Abstract
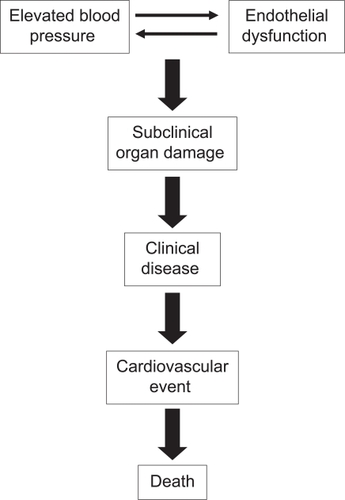
The vascular endothelium, the largest “organ” in the body, synthesizes and releases a wide spectrum of vasoactive substances into the circulation. Endothelial dysfunction links hypertension and other cardiovascular (CV) risk factors that promote the development of atherosclerotic plaque, CV disease, and fatal and nonfatal CV events. Blood pressure (BP) reduction is the most effective way to reduce CV risk in patients with hypertension, but it is unknown whether endothelial dysfunction is a cause or consequence of hypertension. Renin–angiotensin–aldosterone system blockers improve endothelial function and have favorable vascular, metabolic, cardiac, and renoprotective effects that are independent of BP reduction. Olmesartan effectively reduces BP and also has vasoprotective properties, including reductions in endothelial dysfunction and inflammation, prevention of microalbuminuria, and reversal of vascular remodeling. Large-scale, long-term studies are needed to confirm that olmesartan has vasoprotective effects that are independent of BP control and to determine whether these pleiotropic effects translate into improved CV disease outcomes.
Introduction
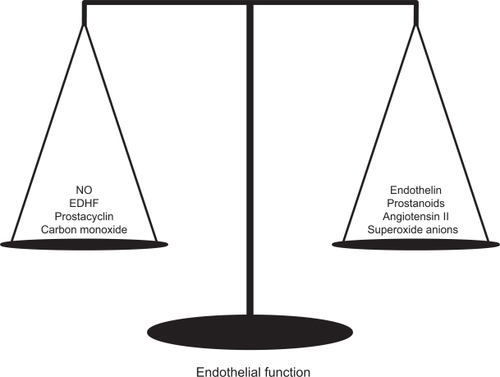
The vascular endothelium is a thin layer of cells that line the interior surface of blood vessels separating circulating blood from layers of vascular smooth muscle cells (VSMC). Since the initial description of endothelium-derived relaxing factor (EDRF) by Furchgott and Zawadzki in 1980,Citation1 the endothelium has been considered the largest “organ” in the body. The endothelium synthesizes and releases a wide spectrum of vasoactive substances. This cellular monolayer both produces and responds to agents that regulate vascular tone, platelet aggregation, oxidative stress, inflammation, thrombogenicity, and VSMC proliferation.Citation2 These include the vasodilators, such as nitric oxide (NO), prostacyclin, endothelium-derived hyperpolarizing factor, and carbon monoxide, and the vasoconstrictors, such as endothelin, superoxide anions (O2−), prostanoids, and angiotensin II (Ang II). Both vasodilators and vasoconstrictors are tonically active, and the balance between those factors determines the normal or pathological state of the vasculature ().
Figure 1 Agents that act continuously in the tone regulation of the vasculature.
Endothelial dysfunction is the common link between cardiovascular (CV) risk factors, such as hypertension and diabetes, and CV diseases.Citation3 For example, coronary vascular endothelial dysfunction independently predicts acute CV events in patients with and without atherosclerotic coronary artery disease.Citation4 In this article, we discuss the relation between endothelial function, blood pressure (BP), and CV outcomes, with emphasis on the protective effects of the angiotensin receptor blocker (ARB) olmesartan medoxomil.
Endothelial function
Nitric oxide and oxidative stress
NO, originally described as EDRF,Citation1 is a free radical, short-lived, highly permeable gas. It is a potent vasodilator, inhibitor of thrombocyte aggregation and leukocyte adhesion, and suppressor of the migration and proliferation of VSMC. NO is released by endothelial cells in response to activation of a variety of receptors, shear stress, changes in BP, and pulsatile stretch.Citation5 It increases intracellular cyclic guanosine monophosphate concentrations by activating guanylate cyclase, leading to VSMC relaxation. NO is synthesized by NO synthase (NOS) from L-arginine, and its formation can be inhibited by false substrates for NOS such as NG-monomethyl-arginine (L-NMMA).Citation6,Citation7 Thus, L-NMMA can be used as an experimental tool for assessing NO function in animal models of vascular disease.
