Abstract
Background
Add-on prolonged-release melatonin (PRM) in antihypertensive therapy has been shown to ameliorate nocturnal hypertension. Hypertension is a major comorbidity among insomnia patients. The efficacy and safety of PRM for primary insomnia in patients aged 55 years and older who are treated with antihypertensive drugs were evaluated.
Methods
Post hoc analysis of pooled antihypertensive drug-treated subpopulations from four randomized, double-blind trials of PRM and placebo for 3 weeks (N[PRM] = 195; N[placebo] = 197) or 28 weeks (N[PRM] = 157; N[placebo] = 40). Efficacy measurements included Leeds Sleep Evaluation Questionnaire scores of quality of sleep and alertness and behavioral integrity the following morning after 3 weeks, and sleep latency (daily sleep diary) and Clinical Global Impression of Improvement (CGI-I) after 6 months of treatment. Safety measures included antihypertensive drug-treated subpopulations from these four and three additional single-blind and open-label PRM studies of up to 1 year (N[PRM] = 650; N[placebo] = 632).
Results
Quality of sleep and behavior following wakening improved significantly with PRM compared with placebo (P < 0.0001 and P < 0.0008, respectively). Sleep latency (P = 0.02) and CGI-I (P = 0.0003) also improved significantly. No differences were observed between PRM and placebo groups in vital signs, including daytime blood pressure at baseline and treatment phases. The rate of adverse events normalized per 100 patient-weeks was lower for PRM (3.66) than for placebo (8.53).
Conclusions
The findings demonstrate substantive and sustained efficacy of PRM in primary insomnia patients treated with antihypertensive drugs. PRM appears to be safe for insomnia in patients with cardiovascular comorbidity.
Introduction
Insomnia, defined as difficulties initiating or maintaining sleep or nonrestorative sleep associated with significant daytime distress (Diagnostic and Statistical Manual of Mental Disorders, 4th ed [DSM-IV]), occurs in about 30% of subjects aged 55 years and older.Citation1–Citation6 One of the major health issues found in the 55+ years population is hypertension.Citation7,Citation8 The prevalence of hypertension is significantly higher among insomnia patients (~44%) as compared with good sleepers (~19%), suggesting a cross-talk between sleep and blood pressure (BP) control.Citation9 In particular, higher systolic BP and lower day-to-night systolic BP dipping were reported in normotensive insomniacs as compared with in normotensive good sleepers.Citation10 Furthermore, short sleep duration and insomnia were found to be risk factors for hypertension, as assessed in middle-aged subjects and depressed patients.Citation11,Citation12 In the elderly, it was shown that impaired sleep architecture as expressed by decreased slow-wave sleep increases the risk of developing hypertension.Citation13 The blunted nocturnal BP dip and the resulting nocturnal hypertension have severe consequences and are considered major risk factors for cardiovascular events.Citation14 Accordingly, a recent Dutch population-based cohort study of 20,432 men and women aged 20–65 years revealed that short sleepers with poor sleep quality had a 63% higher risk of cardiovascular disease (CVD) and a 79% higher risk of coronary heart disease compared with normal sleepers with good quality sleep.Citation15 Add-on controlled-release and prolonged-release melatonin (PRM) preparations for antihypertensive therapy have been shown to ameliorate nocturnal hypertension.Citation16 It is therefore important to find out whether such preparations would effectively treat insomnia in patients who have cardiovascular comorbidity.
PRM (Circadin®, Rad Neurim Pharmaceuticals EEC Ltd, Reading, UK) is a new drug licensed to treat primary insomnia in patients aged 55 years and older. It is designed to mimic the release pattern of endogenous melatonin, a hormone that regulates sleep and circadian rhythms.Citation17 There is an age-related decline in the robustness of the biological clock and melatonin production, thus depriving the brain of an important sleep regulator.Citation18–Citation21 In patients aged 55 years and over who suffer from poor sleep quality, melatonin production is even lower than in healthy elderly without such a complaint.Citation22,Citation23 PRM (2 mg) has been shown to be effective in improving the patient-reported quality of sleep and morning alertness as well as sleep latency in insomnia patients.Citation24–Citation28 It was thus pertinent to check whether add-on of PRM improves quality of sleep, sleep latency, and next-day alertness in patients aged 55 and older with primary insomnia who are treated with antihypertensive drugs. The safety of PRM in this population was also of interest because of potential drug interactions with medications used for the treatment of CVD, including hypertension.
