotting and inhibitory factors, as well as decreased clearance of activated factors, leading to accelerated intravascular coagulation. There is also decreased clearance of t-PA and PAI-1 from the circulationCitation89 and decreased hepatic synthesis of alpha-2-antiplasmin and TAFI.Citation90 As a result, there is a rebalanced state between pro- and antifibrinolytic factors, which leads to hyperfibrinolysis in up to 30%–46% of patients with end-stage liver disease.Citation91 This hypercoagulable state with excessive platelet consumption plays a role in the development of thrombocytopenia in cirrhosis and is supported by studies of platelet kinetics analysis.Citation92 A retrospective autopsy study of patients with liver disease found that platelet counts in patients with thrombotic complications were lower in those without thrombosis, further suggesting that increased thrombosis consumes platelets.Citation93 Antifibrinolytic agents, such as tranexamic acid, aprotinin, and epsilon-aminocaproic acid, have been shown to reduce intraoperative bleeding in liver transplantation, as well as in cirrhotic patients with bleeding associated with hyperfibrinolysis.Citation94–Citation97
Bacterial translocation
Bacterial translocation associated with endotoxemia is common in cirrhosis and can accelerate platelet consumption and the development of thrombocytopenia. High levels of circulating endotoxins are observed in cirrhosis, even in those not clinically infected.Citation98 Kalambokis and TsianosCitation99 first postulated the role of endotoxin in the pathophysiology of thrombocytopenia in cirrhosis: intestinal bacterial overgrowth and altered gut permeability allow bacterial translocation of microorganisms from the intestinal lumen into the portal circulation.Citation100 Impairment of the reticuloendothelial system,Citation101 along with portosystemic shunting, accounts for its presence in the systemic circulation.Citation102 Endotoxin accelerates the release of proinflammatory cytokines (tumor necrosis factor-alpha [TNF-α] and interleukins [IL-3, IL-6, and IL-11]).Citation103 The various cytokines are important regulators of inflammation, cell growth, and maturation; they have key roles in thrombopoiesis and are elevated in cirrhotic patients in proportion to the degree of liver disease.Citation104 In patients with alcoholic cirrhosis, endotoxin levels are significantly higher among those with thrombocytopenia than in those without thrombocytopenia, and platelet counts are inversely correlated with endotoxin levels.Citation105
Endotoxin stimulates B-cell activity and production of IgG, including PAIgG, which increases the removal of platelets from the circulation.Citation99 It contributes to thrombocytopenia by triggering disseminated intravascular coagulation (DIC), platelet activation, aggregation, and platelet toll-like receptors.Citation106 Platelet consumption from activation of platelet–monoctye aggregates is induced by endotoxin,Citation107 and endotoxemia impairs ADAMTS13 activity and promotes thrombotic complications and thrombocytopenia.Citation57
TNF-α and other inflammatory cytokines suppress hepatic production of TPO,Citation105 inhibit the growth and differentiation of megakaryocytes,Citation108 and induce platelet apoptosis.Citation109 Finally, TNF-α induces vascular nitric oxide production,Citation110 which is the main mediator for the development of portal hypertension, and suppresses TPO production.Citation111
Cirrhosis may also predispose patients to an excessive response to lipopolysaccharide (LPS), a component of cell walls of Gram-negative bacteria, which directly increases platelet aggregation in animal models. In experimental animals and in human cells from cirrhotic patients ex vivo, LPS induces higher levels of TNF-α and IL-6 than noncirrhotic controls.Citation112
Bacterial infection