NO plays an important role in BP regulation, thrombosis, and atherosclerosis. Experimentally induced hypertension promotes the release of NO, and decreases in BP suppress NO release, thus promoting vascular homeostasis. Inhibitors of NO production induce sustained hypertension when administered to animals, suggesting that the CV system is exposed to continuous tonic NO-dependent vasodilator tone.Citation8
Endothelial dysfunction associated with systemic hypertension is characterized by impaired NO bioactivity related to increased oxidative stress. Oxidative stress, a condition which occurs when the production of free radicals exceeds the body’s ability to neutralize and eliminate them, is believed to be a major cause of impaired NO bioactivity. Reactive oxygen species (ROS), including superoxide anions (O2−), scavenge
NO and reduce its bioavailability. ROS are generated from both nonenzymatic and enzymatic sources. Nicotinamide adenine dinucleotide phosphate(NAD(P)H) oxidase and cyclooxygenases are the major sources of O2−.Citation9 Thus, ROS, particularly O2−, contribute to the vasoconstrictor effect of Ang II via their interaction with endothelium-derived NO. In addition to the scavenging effect of ROS, the reduced NO bioavailability observed in patients with hypertension compared with normotensive controls is due to a deficiency in NO synthesis (NOS activity).Citation10
Some antihypertensive agents, such as the third generation vasodilating β blocker nebivolol, angiotensin-converting enzyme inhibitors (ACEIs), ARBs, and calcium channel blockers (CCBs), can improve endothelial function by increasing NO bioavailability or decreasing oxidative stress.Citation9 In addition, statins have a modest antihypertensive effect that has been attributed to potentiation of NO.Citation11 Pharmacological inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase with statins stimulates endothelial NOS (eNOS), attenuating the expression of caveolin-1 and reducing the abundance of caveolae in endothelial cells. Caveolin plays a role in endothelial function, binding directly to and inhibiting eNOS, thus decreasing NO availability. Thus, attenuation of caveolin expression and related stimulation of eNOS may explain the BP-reducing effect of statins.
Clinical assessment of endothelial function
Several techniques are available for the assessment of endothelial function in humans (), but all of these have limited applicability in clinical practice. The “perfect” endothelial function test should be cheap, safe, noninvasive, reproducible, repeatable, and standardized among laboratories. However, none of the tests available fulfills these requirements.Citation12 The gold standard for diagnosing endothelial dysfunction is assessment of the response to intraarterial infusion of acetylcholine.Citation3 However, this test is rarely used in clinical practice because of its invasive nature.
Table 1 Methods for measuring endothelial function
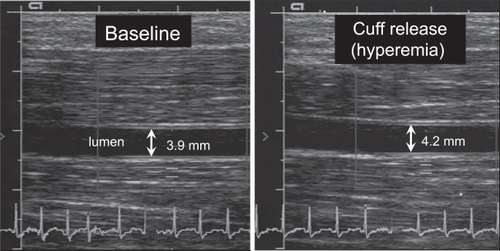
Measurement of flow-mediated vasodilation of the brachial artery by high-resolution ultrasonography is an accepted noninvasive method for assessment of endothelial function.Citation13 The diameter of the brachial artery is measured using high-resolution ultrasound before and after occlusion of the artery for 5 minutes with sphygmomanometer cuff proximal to the brachial artery (). When the cuff is released, the reactive hyperemia causes shear stress in the artery wall and release of NO. The amount of dilation is used as a marker of the endothelial function. Maximum vasodilation capacity is induced using nitroglycerin for comparison. Although noninvasive, this test is time-consuming and operator dependent.
Figure 2 High-resolution ultrasound images showing the brachial artery before (left) and after (right) arterial occlusion.
Endothelial dysfunction contributes to arterial stiffness (reduction of elasticity), and estimation of arterial stiffness by pulse wave analysis has been used as marker of endothelial dysfunction. Increased pulse wave velocity (PWV) and altered wave reflection are important determinants of increased systolic and pulse pressure and provide evidence of CV disease.Citation14,Citation15 With each ejection of blood from the left ventricle, a pressure (pulse) wave is generated and travels from the heart to the periphery at a finite speed that depends on the elastic properties of the conduit arteries. Increased arterial stiffness is correlated with elevated PWV. The pulse wave is reflected at any point of discontinuity in the arterial tree and returns to the aorta and left ventricle. The timing of the wave reflection depends on both the elastic properties and the length of the conduit arteries. Pulse wave analysis provides the augmentation index (AIx) that is a surrogate of wave reflection and is defined as augmented pressure (magnitude of wave reflection) divided by pulse pressure.Citation16
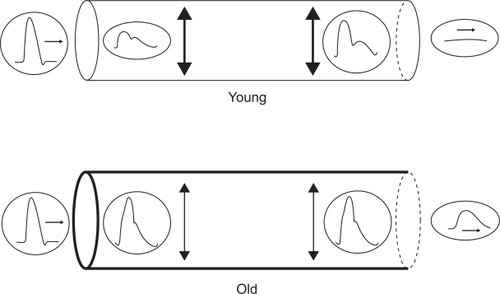
In younger persons (, top panel), PWV is sufficiently slow (approximately 5 m/s) that the reflected wave reaches the aortic valve after closure, leading to a higher diastolic BP and enhancing coronary perfusion by providing a “boosting” effect. In older persons, particularly if they are hypertensive, PWV is greatly increased (approximately 20 m/s) due to central arterial stiffening. At this speed, the reflective wave reaches the aortic valve before closure, leading to a higher systolic BP, pulse pressure, and afterload and a lower diastolic BP (, bottom panel). Coronary perfusion pressure is compromised in some cases secondary to low diastolic BP.