The efficacy and safety of treatment with PRM and placebo were thus compared in a subpopulation from PRM clinical trials of the insomnia patients aged 55 years and older treated with antihypertensive drugs when entering the studies. A post hoc, pooled analysis of four randomized, double-blind trials (short-term 3-week studies and a long-term 6-month study)Citation24–Citation28 with similar designs compared the efficacy of PRM and placebo (3 weeks up to 6 months) in the treatment of primary insomnia in this subpopulation. Safety measures included vital signs as well as the frequencies of adverse events (AEs) along with general safety measures in all patients with insomnia from these four and three additional single-blind and open-label PRM trials that had a recorded history of any cardiovascular abnormalities when entering the trials.
Methods
Studies: efficacy analysis
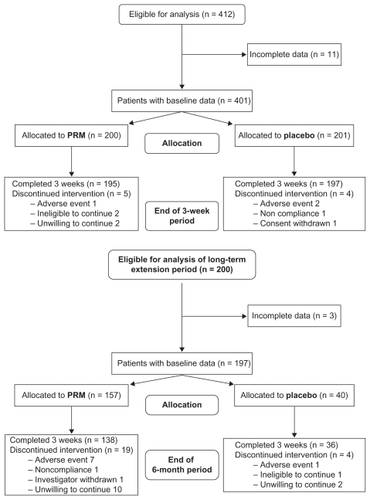
Data from four clinical trials conducted between 1998 and 2008 were used for the efficacy analysis.Citation24–Citation28 These trials shared the same basic design, which included 1–2 weeks of a single-blind placebo run-in period followed by a 3-week randomized, double-blind treatment period. Patients were instructed to take either PRM 2 mg (Circadin®) or a placebo tablet daily 2 hours before bedtime. Efficacy parameters were measured at the beginning (baseline) and at the end of the 3-week double-blind period. In one of these studies,Citation27 the double-blind treatment was then continued for 6 months, wherein patients randomized to PRM continued and those randomized to placebo were re-randomized to PRM and placebo for 6 months (). Another studyCitation24 included a 6- to 12-month open-label extension phase of PRM 2 mg treatment, which contributed safety data.
Figure 1 Overall patient disposition in efficacy analysis. Analysis of the short-term period included eligible patients who completed 3 weeks of double-blind treatment with prolonged-release melatonin (PRM) or placebo. Analysis of the long-term period included patients of one studyCitation27 re-randomized to PRM and placebo for 26 weeks of treatment.
Studies: safety analysis
Safety analysis was performed for all patients included in the efficacy analysis. For the sake of completeness of the data, the analysis included also all patients with insomnia and any recorded CVD aged 55 years and older from two additional single-blind safety trialsCitation23,Citation26 and a long-term open-label extension phase of one study.Citation24 In these trials, patients aged 18–80 years participated, and PRM doses used were 0.1 mg, 1 mg, 2 mg, and 5 mg daily for 3–52 weeks. Altogether, the number of patients with any recorded CVD included in the safety analysis was N[PRM] = 650 and N[placebo] = 632.
All study protocols were approved by local ethics committees and complied with Good Clinical Practice standards stated in the Declaration of Helsinki 1975.