Thrombocytopenia commonly develops in patients with infection, especially sepsis. In a retrospective review of all patients admitted to a Medical Intensive Care Unit with severe sepsis or septic shock, thrombocytopenia developed in 47.6% of patients.Citation113 Infection is more common in patients with cirrhosis than in the general population. The overall incidence of infection in patients with liver disease has been estimated to be up to 47%.Citation114 Multiple sources of infection are common in advanced cirrhosis, including spontaneous bacterial peritonitis, urinary tract infection, and pneumonia. Patients with cirrhosis have an increased risk of developing sepsis, sepsis-induced organ failure, and sepsis-related death.Citation115
Mechanisms by which sepsis lead to thrombocytopenia include intensification of the adverse effects of endotoxemia. TNF-α is increased in patients with sepsis, and TNF-α levels are higher in patients with sepsis with thrombocytopenia.Citation116 TNF-α released during infection can trigger a DIC-like picture with hyperfibrinolysis with increased platelet activation and adhesion to endothelium.Citation117 TNF-α triggers platelet activation and amplifies platelet response to collagen in vitro.Citation118 Activation of the coagulation system in sepsis results in fibrin clot formation and the consumption of platelets.Citation119
Immune mechanisms have also been implicated.Citation120 PF4 forms immune complexes with heparin and other polyanions, in addition to binding bacteria, exposing neoantigen(s), and inciting antibody formation. Specifically, PF4 has been demonstrated to bind the negatively charged LPS on Gram-negative bacteria.Citation121 This PF4/LPS complex is immunogenic and can elicit cross-reacting antibodies against the PF4/heparin complex, resulting in a spontaneous HIT-like picture. Accordingly, anti-PF4/heparin antibody titers are higher in patients with bacteremia.Citation122 Finally, endogenous heparinoids can also be detected in cirrhotic patients in the setting of infection and disappear following its resolution. Although they are associated with impaired coagulation, an effect on platelet count has not been detected.Citation123
Conclusion
Thrombocytopenia is common in CLD of all etiologies and is a complicated and multifactorial phenomenon. Recent advances in elucidating the pathways of platelet production and consumption have led to significant improvements in our understanding of thrombocytopenia in CLD. An in-depth understanding of the pathophysiology of the thrombocytopenia of CLD is crucial when considering treatment strategies.
Acknowledgments
The authors are grateful to Nadia Nieves for assistance with development of the figures.
Disclosure
Samuel Sigal received research support from GSK. Oscar Mitchell, David Feldman, and Marla Diakow report no conflicts of interest in this work.
References
- QamarAAGraceNDGroszmannRJIncidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosisClin Gastroenterol Hepatol20097668969519281860
- BashourFNTeranJCMullenKDPrevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver diseaseAm J Gastroenterol200095102936293911051371
- SheikhMYRaoufiRAtlaPRPrevalence of cirrhosis in patients with thrombocytopenia who receive bone marrow biopsySaudi J Gastroenterol201218425726222824769
- PoynardTBedossaPAge and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study GroupsJ Viral Hepat1997431992089181529
- HayashiHBeppuTShirabeKManagement of thrombocytopenia due to liver cirrhosis: a reviewWorld J Gastroenterol201420102595260524627595
- AsterRHPooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopeniaJ Clin Invest19664556456575327481
- WendlingFTambourinPThe oncogene V-MPL, a putative truncated cytokine receptor which immortalizes hematopoietic progenitorsNouv Rev Fr Hematol19913321451461662802
- VigonIMornonJPCocaultLMolecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamilyProc Natl Acad Sci U S A19928912564056441608974
- MethiaNLouacheFVainchenkerWOligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesisBlood1993825139514017689867
- LokSKaushanskyKHollyRDCloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivoNature199436964815655688202158
- de SauvageFJHassPESpencerSDStimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligandNature199436964815335388202154
- StoffelRWiestnerASkodaRCThrombopoietin in thrombocytopenic mice: evidence against regulation at the mRNA level and for a direct regulatory role of plateletsBlood19968725675738555478
- FielderPJGurneyALStefanichERegulation of thrombopoietin levels by c-mpl-mediated binding to plateletsBlood1996876215421618630374
- SaurSJSangkhaeVGeddisAEUbiquitination and degradation of the thrombopoietin receptor c-MplBlood201011561254126319880496
- SattlerMDurstinMAFrankDAThe thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinasesExp Hematol1995239104010487543416
- RouyezMCBoucheronCGisselbrechtSControl of thrombopoietin-induced megakaryocytic differentiation by the mitogen-activated protein kinase pathwayMol Cell Biol1997179499150009271377
- GurneyALCarver-MooreKde SauvageFJThrombocytopenia in c-mpl-deficient miceScience19942655177144514478073287
- KawasakiTTakeshitaASoudaKSerum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosisAm J Gastroenterol19999471918192210406260
- GianniniEBottaFBorroPRelationship between thrombopoietin serum levels and liver function in patients with chronic liver disease related to hepatitis C virus infectionAm J Gastroenterol200398112516252014638357
- Peck-RadosavljevicMZacherlJMengYGIs inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver?J Hepatol19972711271319252085
- MartinTG3rdSombergKAMengYGThrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantationAnn Intern Med199712742852889265428
- KoikeYYoneyamaAShiraiJEvaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrationsThromb Haemost1998796110611109657432
- ZeldisJBMugishimaHSteinbergHNIn vitro hepatitis B virus infection of human bone marrow cellsJ Clin Invest19867824114173090103
- WangCSYaoWJWangSTStrong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infectionClin Infect Dis200439679079615472809
- BordinGBallareMZigrossiPA laboratory and thrombokinetic study of HCV-associated thrombocytopenia: a direct role of HCV in bone marrow exhaustion?Clin Exp Rheumatol199513Suppl 13S39S438730475
- Garcia-SuarezJBurgaletaCHernanzNHCV-associated thrombocytopenia: clinical characteristics and platelet response after recombinant alpha2b-interferon therapyBr J Haematol200011019810310930984
- LindenbaumJLieberCSHematologic effects of alcohol in man in the absence of nutritional deficiencyN Engl J Med196928173333385794307
- CowanDHHinesJDThrombocytopenia of severe alcoholismAnn Intern Med197174137435539275
- GrossSKeeferVNewmanAJThe platelets in iron-deficiency anemia. I. The response to oral and parenteral ironPediatrics19643431532314211098
- EvstatievRBukatyAJimenezKIron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietinAm J Hematol201489552452924464533
- ChoiSISimoneJVPlatelet production in experimental iron deficiency anemiaBlood19734222192284793111
- de KlerkGRosengartenPCVetRJSerum erythropoietin (EST) titers in anemiaBlood1981586116411707306704
- LooMBeguinYThe effect of recombinant human erythropoietin on platelet counts is strongly modulated by the adequacy of iron supplyBlood199993103286329310233880
- ParkCHValoreEVWaringAJHepcidin, a urinary antimicrobial peptide synthesized in the liverJ Biol Chem2001276117806781011113131
- NemethETuttleMSPowelsonJHepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalizationScience200430657042090209315514116
- PigeonCIlyinGCourselaudBA new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overloadJ Biol Chem2001276117811781911113132
- GeddisAEKaushanskyKCross-reactivity between erythropoietin and thrombopoietin at the level of Mpl does not account for the thrombocytosis seen in iron deficiencyJ Pediatr Hematol Oncol20032511919920 author reply 92014608207
- RoettoAPapanikolaouGPolitouMMutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosisNat Genet2003331212212469120