Figure 3 Distensibility and pulse wave velocity (PWV). Simple tubular models of the arterial system, connecting the heart (left) to the peripheral circulation (right). Top: normal distensibility and normal PWV in a young subject. Bottom: decreased distensibility with increased PWV in an old subject. Copyright © 2007. Reproduced with permission from O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50(1):1–13.
Endothelial dysfunction: from hypertension to CV disease
Endothelial dysfunction links hypertension with other CV risk factors that promote the development of atherosclerotic plaque, CV disease, and fatal and nonfatal CV events ().Citation17,Citation18 Multiple studies have demonstrated impaired endothelial function in patients with hypertension. Endothelium-dependent vasodilation in response to acetylcholine infusion in the forearm and coronary circulations is impaired in patients with elevated BP.Citation19–Citation22 However, it is unknown whether endothelial dysfunction is a cause or consequence of hypertension. Normotensive offspring of hypertensive parents exhibits impaired endothelium-dependent vasodilatation in response to acetylcholine.Citation23 Derangement of endothelial function in normotensive offspring of hypertensive parents is also demonstrated by decreased vasoconstriction in response to inhibition of NOS activity.Citation24,Citation25 Together, these findings support the interpretation that endothelial dysfunction is an antecedent rather than a consequence of hypertension.
The renin–angiotensin–aldosterone system (RAAS), the main regulator of sodium and fluid balance in normal subjects and one of the major contributors to the pathogenesis of hypertension, also plays an important role in the pathogenesis of endothelial dysfunction ().Citation26 Ang II increases oxidative stress by stimulating NAD(P)H oxidase, the main source of ROS in the vasculature, and accelerates senescence of endothelial progenitor cells (EPCs).Citation27 EPCs, a rare population of cells that circulate in the blood with the ability to differentiate into endothelial cells, are a marker for vascular function and cumulative CV risk.Citation28 Patients with coronary artery disease have lower circulating levels of EPCs and those with hypertension have accelerated senescence of EPCs.Citation29 This deficiency in EPC numbers/function may contribute to the development of endothelial function in these conditions.
Figure 5 Mechanisms of action of angiotensin receptor blockers (ARBs).
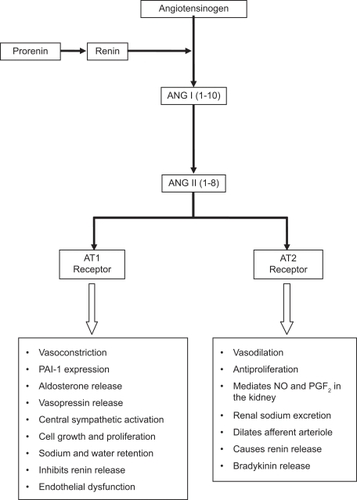
Preclinical studies have demonstrated that activation of the Ang II type 1 (AT1) receptor contributes to the development of atherosclerosis by mediating endothelial dysfunction. Compared with wild type mice, apolipoprotein E knockout mice (ApoE−/−) have significantly greater O2− formation, greater impairment in endothelium-dependent vasodilation, and more extensive atherosclerotic lesions development when fed a high cholesterol diet.Citation30 In contrast, AT1 knockout mice (AT1−/−) have lower oxidative stress, reduced endothelial dysfunction, and less atherosclerotic plaque formation than wild type mice independently of BP and plasma cholesterol levels. Offspring of ApoE × AT1−/− matings has significantly lower O2− and BP levels than ApoE−/− mice and do not develop atherosclerosis. These findings support the functional significance of Ang II in the generation of oxidative stress and atherosclerosis in this model.
RAAS blockers improve endothelial function and have favorable vascular, metabolic, cardiac, and renoprotective effects. ARBs reduce BP by selectively blocking the binding of Ang II to the AT1 receptors in VSMC and other cell types.Citation31 ARBs also reduce CV morbidity and mortality in patients with hypertension, left ventricular hypertrophy, diabetes, renal disease, and congestive heart failure.Citation32–Citation34 Olmesartan medoxomil is a long-acting ARB approved for the treatment of mild to severe hypertension that has vasoprotective properties.