Subjects
In the included double-blind PRM trials, eligible patients were men and women aged 55–80 years suffering from primary insomnia according to DSM-IV criteria and for whom this was the main consultation complaint. A four-step process was used for screening out patients with secondary sleep disorders, including depression and other sleep disorders. First, a Sleep History Questionnaire (SHQ) was used. This SHQ, which was adopted from the Management of Insomnia Guidelines for Clinical Practice of the World Psychiatric Association, characterizes the primary sleep complaint and also helps in differentiating primary insomnia from secondary insomnia due to medical and psychiatric disorders (including depression and anxiety) and specific insomnia disorders like circadian rhythm disorders, movement disorders, parasomnias, and breathing-related sleep disorders. Second, the SHQ was performed at the screening visit by a qualified clinician. Third, in order to rule out psychiatric disorders, including depression anxiety and dementia, the patients went through a detailed psychological assessment that included the Raskin Depression Scale, Covi Anxiety Scale, and Mini-mental State Examination at Visit 1. Finally, patients who were using psychotropic treatments (neuroleptics, antiepileptics, barbiturates, antidepressants, anxiolytics, and lithium) in the previous 3 months before the study were excluded. A positive drug screen on Visit 2 for benzodiazepines, barbiturates, sedating antihistamines, hydroxyzine, doxylamine, zaleplon, zopiclone, or zolpidem led to immediate exclusion. In addition, patients had to report any concomitant medication, and any patient who reported using any psychotropic treatment as detailed was not randomized in the study. For the purpose of the current analysis, patients were selected if their medical history included hypertension and if they received antihypertensive medication before and during the study.
Efficacy measures
The primary efficacy measures in the short-term (3 weeks) studies were the improvements in quality of sleep and morning alertness as assessed by the Leeds Sleep Evaluation Questionnaire (LSEQ). The LSEQ is a widely used standardized instrument for the measurement of sleep difficulties in clinical settings.Citation29 It is a retrospective instrument by which the patients are asked to contrast aspects of their current sleep with those at the time before they joined the study. The LSEQ comprises ten individual visual analog scales (100 millimeters) shown by factor analysis to assess four discrete domains that are used independently to assess the following aspects of sleep and daytime behavior: getting to sleep, quality of sleep (QOS), awakening from sleep, and behavior following wakening (BFW).Citation30,Citation31 The QOS domain is the mean of Questions 4 and 5, which relate to the question “How would you describe the quality of your sleep compared with normal sleep?”. Alertness and behavioral integrity the following morning (BFW) is the mean of Questions 8, 9, and 10 (“How do you feel when you wake up?, How do you feel now?, How would you describe your balance and coordination upon awakening?”). The LSEQ is used in a repetitive manner, yielding a series of measurements, and the difference between current and preceding measurements is used in drug efficacy evaluations.Citation29 Patients were asked to fill in the LSEQ 2 hours after awakening and to evaluate their quality of sleep and morning behaviors as compared with the respective values before starting run-in. Patients in all four studies completed the LSEQ during the last 3 days of the run-in period (baseline measurement) and the last 3 days of the 3-week treatment period. The changes in each parameter averaged over three consecutive days from run-in placebo (baseline) to end of the 3-week treatment were calculated for each patient. In the long-term study,Citation27 patients completed a daily sleep diary. The main efficacy parameter in this study was the patient-reported time taken to fall asleep (sleep latency) measured over the last 7 days of baseline and treatment period. The global improvement in patients’ health status, assessed in each patient using Clinical Global Impression of Improvement (CGI-I),Citation32 is also presented as a measure of overall benefit to the patients. The CGI rating scales are commonly used measures of symptom severity, treatment response, and efficacy of treatments. This is a validated subjective scale that requires the user of the scale to compare the subjects with typical patients in the clinician experience. The CGI-I is a seven-point scale that requires the clinician to assess how much the patient’s illness has improved or worsened relative to a baseline state at the beginning of the intervention and can be rated as 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; or 7, very much worse.
Safety
Vital signs, including BP (daytime), were measured at each visit as a measure of safety and tolerability. AE incidences were pooled, including those in patients for whom cardiovascular abnormality was recorded in either their medical history or the pretreatment physical examination. In an attempt to adjust for differences in duration of exposure between short- and long-term treatment periods with PRM and placebo, the incidences of AEs normalized for the exposure period (per 100 patient-weeks) were also presented.