- DeugnierYTurlinBle QuilleucDA reappraisal of hepatic siderosis in patients with end-stage cirrhosis: practical implications for the diagnosis of hemochromatosisAm J Surg Pathol19972166696759199645
- JaroszewiczJRogalskaMFlisiakRSerum prohepcidin reflects the degree of liver function impairment in liver cirrhosisBiomarkers200813547848518979640
- AllenDWManningNAbnormal phospholipid metabolism in spur cell anemia: decreased fatty acid incorporation into phosphatidylethanolamine and increased incorporation into acylcarnitine in spur cell anemia erythrocytesBlood1994844128312878049442
- PascoeAKerlinPSteadmanCSpur cell anaemia and hepatic iron stores in patients with alcoholic liver disease undergoing orthotopic liver transplantationGut199945230130510403746
- ConnellWRKammMARitchieJKBone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experienceGut1993348108110858174958
- DuttaTKBadheBACiprofloxacin-induced bone marrow depressionPostgrad Med J19997588757157310616701
- YamaneANakamuraTSuzukiHInterferon-alpha 2b-induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytesBlood2008112354255018523149
- SataMYanoYYoshiyamaYMechanisms of thrombocytopenia induced by interferon therapy for chronic hepatitis BJ Gastroenterol19973222062109085169
- Peck-RadosavljevicMWichlasMPidlichJBlunted thrombopoietin response to interferon alfa-induced thrombocytopenia during treatment for hepatitis CHepatology1998285142414299794931
- JandlJHAsterRHIncreased splenic pooling and the pathogenesis of hypersplenismAm J Med Sci196725343833985336447
- MorrisPWPattonTBBalintJAPortal hypertension, congestive splenomegaly, and portacaval shuntGastroenterology19624255555914476275
- RiosRSangroBHerreroIThe role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosisAm J Gastroenterol200510061311131615929762
- SiedleckiCALestiniBJKottke-MarchantKKShear-dependent changes in the three-dimensional structure of human von Willebrand factorBlood1996888293929508874190
- AryaMAnvariBRomoGMUltralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezersBlood200299113971397712010796
- LianECPathogenesis of thrombotic thrombocytopenic purpura: ADAMTS13 deficiency and beyondSemin Thromb Hemost200531662563216388413
- TsaiHMPhysiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ionBlood19968710423542448639782
- RuggeriZMMechanisms of shear-induced platelet adhesion and aggregationThromb Haemost19937011191238236086
- UemuraMFujimuraYMatsumotoMComprehensive analysis of ADAMTS13 in patients with liver cirrhosisThromb Haemost20089961019102918521503
- UemuraMFujimuraYKoSPivotal role of ADAMTS13 function in liver diseasesInt J Hematol2010911202920054668
- OdaAMiyakawaYDrukerBJThrombopoietin primes human platelet aggregation induced by shear stress and by multiple agonistsBlood19968711466446708639835
- PereiraJAccatinoLAlfaroJPlatelet autoantibodies in patients with chronic liver diseaseAm J Hematol19955031731787485078
- SanjoASatoiJOhnishiARole of elevated platelet-associated immunoglobulin G and hypersplenism in thrombocytopenia of chronic liver diseasesJ Gastroenterol Hepatol200318663864412753144
- AokiYHiraiKTanikawaKMechanism of thrombocytopenia in liver cirrhosis: kinetics of indium-111 tropolone labelled plateletsEur J Nucl Med19932021231298440268
- MacchiLClofent-SanchezGMaritGPAICA: a method for characterizing platelet-associated antibodies – its application to the study of idiopathic thrombocytopenic purpura and to the detection of platelet-bound c7E3Thromb Haemost1996766102010298972027
- van LeeuwenEFvan der VenJTEngelfrietCPSpecificity of autoantibodies in autoimmune thrombocytopeniaBlood198259123267032627
- CinesDBBlanchetteVSImmune thrombocytopenic purpuraN Engl J Med200234613995100811919310
- GeorgeJNWoolfSHRaskobGEIdiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of HematologyBlood19968813408704187
- BassendineMFCollinsJDStephensonJPlatelet associated immunoglobulins in primary biliary cirrhosis: a cause of thrombocytopenia?Gut19852610107410794054707