Olmesartan
Pharmacology
Olmesartan medoxomil is an inactive prodrug that is rapidly and completely bioactivated by ester hydrolysis in the gut wall to the pharmacologically active compound olmesartan. Its peak plasma concentrations are achieved between 1 and 3 hours with an elimination half-life of 12–18 hours.Citation35,Citation36 The absolute bioavailability of olmesartan medoxomil after oral administration is 26%–28.6%, and the steady-state plasma concentrations are reached within the first few days. Accumulation is not noted on long-term dosing. Olmesartan is excreted unchanged in the urine (35%–50%) and in the bile. Olmesartan has minimal or no inhibitory activity on human cytochrome P450.Citation35,Citation36 A unique mechanism of binding to the AT1 receptor appears to contribute to the sustained duration of AT1 receptor blockade seen with olmesartan.Citation37,Citation38 This involves the “double chain domain”, whereby olmesartan binds to the receptor at two sites, a −OH group and an α-COOH group, whereas other ARBs bind only at the −OH group. Whether the more sustained inhibition of the pressor effects of infused angiotensin seen with olmesartan compared with other ARBs is secondary to the “double chain domain” is unknown.
Protective effects of olmesartan
Blood pressure
Olmesartan reduces BP rapidly and effectively in hypertensive patients. An analysis of seven randomized, double-blind, placebo- controlled, parallel group studies compared the safety (n = 2,540) and efficacy (n = 2,145) of olmesartan monotherapy with placebo in patients with essential hypertension (sitting diastolic BP ≥100 mm Hg and ≥115 mm Hg).Citation39 Olmesartan produced dose-dependent reductions in both diastolic and systolic BP within 1 week of initiating treatment, and the response was nearly maximal at 2 weeks. There was no difference in efficacy between younger (<65 years) and older (>65 years) patients.
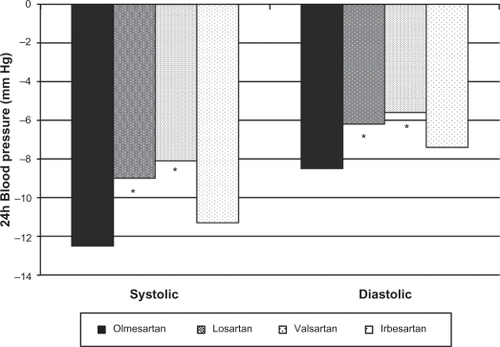
Olmesartan reduces BP more effectively than other ARBs when administered at traditionally recommended starting doses.Citation40 A 588 patient, multicenter, randomized, double-blind trial compared once-a-day therapy with the recommended starting doses of olmesartan (20 mg), losartan (50 mg), valsartan (80 mg), and irbesartan (150 mg) in patients with high BP (cuff diastolic BP ≥100 mm Hg and ≤115 mm Hg, and a mean daytime diastolic BP ≥90 mm Hg and ≤120 mm Hg). A significantly greater reduction in sitting cuff diastolic BP at trough was demonstrated with olmesartan (11.5 mm Hg) compared with losartan, valsartan, and irbesartan (8.2, 7.9, and 9.9 mm Hg, respectively, P < 0.005 olmesartan vs losartan; P < 0.05 olmesartan vs valsartan and irbesartan).Citation41 Reductions in cuff systolic BP with the 4 ARBs had the same numerical trend, although differences between treatments were not statistically significant due to variability in the data. The reduction in mean 24-hour diastolic BP with olmesartan (8.5 mm Hg) was significantly greater than reductions with losartan and valsartan (6.2 and 5.6 mm Hg, respectively, P < 0.05) and showed a trend toward significance when compared with irbesartan (7.4 mm Hg; P =0.087) (). The reduction in mean 24-hour systolic BP with olmesartan (12.5 mm Hg) was significantly greater than those with losartan and valsartan (9.0 and 8.1 mm Hg, respectively) and equivalent to the reduction with irbesartan (11.3 mm Hg). All drugs were well tolerated.
Figure 6 Reductions in mean 24-hour systolic and diastolic blood pressure with 4 different ARBs.
Note: *P < 0.05 compared with olmesartan.