Statistical analysis
Analyzed variables were presented in summary tables providing sample size (N), arithmetic mean, standard deviation, and minimum and maximum values. An analysis of covariance (ANCOVA) model was applied for testing the significance of the differences in efficacy measures between the study groups adjusted to baseline value and study. χ2 analysis was applied for comparing responder rates assessed by the CGI-I score between PRM and placebo groups after 6 months of treatment. A responder was defined as a patient who scored 1 (very much improved) or 2 (improved) in CGI-I at the end of the 6-month period. Student’s t-test analysis was applied for comparing mean group BP (daytime measurement) at baseline and after 3 weeks (for all studies) and 6 months (long-term study).Citation27,Citation28 Effect size was calculated using Cohen’s d.Citation33 All tests applied were two-tailed, and a P value ≤5% was considered statistically significant. The data were analyzed using SAS® (v 9.1; SAS Institute, Cary, NC).
Results
Efficacy
A total of 412 primary insomnia patients (139 men, 273 women) who had a medical history of hypertension and concomitant therapy records of hypertension (with or without other CVD) were included in the PRM trials. Of these, 392 completed the 3-week randomized, double-blind treatment period, had valid efficacy data, and were included in the analysis (see for full details). All of these subjects were concomitantly treated with at least one antihypertensive drug (26% with ß-blockers, 53% with renin-angiotensin system inhibitors, and 33% with calcium channel blockers). The majority of patients received two or more kinds of antihypertensive medications concomitantly, and the minority (19.6%; N = 77) received one kind of antihypertensive medication (3.8% ß-blockers, 6.1% angiotensin-converting enzyme inhibitors, 3.6% calcium channel inhibitors, and 6.1% other kind of medication such as diuretics and serum lipid-reducing agents).
The effects of PRM (Circadin®, 2 mg) and placebo treatment on sleep quality in this population are presented in . The patients have demonstrated a statistically significant improvement in QOS with PRM for 3 weeks compared with placebo with a mean improvement (decrease) from baseline of 9.2 with PRM versus 3.7 millimeters with placebo (df = 1; F = 16.67; P < 0.0001, ANCOVA adjusted to baseline and study). The effect of PRM on BFW the following morning is presented in . A statistically significant improvement in BFW with PRM for 3 weeks compared with placebo was found, with a mean improvement (decrease) from baseline of 7.2 with PRM versus 3.0 millimeters with placebo (df = 1; F = 11.9; P < 0.0008, ANCOVA adjusted to baseline and study). The corresponding effect size (Cohen’s d) was 0.35 for QOS and 0.3 for BFW.
Table 1 Improvement in quality of sleep with PRM compared to placebo (3 weeks)
Table 2 Efficacy of PRM vs placebo: improvement in morning alertness compared to placebo (3 weeks)
By the end of the 6-month treatment period, the mean improvement (decrease) in patients’ evaluated sleep latency (reported in the daily sleep diary) was significantly higher with PRM (25.89 minutes) than with placebo (7.54 minutes) (df = 1; F = 8.74; P = 0.02, ANCOVA) (). The Cohen’s d effect size was 0.39.
Table 3 Efficacy of prolonged-release melatonin (PRM) compared with placebo (6 months): improvement in sleep latency (daily sleep diary)
Following the 6-month treatment period, 38.9% of the patients improved or very much improved in CGI-I as compared with 12% of placebo-treated patients (χ2 = 7.87; P = 0.0003).
The association between the concomitant antihypertensive therapy and response to PRM could not be obtained, because only ~20% of the patients included in the analysis had been treated with one kind of antihypertensive medication, and the vast majority (~80%) of the patients had been treated with more than one antihypertensive medication concomitantly. For about 26% of patients the antihypertensive medications included ß-blockers. No significant differences in response were found between patients who, among other drugs, received ß-blockers and those who did not.
Safety
No significant differences were found in vital signs, including BP, between insomnia patients with antihypertensive drugs receiving PRM or placebo in the efficacy population at the baseline visit or after 3-week or 6-month PRM treatment ().
