Abstract
Combined therapy is required in the majority of patients with hypertension to achieve blood pressure (BP) targets. Although different antihypertensive drugs can be combined, not all combinations are equally effective and safe. In this context, the combination of a renin angiotensin system inhibitor with a diuretic, usually a thiazide, particularly hydrochlorothiazide (HCTZ) or thiazide-like diuretics, such as chlorthalidone or indapamide, is recommended. However, not all diuretics are equal. Although HCTZ, chlorthalidone, and indapamide as add-on therapy effectively reduce BP levels, the majority of studies have obtained greater BP reductions with chlorthalidone or indapamide than with HCTZ. Moreover, there are data showing benefits with chlorthalidone or indapamide beyond BP. Thus, chlorthalidone seems to have pleiotropic effects beyond BP reduction. Moreover, compared with placebo, chlorthalidone has small effects on fasting glucose and total cholesterol, and compared with HCTZ, chlorthalidone achieves significantly lower total cholesterol and low-density lipoprotein cholesterol levels. Similarly, indapamide has demonstrated no negative impact on glucose or lipid metabolism. More importantly, although head-to-head clinical trials comparing the effects of indapamide or chlorthalidone with HCTZ are not available, indirect comparisons and post hoc analyses suggest that the use of chlorthalidone or indapamide is associated with a reduction in cardiovascular events. Despite this, the most frequent diuretic used in clinical practice as add-on therapy for hypertension is HCTZ. The purpose of this review is to update the published data on the efficacy and safety of HCTZ, chlorthalidone, and indapamide as add-on therapy in patients with hypertension.
Importance of blood pressure control
Hypertension is one of the most important risk factors for the development of cardiovascular disease, including stroke, ischemic heart disease, heart failure, and chronic kidney disease.Citation1 Approximately 54% of stroke, 47% of ischemic heart disease, and 13.5% of total deaths are attributable to hypertension worldwide.Citation2 This is very relevant, given that more than one third of adults have hypertension.Citation3 Noteworthy is that although these numbers increase markedly with age, hypertension has become increasingly common in the younger age groups in recent years.Citation4
Decreasing blood pressure (BP) levels to recommended targets is essential to improve the cardiovascular prognosis in the hypertensive population. The reduction of coronary heart disease mortality observed in a number of countries has been at least in part associated with improved medical treatment and control of risk factors, particularly with regard to systolic BP and total cholesterol.Citation5,Citation6 Data from INVEST (the INternational VErapamil SR-Trandolapril STudy) showed that hypertensive patients with ischemic heart disease and a higher proportion of visits in which BP control was attained had a 32% reduction in the risk of myocardial infarction and a 50% reduction in the risk of stroke.Citation7
In recent years, there has been an improvement in BP control rates worldwide.Citation8–Citation12 This improvement has been attributed mainly to increased use of antihypertensive agents, particularly combined therapy.Citation13
Importance of combined therapy in the treatment of hypertension
It has been reported that most patients with hypertension need at least two antihypertensive agents to achieve BP goals, particularly patients at higher risk.Citation14,Citation15 Combining antihypertensive drugs with different mechanisms of action is a logical approach, because hypertension is caused by multifactorial interacting mechanisms.Citation15 As a result, the combination of drugs with different mechanisms of action can enhance the antihypertensive efficacy of each agent in monotherapy when combined, and may block counter-regulatory mechanisms, thereby reducing the incidence of side effects.Citation15 Current guidelines recommend the use of combined therapy when monotherapy fails to attain BP goals, and as a first choice in patients with markedly elevated BP, particularly those at high or very high cardiovascular risk.Citation16,Citation17
Although different antihypertensive drugs can be combined, not all combinations are equally effective and safe. In this context, the combination of a renin angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor [ACEi] or an angiotensin receptor blocker [ARB]) with a diuretic, usually a thiazide or thiazide-like agent, is specifically recommended.Citation16,Citation17 In fact, both types of drugs have synergistic mechanisms of action. The thiazides enhance the activity of the renin angiotensin system, increasing the efficacy of renin angiotensin system inhibitors. Moreover, ACEi and ARB reduce the risk of the side effects associated with diuretics, including hypokalemia and metabolic disturbances (ie, hyperglycemia, insulin resistance, and hyperuricemia).Citation18–Citation21 The combination of a renin angiotensin system inhibitor and a diuretic is very common in clinical practice. Thus, in Spain, when combined therapy is required, the combination preferred by general practitioners for most patients is a renin angiotensin system blocker and a diuretic.Citation12
Hydrochlorothiazide (HCTZ), chlorthalidone, and indapamide have been diuretics the most frequently used in combination with an ACEi or ARB. However, in clinical practice, the majority of fixed combinations containing a renin angiotensin system inhibitor and a diuretic have included HCTZ.Citation12,Citation14 Are there differences between these diuretics? The aim of this review was to analyze the available evidence regarding the efficacy and safety of these drugs alone and as add-on therapy in patients already taking agents that block the renin angiotensin system.