- ArakawaYAmakiSMiyakawaHPBC-AIH overlap syndrome with concomitant ITP and Hashimoto’s disease with positivity for anti-centromere antibodyJ Gastroenterol200439549049515175950
- PawlotskyJMBouvierMFromontPHepatitis C virus infection and autoimmune thrombocytopenic purpuraJ Hepatol19952366356398750160
- ChiaoEYEngelsEAKramerJRRisk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infectionArch Intern Med2009169435736319237719
- MisianiRBellavitaPFeniliDHepatitis C virus infection in patients with essential mixed cryoglobulinemiaAnn Intern Med199211775735771326246
- SteeperTAHorwitzCAMooreSBSevere thrombocytopenia in Epstein-Barr virus-induced mononucleosisWest J Med198915021701732543142
- WekslerBBReview article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver diseaseAliment Pharmacol Ther200726Suppl 1131917958515
- MayoMJExtrahepatic manifestations of hepatitis C infectionAm J Med Sci2003325313514812640289
- NagamineTOhtukaTTakeharaKThrombocytopenia associated with hepatitis C viral infectionJ Hepatol19962421351408907565
- HamaiaSLiCAllainJPThe dynamics of hepatitis C virus binding to platelets and 2 mononuclear cell linesBlood20019882293230011588022
- PanzerSSeelEIs there an increased frequency of autoimmune thrombocytopenia in hepatitis C infection? A reviewWien Med Wochenschr200315319–2041742014648921
- VisentinGPLiuCYDrug-induced thrombocytopeniaHematol Oncol Clin North Am2007214685696vi17666285
- GeorgeJNRaskobGEShahSRDrug-induced thrombocytopenia: a systematic review of published case reportsAnn Intern Med1998129118868909867731
- DourakisSPDeutschMHadziyannisSJImmune thrombocytopenia and alpha-interferon therapyJ Hepatol19962569729759007728
- ArenaRCecinatoPLisottiASevere immune thrombocytopenia after peg-interferon-alpha2a, ribavirin and telaprevir treatment completion: a case report and systematic review of literatureWorld J Hepatol20157121718172226140092
- AlberioLKimmerleSBaumannARapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopeniaAm J Med2003114752853612753876
- NewmanPMChongBHHeparin-induced thrombocytopenia: new evidence for the dynamic binding of purified anti-PF4-heparin antibodies to platelets and the resultant platelet activationBlood200096118218710891449
- AsterRHBougieDWDrug-induced immune thrombocytopeniaN Engl J Med2007357658058717687133
- WarkentinTEDrug-induced immune-mediated thrombocytopenia – from purpura to thrombosisN Engl J Med2007356989189317329695
- WimanBCollenDMolecular mechanism of physiological fibrinolysisNature19782725653549550151233
- HerschSLKunelisTFrancisRBJrThe pathogenesis of accelerated fibrinolysis in liver cirrhosis: a critical role for tissue plasminogen activator inhibitorBlood1987695131513192436684
- CollenDIdentification and some properties of a new fast-reacting plasmin inhibitor in human plasmaEur J Biochem1976691209216136345
- BajzarLThrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathwayArterioscler Thromb Vasc Biol200020122511251811116046
- LeiperKCrollABoothNATissue plasminogen activator, plasminogen activator inhibitors, and activator-inhibitor complex in liver diseaseJ Clin Pathol19944732142178163691
- Van ThielDHGeorgeMFareedJLow levels of thrombin activatable fibrinolysis inhibitor (TAFI) in patients with chronic liver diseaseThromb Haemost200185466767011341503
- KujovichJLHemostatic defects in end stage liver diseaseCrit Care Clin200521356358715992673
- SteinSFHarkerLAKinetic and functional studies of platelets, fibrinogen, and plasminogen in patients with hepatic cirrhosisJ Lab Clin Med19829922172307061918
- IkuraYOhsawaMOkadaMThe significance of platelet consumption in the development of thrombocytopenia in patients with cirrhosisAm J Med Sci2013346319920323979210
- BoylanJFKlinckJRSandlerANTranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantationAnesthesiology199685510431048 discussion 1030A–1031A8916821
- PorteRJMolenaarIQBegliominiBAprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. EMSALT Study GroupLancet200035592121303130910776742