Table 4 Blood pressure measurement taken at baseline, 3 weeks, and 6 months as part of safety vital signs collection
For the AE analysis, altogether, 650 PRM-treated patients and 632 placebo-treated patients with a recorded history of cardiovascular abnormalities or who were diagnosed prior to the trials were evaluated. No changes in vital signs were observed with PRM in this population. The overall rate of AEs recorded in these patients was somewhat higher in PRM (41.8%) versus placebo-treated patients (36.6%). However, when normalized per exposure time, the AE occurrences per 100 patient-weeks was much lower for PRM (3.66) than for placebo (8.53). As can be seen in , the main contributors to the differences in AEs with PRM versus placebo per 100 patient-weeks are gastrointestinal (0.86 vs 1.84), infections and infestations (0.95 vs 1.36), nervous system disorders (0.95 vs 2.66), and psychiatric disorders (0.41 vs 1.81), all of which are lower with PRM than with placebo treatment. Cardiovascular-related AEs were rare (<0.3%), with no significant differences between PRM and placebo.
Table 5 Adverse events during treatment with PRM and placebo in insomnia patients with and without history of cardiovascular abnormalities
Discussion
The results of the current post hoc analysis using the primary efficacy endpoints from the individual studies demonstrate that in insomnia patients aged 55 years and older with a history of hypertension and concomitant treatment with antihypertensive drugs, treatment with PRM improves sleep quality and next-day alertness significantly more than placebo. Long-term benefit to these patients was also demonstrated by the significantly greater improvements in sleep latency treated for 6 months with PRM compared with placebo. The effect size of ~0.35 obtained with PRM in these three sleep variables in comparison with placebo is considered medium,Citation33 quite comparable with those of hypnotics,Citation34 and well within the range of effect sizes found with central nervous system drugs,Citation35,Citation36 and is therefore of clear clinical relevance. Benefit to patients is confirmed by the higher percentage of patients who improved or very much improved in CGI-I with PRM compared with placebo following 6 months of treatment. The safety profile of PRM in this population is benign compared with placebo. This implies that add-on PRM therapy does not present significant risks of detrimental drug interactions with the main drugs used to treat CVD, including hypertension, for long-term periods.
Because hypertension is linked to insomnia,Citation9 the efficacy of PRM in improving sleep and morning alertness in patients with insomnia may in part be due to lowering nocturnal BP. Notably, in a 24-hour ambulatory BP monitoring study, 53% of the patients treated with antihypertensive drugs demonstrated nondipping/nocturnal hypertension despite pharmacotherapy,Citation37 suggesting that antihypertensive treatment does not restore proper circadian rhythms in BP. PRM and other controlled-release melatonin formulations (2–3 mg) but not immediate-release formulations have been consistently shown to reduce nocturnal BP.Citation16 The other way around is less likely, as improvement in sleep alone, such as with benzodiazepine/benzodiazepine-like hypnotics, does not improve nocturnal hypertension. In fact, zolpidem, the most commonly used hypnotic drug, does not lessen, and can even increase, nocturnal BP.Citation38,Citation39
A limitation in our study is that no measurements of nocturnal BP were taken in the clinical trials included in the combined analysis. However, it is important to note that BP (daytime) measured in all of the studies as part of general safety vital sign assessments was not impaired with PRM compared with placebo, suggesting that PRM did not reduce the efficacy of the antihypertensive therapy. These data agree well with studies on the effects of PRM and controlled-release melatonin preparations in patients with nocturnal hypertension, all of which showed a decrease in BP during the night and no change during the day (reviewed by Grossman et al).Citation16 Therefore, it is possible that the improvement in nocturnal hypertension augments the soporific effects of PRM in insomnia patients who are treated with antihypertensive drugs. Both effects may nevertheless be related to the effects of PRM on the biological clock regulating the day–night cycles in sleep and wakefulness and BP. An ambulatory BP monitoring study examining the effect of PRM on insomnia patients with hypertension to shed more light on the inter-relation between insomnia and nocturnal BP is thus warranted.