Pharmacokinetics of hydrochlorothiazide, chlorthalidone, and indapamide
HCTZ is a thiazide that is rapidly absorbed after oral intake and reaches peak concentrations in about 2 hours. It has been calculated that the half-life of HCTZ is approximately 8–15 hours with long-term dosing. It is eliminated unchanged in the urine. Different studies have shown that the pharmacodynamic response of HCTZ is much longer than expected from its half-life, supporting once-daily dosing of this drug. On the other hand, it has been reported that doses higher than 25 mg do not markedly increase the antihypertensive efficacy of HCTZ, but are associated with a higher risk of hypokalemia. In contrast, HCTZ doses of 12.5 mg daily, despite being less effective than 25 mg daily, cause less hypokalemia.Citation22–Citation28
Chlorthalidone is a thiazide-like diuretic. After oral intake, peak serum concentrations are achieved in 2–6 hours. The half-life of chlorthalidone is approximately 42 (range 29–55) hours, reaching 45–60 hours after long-term dosing. However, interindividual variability in the half-life of chlorthalidone is wide. Chlorthalidone is excreted unchanged by the kidneys. The natriuretic effect of chlorthalidone is maximal at 18 hours and lasts more than 48 hours. Comparing different doses of chlorthalidone, it has been observed that 25 mg daily is nearly as effective as higher doses, but with less risk of hypokalemia. Other studies have shown that chlorthalidone doses of 12.5 mg and 25 mg daily offer the best efficacy and safety (in terms of hypokalemia) profiles.Citation22,Citation29,Citation30
Indapamide is a thiazide-like diuretic agent acting in the proximal segment of the distal tubule, mainly on sodium and chloride excretion and with a lesser effect on potassium or uric acid urine excretion. Indapamide reduces vascular reactivity to pressor amines. It is rapidly absorbed after oral ingestion and is metabolized predominantly in the liver, mainly by cytochrome P450 (CYP)2C9 and CYP3A4 isozymes and by cytosolic hydrolysis enzymes. The plasma elimination half-life is biphasic (14 and 25 hours), and the main route of elimination is via the urine.Citation21,Citation31,Citation32
Efficacy and safety of HCTZ as add-on therapy
The addition of HCTZ to an ACEi or ARB as a free or fixed combination has been widely investigated and used in clinical practice. In one study, patients with uncontrolled BP despite treatment with HCTZ 25 mg daily were randomized to receive amiloride 2.5–5 mg/day or enalapril 10–20 mg/day. After 12 weeks of treatment, the addition of enalapril was more effective than amiloride for lowering BP, as measured by ambulatory BP monitoring and office systolic BP.Citation33
INCLUSIVE (Irbesartan/HCTZ Blood Pressure Reductions in Diverse Patient Populations) was a multicenter, prospective, open-label, single-arm study that aimed to determine the efficacy and safety of a fixed combination of irbesartan and HCTZ in patients with uncontrolled systolic BP after at least 4 weeks of antihypertensive monotherapy. Treatment was sequential, ie, placebo (4–5 weeks), HCTZ 12.5 mg (2 weeks), irbesartan/HCTZ 150/12.5 mg (8 weeks), and irbesartan/HCTZ 300/25 mg (8 weeks). At the end of the study, more than 75% of patients who had been uncontrolled on monotherapy achieved their target systolic BP. All the treatments were well tolerated.Citation34
A number of studies have analyzed the efficacy of combining candesartan with HCTZ.Citation35–Citation38 In a dose-response analysis of a combination of candesartan (2–32 mg) and HCTZ (6.25–25 mg) performed in 4,632 patients with mild to moderate hypertension, the effects of this combination were dose-related over a wide range of doses and additive.Citation35
In the OLMEBEST study, the question of whether dose titration of olmesartan medoxomil (to 40 mg once daily) and olmesartan medoxomil/HCTZ combination therapy (to 20/12.5 mg once daily) was therapeutically equivalent was investigated in 2,306 patients with mild to moderate hypertension that was not controlled on low-dose olmesartan medoxomil monotherapy (20 mg once daily). At the end of the study, both strategies were effective and well tolerated.Citation39
Efficacy and safety of chlorthalidone