The differences in BP reduction achieved with olmesartan compared with losartan and valsartan are attenuated when doses of the other ARBs are increased. In a 12-week, randomized, double-blind, forced-titration study, 723 hypertensive patients were assigned to receive olmesartan 20 mg, losartan 50 mg, valsartan 80 mg, or placebo, all once daily.Citation42 Doses were titrated to 40, 100, and 160 mg once daily for olmesartan, losartan, and valsartan, respectively, after 4 weeks of treatment. At week 8, doses were titrated to 50 mg twice daily for losartan and 320 mg once daily for valsartan. Olmesartan remained at 40 mg once daily. Compared with placebo, all 3 medications significantly reduced mean seated diastolic BP from baseline. At week 8, patients receiving olmesartan 40 mg once daily had significantly greater reductions in mean seated diastolic BP than those receiving losartan (−15.2/−12.9 vs−10.9/−9.4 mm Hg, respectively, P < 0.001). There was no significant difference compared with valsartan. A significantly greater percentage of patients achieved BP goals (<140/90 mm Hg) with olmesartan compared with losartan and valsartan (39.7%, 19.8%, and 29.0%, respectively, P < 0.001 vs losartan and P = 0.031 vs valsartan). Olmesartan did not reduce mean seated systolic BP significantly compared with valsartan.
Microalbuminuria
Microalbuminuria is an early sign of glomerular endothelial dysfunction and is associated with progressive glomerulosclerosis, renal function loss, and development of overt pro-teinuria. Microalbuminuria is also associated with increased CV morbidity and mortality in both diabetic and nondiabetic subjects.Citation43–Citation47
The Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study,Citation48 conducted in Europe, included 4,449 men and women. Participants had type 2 diabetes, normal kidney function, and at least one additional CV risk factor, including hypertension, but without microalbuminuria.Citation49 They were randomly assigned to receive olmesartan 40 mg daily (n = 2,232) or placebo (n = 2,215), in addition to other antihypertensive medications (except ACEIs or ARBs) during the study in order to reach the target BP of <130/80 mm Hg. Primary end point was time to onset of microalbuminuria, and secondary end points included renal and CV events. The mean baseline BP was 136/81 mm Hg. After 48 months, nearly 80% and 71% of patients in the olmesartan and placebo groups, respectively, reached target BP (<130/80 mm Hg). Patients in the olmesartan group were 23% less likely than those in the placebo group to develop microalbuminuria at 48 months (8.2% vs 9.8%, hazard ratio 0.77; 95% confidence interval: 0.63–0.94, P = 0.01). Overall, CV morbidity and mortality were similar (3.6% vs 4.1% in olmesartan vs placebo), but CV deaths occurred more frequently in the olmesartan group (n = 15) than in the placebo group (n = 3, P = 0.01). The increased risk of CV mortality with olmesartan relative to placebo was attributed by the authors to possible hypotensive episodes in subjects with preexisting CV disease.
Inflammation, vascular remodeling, and endothelial function
Olmesartan has vasoprotective and anti-inflammatory effects that are unrelated to BP reduction. In the EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) study, investigators compared the anti-inflammatory effects of olmesartan alone and combined with pravastatin to placebo in patients with essential hypertension (sitting diastolic BP ≥95 mm Hg and ≤100 mm Hg) and microinflammation. Microinflammation was defined as high-sensitivity C-reactive protein (CRP) ≥3 mg/L, and detectable serum interleukin-6 (IL-6) and intercellular adhesion molecule-1.Citation50 Patients were randomized to olmesartan medoxomil 20 mg/d or placebo for 12 weeks. Hydrochlorothiazide 12.5–25 mg/d was added if diastolic BP ≥90 mm Hg. All patients received pravastatin 20 mg/d after 6 weeks of double-blind treatment for the remainder of the study. Olmesartan treatment significantly reduced serum levels of high-sensitivity CRP, high-sensitivity tumor necrosis factor-α (TNF-α), IL-6, and monocyte chemotactic protein-1 compared with placebo independently of BP reduction. Treatment with pravastatin alone did not significantly alter inflammation markers. This study demonstrated that olmesartan significantly reduces biochemical markers of inflammation by as early as 6 weeks of treatment, resulting in possible additional CV benefits independent of BP reduction.
Olmesartan has a remedial effect on the remodeling of resistance vessels that result from hypertension-related target organ damage. The Vascular Improvement with Olmesartan medoxomil Study (VIOS) tested the hypothesis that, independent of BP control, suppression of the RAAS with olmesartan reverses abnormal remodeling of resistance vessels and has favorable effects on central hemodynamics compared with suppression of sympathetic drive with the β blocker atenolol.Citation51 Nondiabetic patients (n = 100) with stage 1 hypertension were randomized to either olmesartan 20 mg/d or atenolol 50 mg/d.Citation52 The doses of olmesartan and atenolol were increased to 40 mg and 100 mg, respectively, with subsequent addition of other agents (hydrochlorothiazide, amlodipine, or hydralazine) if BP >140/90 mm Hg. Subcutaneous gluteal resistance arteries were examined on a pressurized myograph to evaluate vascular structure at baseline and after 1 year of treatment. Before starting treatment, the wall width, media cross sectional area, and wall-to-lumen (W/L) ratio of resistance arteries were all significantly higher in patients with hypertension than in normotensive control subjects. In the presence of BP reduction to near normal levels in both groups, olmesartan (n = 27), but not atenolol (n = 22), significantly reduced all arteriolar dimensions. The mean W/L ratio in patients who received olmesartan was similar to that in normotensive controls. Further, AIx, a marker of vascular stiffness and endothelial function discussed previously, fell significantly with olmesartan, but remained unchanged with atenolol. Central aortic pressure decreased significantly in both groups without differences between groups.