All of the patients in the hypertensive insomnia subpopulation in our studies were treated with antihypertensive medications (mostly ß-blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors). Nevertheless, because most of the patients received two and sometimes three or four antihypertensive medications, we could not determine whether the improvement in sleep and daytime parameters with PRM was specifically associated with the concomitant use of a particular antihypertensive drug. Specifically, ß-blockers are known to reduce endogenous melatonin secretionCitation40 and induce insomnia.Citation41 We did not observe major differences in response to PRM between patients treated with ß-blockers or not treated with ß-blockers. This is perhaps because melatonin is low in patients with hypertension or coronary heart disease,Citation42–Citation45 or because some of the ß-blockers used do not affect melatonin.Citation46,Citation47 Altogether, PRM appears to be effective and safe for the treatment of insomnia in patients with insomnia and cardiovascular comorbidity, including hypertension, and may therefore serve as the first choice of hypnotics for patients with hypertension.
Acknowledgment/disclosure
All the studies described in this article were supported by Neurim Pharmaceuticals (1991) Ltd, the manufacturer of PRM (Circadin®). The authors report no other conflicts of interest in this work.
References
- BliwiseDLKingACHarrisRBHaskellWLPrevalence of self-reported poor sleep in a healthy population aged 50–65Soc Sci Med199234149551738856
- FullertonDSThe economic impact of insomnia in managed care: a clearer picture emergesAm J Manag Care200612Suppl 8S24625216686594
- LegerDPoursainBNeubauerDUchiyamaMAn international survey of sleeping problems in the general populationCurr Med Res Opin200824130731718070379
- OhayonMMSagalesTPrevalence of insomnia and sleep characteristics in the general population of SpainSleep Med201011101010101821093362
- RothTInsomnia: definition, prevalence, etiology, and consequencesJ Clin Sleep Med20073Suppl 5S71017824495
- RothTRoehrsTInsomnia: epidemiology, characteristics, and consequencesClinical Cornerstone Chronic Insomnia200353515
- EganBMZhaoYAxonRNUS trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008JAMA2010303202043205020501926
- Wolf-MaierKCooperRSBanegasJRHypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United StatesJAMA2003289182363236912746359
- RothTComorbid insomnia: current directions and future challengesAm J Manag Care2009Suppl 15S61319298104
- LanfranchiPAPennestriMHFradetteLDumontMMorinCMMontplaisirJNighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular riskSleep200932676076619544752
- GangwischJEMalaspinaDPosnerKInsomnia and sleep duration as mediators of the relationship between depression and hypertension incidenceAm J Hypertens2010231626919893498
- GangwischJEHeymsfieldSBBoden-AlbalaBShort sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination SurveyHypertension5200647583383916585410
- FungMMPetersKRedlineSDecreased slow wave sleep increases risk of developing hypertension in elderly menHypertension201158459660321876072
- HermidaRCAyalaDEMojonAFernandezJRDecreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular riskJ Am Coll Cardiol201158111165117321884956
- Hoevenaar-BlomMPSpijkermanAMWKromhoutDvan den BergJFVerschurenWMMSleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN StudySleep2011
- GrossmanELaudonMZisapelNEffect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trialsVasc Health Risk Manag2011757758421966222
- WadeAZisapelNLemoinePProlonged-release melatonin for the treatment of insomnia: targeting quality of sleep and morning alertnessAging Health200811121
- CzeislerCADuffyJFShanahanTLStability, precision, and near-24-hour period of the human circadian pacemakerScience199928454232177218110381883
- HofmanMASwaabDFAlterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with agingBrain Res19946511–21341427922560
- MahlbergRKienastTHadelSHeidenreichJOSchmitzSKunzDDegree of pineal calcification (DOC) is associated with polysomnographic sleep measures in primary insomnia patientsSleep Med200910443944518755628