- DalmauASabateAKooMThe prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative studyLiver Transpl200410227928414762867
- GunawanBRunyonBThe efficacy and safety of epsilon-aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysisAliment Pharmacol Ther200623111512016393288
- LumsdenABHendersonJMKutnerMHEndotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosisHepatology1988822322363281884
- KalambokisGTsianosEVEndotoxaemia in the pathogenesis of cytopenias in liver cirrhosis. Could oral antibiotics raise blood counts?Med Hypotheses201176110510920832949
- WiestRGarcia-TsaoGBacterial translocation (BT) in cirrhosisHepatology200541342243315723320
- KuratsuneHKodaTKurahoriTThe relationship between endotoxin and the phagocytic activity of the reticuloendothelial systemHepatogastroenterology198330379826193047
- Garcia-TsaoGWiestRGut microflora in the pathogenesis of the complications of cirrhosisBest Pract Res Clin Gastroenterol200418235337215123075
- DeviereJContentJDenysCExcessive in vitro bacterial lipopolysaccharide-induced production of monokines in cirrhosisHepatology19901146286342184115
- LinRSLeeFYLeeSDEndotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulationJ Hepatol19952221651727790704
- KalambokisGNMouzakiARodiMRifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemiaLiver Int201232346747522098272
- ItohHCicalaCDouglasGJPlatelet accumulation induced by bacterial endotoxin in ratsThromb Res19968364054198885136
- PanasiukAZakJKasprzyckaEBlood platelet and monocyte activations and relation to stages of liver cirrhosisWorld J Gastroenterol200511182754275815884116
- JelkmannWWolffMFandreyJModulation of the production of erythropoietin by cytokines: in vitro studies and their clinical implicationsContrib Nephrol19908768772128766
- LiJXiaYBertinoAMThe mechanism of apoptosis in human platelets during storageTransfusion200040111320132911099659
- NathanCNitric oxide as a secretory product of mammalian cellsFASEB J1992612305130641381691
- SchobersbergerWHoffmannGFandreyJNitric oxide donors suppress erythropoietin production in vitroPflugers Arch199643269809858781191
- GustotTDurandFLebrecDSevere sepsis in cirrhosisHepatology20095062022203319885876
- VenkataCKashyapRFarmerJCThrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcomeJ Intensive Care201311925810916
- CalyWRStraussEA prospective study of bacterial infections in patients with cirrhosisJ Hepatol19931833533588228129
- ForemanMGManninoDMMossMCirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge SurveyChest200312431016102012970032
- WangYQWangBLiangYRole of platelet TLR4 expression in pathogensis of septic thrombocytopeniaWorld J Emerg Med201121131725214976
- GawazMDickfeldTBognerCPlatelet function in septic multiple organ dysfunction syndromeIntensive Care Med19972343793859142575
- PignatelliPDe BiaseLLentiLTumor necrosis factor-alpha as trigger of platelet activation in patients with heart failureBlood200510661992199415956282
- WarkentinTEAirdWCRandJHPlatelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndromeHematology Am Soc Hematol Educ Program200349751914633796
- KalambokisGTsianosEVThrombocytopenia associated with chronic liver disease: effects of rifaximin on platelet countAm J Gastroenterol2010105122705270721131941
- KrauelKHackbarthCFurllBHeparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodiesBlood201211951248125522049520
- PongasGDasguptaSKThiagarajanPAntiplatelet factor 4/heparin antibodies in patients with gram negative bacteremiaThromb Res2013132221722023830968
- MontaltoPVlachogiannakosJCoxDJBacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective studyJ Hepatol200237446347012217599
- Spleen [webpage on the Internet]Redondo Beach, CAPacific Health and Wellness2014 Available from: http://phaws.com/spleen/Accessed February 18, 2016
- Liver and Cirrhosis [webpage on the Internet]Seattle, WACognition Studio Available from: http://cognitionstudio.com/Accessed March 23, 2016