Endothelial function has been shown to improve with olmesartan treatment independent of BP reduction. A prospective study assessed endothelium-dependent coronary dilation in 26 untreated hypertensive patients.Citation53 Changes of corrected myocardial blood flow (ΔMBF) and coronary vascular resistance (ΔCVR) from rest to cold pressor were measured by using 15O-water and positron emission tomography before and after 12 weeks of treatment. Patients initially received olmesartan 20 mg or amlodipine 5 mg daily. After 1 month, doses were doubled if BP was >140/90 mm Hg, or halved if systolic BP <110 mm Hg to olmesartan 40 mg or 10 mg or amlodipine 10 mg or 2.5 mg, respectively. Blood biomarkers, including lipids, glucose, insulin, high-sensitivity CRP, IL-6, TNF-α, and superoxide dismutase (SOD), were also measured. Mean dose was olmesartan 27.7 mg and amlodipine 5.6 mg at the end of 12 weeks. ΔMBF tended to be greater, and ΔCVR was significantly decreased in the olmesartan group, but did not change in patients treated with amlodipine. Serum SOD activity tended to increase with olmesartan, but not with amlodipine. The authors concluded that olmesartan, but not amlodipine, improved endothelium-dependent coronary function, and that these beneficial effects of olmesartan on coronary vasomotion might be mediated via an antioxidant property of the ARB.
CV outcomes
Olmesartan has been shown to reduce the volume of atherosclerotic plaque in patients with hypertension independent of BP reduction. In the Multicenter Olmesartan atherosclerosis Regression Evaluation (MORE) study, carotid intima media thickness (IMT) and plaque volume were evaluated in 165 hypertensive patients (systolic/diastolic BP 140–180/90–105 mm Hg) with carotid artery disease and increased CV risk.Citation54 Carotid artery disease was defined as IMT 0.8–1.6 mm and at least one plaque (volume 4–500 μL) in the common carotid artery or the carotid bulb. Patients were randomized to double-blind treatment with either olmesartan 20 mg/d or atenolol 50 mg/d. Patients with uncontrolled BP (>140/90 mm Hg) after 4 weeks of treatment were titrated to olmesartan 40 mg or atenolol 100 mg once daily. Hydrochlorothiazide 12.5 mg with up-titration to 25 mg after another 4 and 8 weeks was added, if BP remained uncontrolled. After 2 years, although both treatments reduced IMT similarly, preferential decreases in volume of larger plaques (≥33.7 μL) were seen with olmesartan compared with atenolol (=11.5 vs +0.6 μL, P = 0.023). There were more men (73% vs 50%), current smokers (38% vs 31%), and patients with history of CV disease (14% vs 9%) in the atenolol than in the olmesartan group. The reductions in large plaques occurred despite similar reductions in BP, suggesting an antiatherosclerotic action of olmesartan that is independent of its BP lowering effect.
More recently, the results of the Impact of OLmesarten on progression of coronary atherosclerosis, evaluation by IntraVascular UltraSound (OLIVUS) study, showed that the ARB olmesartan has a positive effect on coronary atherosclerotic plaque.Citation55 In this prospective, randomized multicenter trial, 247 patients with stable angina and native coronary artery disease underwent percutaneous coronary intervention for culprit lesions. Intravascular ultrasound (IVUS) examination was performed in the nonculprit lesions (stenosis <50%) before and after 12–16 months of the randomized treatment regimens. Patients were randomly assigned to control (without treatment with ACEIs or ARBs) or olmesartan 10–40 mg titrated to maximally tolerated dose by 8 weeks. Patients were also treated with a combination of β blockers, CCBs, diuretics, nitrates, glycemic control agents, and/or statins per physician’s guidance. Follow-up IVUS showed significantly decreased progression in total atheroma volume (0.6% vs 5.4%, P < 0.05) and percent change in percent atheroma volume (−0.7% vs 3.1%, P < 0.05) in the olmesartan compared with control group. There were no significant differences in systolic and diastolic BP between groups either at baseline or 14-month follow-up.