- WaldhauserFWeiszenbacherGTatzerEAlternations in nocturnal serum melatonin levels in humans with growth and agingJ Clin Endocrinol Metab1988666486523350912
- HaimovILaudonMZisapelNSleep disorders and melatonin rhythms in elderly peopleBMJ19943091678044096
- LegerDLaudonMZisapelNNocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapyAm J Med20041162919514715322
- LemoinePGarfinkelDLaudonMNirTZisapelNProlonged release melatonin for insomnia – an open label long term study of efficacy, safety and withdrawal symptomsTher Clin Risk Manag2011730131121845053
- LemoinePNirTLaudonMZisapelNProlonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effectsJ Sleep Res20071637238018036082
- LuthringerRMuzetMZisapelNStanerLThe effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomniaInt Clin Psychopharmacol200924523924919584739
- WadeAGFordICrawfordGNightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safetyBMC Med2010815120712869
- WadeAGFordICrawfordGEfficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomesCurr Med Res Opin200723102597260517875243
- ZisapelNLaudonMSubjective assessment of the effects of CNS-active drugs on sleep by the Leeds Sleep Evaluation Questionnaire: a reviewHuman Psychopharmacology Clin Exp200217119
- ParrottACHindmarchIThe Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations – a reviewPsychopharmacology (Berl)19807121731796777817
- TarraschRLaudonMZisapelNCross-cultural validation of the Leeds Sleep Evaluation Questionnaire (LSEQ) in insomnia patientsHum Psychopharmacol200318860361014696019
- GuyWECDEU assessment manual for psychopharmacology (rev, 1976KensingtonDHEW publication Biometric laboratory, George Washington University, US Department of Health, Education and Welfare1976
- CohenJStatistical power analysis for the behavioral sciencesHillsdale, NJLawrence Earlbaum Associates1988
- GlassJLanctotKLHerrmannNSprouleBABustoUESedative hypnotics in older people with insomnia: meta-analysis of risks and benefitsBMJ20053317526116916284208
- ClaghornJLKievARickelsKSmithWTDunbarGCParoxetine versus placebo: a double-blind comparison in depressed patientsJ Clin Psychiatry199253124344381487471
- SchneiderLSIsmailMSDagermanKClinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trialSchizophr Bull2003291577212908661
- de la SierraARedonJBanegasJRPrevalence and factors associated with circadian blood pressure patterns in hypertensive patientsHypertension20095346647219171788
- McCannCCQuera-SalvaMABoudetJEffect of zolpidem during sleep on ventilation and cardiovascular variables in normal subjectsFundam Clin Pharmacol1993763053108406294
- RachmaniRShenhavGSlavachevskyILevyZRavidMUse of a mild sedative helps to identify true non-dippers by ABPM: a study in patients with diabetes mellitus and hypertensionBlood Press Monit200492656915096902
- RommelTDemischLInfluence of chronic b-adrenoreceptor blocker treatment on melatonin secretion and sleep quality in patients with essential hypertensionJ Neural Transm1994953948
- TanabeNFujitaTFujiiYOriiTInvestigation of the factors that contribute to the onset of insomnia in hypertensive patients by using a post-marketing surveillance databaseYakugaku Zasshi20111315669677 Japanese21532263
- BruggerPMarktlWHeroldMImpaired nocturnal secretion of melatonin in coronary heart diseaseLancet1995345896214087760612
- JonasMGarfinkelDZisapelNLaudonMGrossmanEImpaired nocturnal melatonin secretion in non-dipper hypertensive patientsBlood Press200312192412699131
- RapoportSIShatalovaAMMalinovskaiaNKVettenbergLMelatonin production in hypertensive patientsKlin Med (Mosk)20007862124 Russian10900864
- SakotnikALiebmannPMStoschitzkyKDecreased melatonin synthesis in patients with coronary artery diseaseEur Heart J199920181314131710462465
- StoschitzkyKSakotnikALercherPInfluence of beta-blockers on melatonin releaseEur J Clin Pharmacol199955211111510335905
- StoschitzkyKStoschitzkyGBrusseeHBonelliCDobnigHComparing beta-blocking effects of bisoprolol, carvedilol and nebivololCardiology2006106419920616679760