Patient perspectives
Adherence rate is inversely related to the number of drugs given and occurrence of adverse effects.Citation56 Adherence to antihypertensive treatment is higher with single daily dose medication that reduces BP with low incidence of side effects. Compliance to the treatment is probably to increase if an antihypertensive agent combines these characteristics and, in addition, if patients are informed about extra protections of the medication.
Conclusion
BP reduction is the most effective way to reduce CV risk in patients with hypertension. Some antihypertensive agents have additional benefits that are independent of BP. Olmesartan effectively reduces BP and also has vasoprotective properties, including reductions in endothelial dysfunction and inflammation, prevention of microalbuminuria, and reversal of vascular remodeling. Large-scale, long-term studies are needed to confirm that olmesartan has vasoprotective effects that are independent of BP control and to determine whether these pleiotropic effects translate into improved CV disease outcomes.
Disclosure
Dr Pimenta reports no conflicts of interest in this work. Dr Oparil has received grants-in-aid from Abbott Laboratories, Astra Zeneca, Boehringer Ingelheim, Bristol Myers-Squibb, Daiichi-Sankyo, Forest Laboratories, GlaxoSmithKline, Novartis, Merck and Co, Pfizer, Sanofi-Aventis, and Schering-Plough, and has served as consultant for Bristol Myers-Squibb, Daiichi Sankyo, Merck and Co, Novartis, Pfizer, Sanofi Aventis, and The Salt Institute.
References
- FurchgottRFZawadzkiJVThe obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholineNature198028857893733766253831
- DeanfieldJEHalcoxJPRabelinkTJEndothelial function and dysfunction: testing and clinical relevanceCirculation2007115101285129517353456
- MunzelTSinningCPostFWarnholtzASchulzEPathophysiology, diagnosis and prognostic implications of endothelial dysfunctionAnn Med200840318019618382884
- HalcoxJPSchenkeWHZalosGPrognostic value of coronary vascular endothelial dysfunctionCirculation2002106665365812163423
- SpiekerLEluscherTFEndothelium in hypertension:nitric oxideOparilSWeberMHypertension: a Companion to Brenner and Rector’s The Kidney2nd edPhiladelphia, PAElsevier Saunders2005146158
- PalmerRMAshtonDSMoncadaSVascular endothelial cells synthesize nitric oxide from L-arginineNature198833361746646663131684
- PalmerRMReesDDAshtonDSMoncadaSL-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxationBiochem Biophys Res Commun19881533125112563390182
- HermannMFlammerALuscherTFNitric oxide in hypertensionJ Clin Hypertens (Greenwich)2006812 Suppl 4172917170603
- BrunnerHCockcroftJRDeanfieldJEndothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of HypertensionJ Hypertens200523223324615662207
- FortePCoplandMSmithLMMilneESutherlandJBenjaminNBasal nitric oxide synthesis in essential hypertensionLancet199734990558378429121259
- FeronODessyCDesagerJPBalligandJLHydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundanceCirculation2001103111311811136695
- DeanfieldJDonaldAFerriCEndothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of HypertensionJ Hypertens200523171715643116
- CelermajerDSSorensenKEGoochVMNon-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosisLancet19923408828111111151359209
- LaurentSCockcroftJvan BortelLExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J200627212588260517000623
- PimentaECalhounDAOparilSEtiology and pathogenesis of systemic hypertensionCrawfordMHDiMarcoJPPaulusWJCardiology3rd edPhiladelphia, PAElsevier2009511522
- O’RourkeMFHashimotoJMechanical factors in arterial aging: a clinical perspectiveJ Am Coll Cardiol200750111317601538
- ChobanianAVAlexanderRWExacerbation of atherosclerosis by hypertension. Potential mechanisms and clinical implicationsArch Intern Med199615617195219568823148
- DzauVJAntmanEMBlackHRThe cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease)Circulation2006114252850287017179034
- LinderLKiowskiWBuhlerFRLuscherTFIndirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertensionCirculation1990816176217672344673
- PanzaJAQuyyumiAABrushJEJrEpsteinSEAbnormal endothelium-dependent vascular relaxation in patients with essential hypertensionN Engl J Med1990323122272355955
- TaddeiSVirdisAMatteiPHypertension causes premature aging of endothelial function in humansHypertension19972937367439052889
- EgashiraKSuzukiSHirookaYImpaired endothelium-dependent vasodilation of large epicardial and resistance coronary arteries in patients with essential hypertension. Different responses to acetylcholine and substance PHypertension19952522012067531175
- StrawnWBChappellMCDeanRHKivlighnSFerrarioCMInhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemiaCirculation2000101131586159310747353
- TaddeiSVirdisAMatteiPArzilliFSalvettiAEndothelium-dependent forearm vasodilation is reduced in normotensive subjects with familial history of hypertensionJ Cardiovasc Pharmacol199220Suppl 12S193S1951282967
- McAllisterASAtkinsonABJohnstonGDHaddenDRBellPMMcCanceDRBasal nitric oxide production is impaired in offspring of patients with essential hypertensionClin Sci (Lond)199997214114710409468
- FerrarioCEffect of angiotensin receptor blockade on endothelial function: focus on olmesartan medoxomilVasc Health Risk Manag20095130131419436655
- ImanishiTHanoTNishioIAngiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stressJ Hypertens20052319710415643130
- HillJMZalosGHalcoxJPCirculating endothelial progenitor cells, vascular function, and cardiovascular riskN Engl J Med2003348759360012584367
- VasaMFichtlschererSAicherANumber and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery diseaseCirc Res2001891E1E711440984
- WassmannSCzechTvan EickelsMFlemingIBohmMNickenigGInhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout miceCirculation2004110193062306715277329
- RuddyMCKostisJBAngiotensin II receptor antagonistsOparilSWeberMAHypertension: a Companion to Brenner and Rector’s The Kidney2nd edPhiladelphia, PAElsevier2005683704
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- PfefferMAMcMurrayJJVelazquezEJValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med2003349201893190614610160
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- LaeisPPuchlerKKirchWThe pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interactionJ Hypertens Suppl2001191S21S3211451211
- SchwochoLRMasonsonHNPharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjectsJ Clin Pharmacol200141551552711361048
- MiuraSFujinoMHanzawaHMolecular mechanism underlying inverse agonist of angiotensin II type 1 receptorJ Biol Chem200628128192881929516690611
- MiuraSKiyaYKanazawaTDifferential bonding interactions of inverse agonists of angiotensin II type 1 receptor in stabilizing the inactive stateMol Endocrinol200822113914617901125
- NeutelJMClinical studies of CS-866, the newest angiotensin II receptor antagonistAm J Cardiol2001878A37C43C
- OparilSSilfaniTNWalkerJFRole of angiotensin receptor blockers as monotherapy in reaching blood pressure goalsAm J Hypertens2005182 Pt 128729415752958
- OparilSWilliamsDChrysantSGMarburyTCNeutelJComparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich)20013528329131811588406
- GilesTDOparilSSilfaniTNWangAWalkerJFComparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertensionJ Clin Hypertens (Greenwich)20079318719517341994
- BianchiSBigazziRCampeseVMMicroalbuminuria in essential hypertension: significance, pathophysiology, and therapeutic implicationsAm J Kidney Dis199934697399510585306
- AdlerAIStevensRJManleySEBilousRWCullCAHolmanRRDevelopment and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64)Kidney Int200363122523212472787
- IrieFIsoHSairenchiTThe relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general populationKidney Int20066971264127116501489
- TonelliMJosePCurhanGSacksFBraunwaldEPfefferMProteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trialBMJ20063327555142616714328
- RuilopeLIzzoJHallerHPrevention of microalbuminuria in patients with type 2 diabetes: what do we know?J Clin Hypertens (Greenwich)201012642243020591087
- HallerHVibertiGCMimranAPreventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) studyJ Hypertens200624240340816508590
- HallerHItoSIzzoJLJrPrevention of microalbuminuria in type 2 diabetes (ROADMAP Trial)J Hypertens201028Suppl Ae233
- FliserDBuchholzKHallerHAntiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammationCirculation200411091103110715313950
- SmithRDYokoyamaHAverillDBThe protective effects of angiotensin II blockade with olmesartan medoxomil on resistance vessel remodeling (The VIOS study): rationale and baseline characteristicsAm J Cardiovasc Drugs20066533534217083268
- SmithRDYokoyamaHAverillDBSchiffrinELFerrarioCMReversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptorsJ Am Soc Hypertens20082316517220409899
- NayaMTsukamotoTMoritaKOlmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patientsJ Am Coll Cardiol200750121144114917868805
- StumpeKOAgabiti-RoseiEZielinskiTCarotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) studyTher Adv Cardiovasc Dis2007129710619124398
- HirohataAYamamotoKMiyoshiTImpact of olmesartan on progression of coronary atherosclerosis: a serial volumetric intravascular ultrasound analysis from the OLIVUS (impact of OLmesarten on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) trialJ Am Coll Cardiol2010551097698220202514
- PimentaEOparilSFixed combinations in the management of hypertension: patient perspectives and rationale for development and utility of the olmesartan-amlodipine combinationVasc Health Risk Manag20084365366418827915