Several studies have analyzed the effects of chlorthalidone on reduction of BP levels and on cardiovascular outcomes. Azilsartan medoxomil is the most recent ARB to reach the market. It is currently available as monotherapy or as a fixed-dose combination with chlorthalidone. In a double-blind factorial study, the efficacy and safety of a fixed-dose combination of azilsartan medoxomil and chlorthalidone were compared with those of its individual components in 1,714 patients with a clinic systolic BP of 160–190 mmHg. Patients were randomized to treatment with azilsartan 0 mg, 20 mg, 40 mg, or 80 mg and/or chlorthalidone 0 mg, 12.5 mg, or 25 mg. After 8 weeks of follow-up, combination treatment with azilsartan and chlorthalidone resulted in a substantially greater reduction in systolic BP than that achieved with either drug alone.Citation40 Improvement in BP control using chlorthalidone has been associated with a regression of left ventricular hypertrophy.Citation41
The SHEP (Systolic Hypertension in the Elderly Program) study was performed to assess the ability of antihypertensive drug treatment to reduce the risk of stroke in 4,736 patients ≥60 years with isolated systolic hypertension. The risk of stroke was reduced by 36% using stepped-care antihypertensive treatment, with low-dose chlorthalidone as the step 1 medication ().Citation42 Moreover, in the SHEP study, low-dose chlorthalidone effectively reduced the risk of cardiovascular events, including cerebrovascular and cardiac events, regardless of the presence of diabetes.Citation43 Additionally, stepped-care treatment based on low-dose chlorthalidone had a strong protective effect with regard to prevention of heart failure, particularly in patients with a previous history of myocardial infarction.Citation44 The effects of active treatment in the participants randomized to active therapy in SHEP were specifically analyzed after a 22-year follow-up, and it was found that stepped-care chlorthalidone therapy for 4.5 years was associated with a longer life expectancy.Citation45
Figure 1 Effects of chlorthalidone on cardiovascular outcomes.
Abbreviations: Chlorthal, chlorthalidone; HCTZ, hydrochlorothiazide; CV, cardiovascular; HF, heart failure; SHEP, Systolic Hypertension in the Elderly Program; vs, versus; NS, not significant.
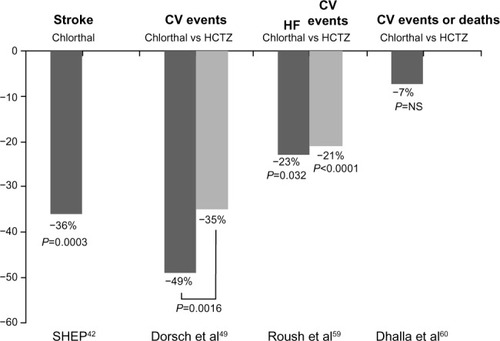
In a prespecified subgroup analysis of 15,638 women and 17,719 men who participated in ALLHAT (the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial), with a total follow-up of 8–13 years (active treatment plus passive surveillance using national administrative databases to ascertain deaths and hospitalizations), the risk of the primary coronary heart disease outcome and any other cardiovascular disease outcome was similar for amlodipine, lisinopril, and chlorthalidone. However, chlorthalidone-based treatment had the lowest risk of heart failure, irrespective of sex.Citation46 Furthermore, in a post hoc analysis of ALLHAT in hypertensive patients with a reduced glomerular filtration rate, the three drugs were similar in terms of reducing the risk of end-stage renal disease or achieving a 50% or greater decrement in glomerular filtration rate.Citation47
Finally, it has been reported that the beneficial effects of chlorthalidone are not limited to its ability to reduce BP, and the pleiotropic effects of chlorthalidone may include improvements in oxidative status, endothelial function, and antiplatelet activity.Citation48 Moreover, studies have shown that, compared with placebo, chlorthalidone has minor effects on fasting glucose and total cholesterol,Citation48 and compared with HCTZ, significantly reduces total cholesterol and low-density lipoprotein cholesterol levels.Citation49
Hydrochlorothiazide versus chlorthalidone: what is the evidence?
The relative antihypertensive potency and relative cardiovascular risk reduction have been investigated for HCTZ and chlorthalidone.Citation50 In a randomized, single-blind, 8-week active treatment crossover study, chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and HCTZ 25 mg/day (force-titrated to 50 mg/day) were compared in untreated hypertensive patients. Compared with HCTZ 50 mg/day, chlorthalidone 25 mg/day reduced ambulatory systolic BP more effectively (mean 24-hour reduction −7.4±1.7 mmHg versus −12.4±1.8 mmHg, respectively, P=0.054; mean nighttime reduction −6.4±1.8 mmHg versus −13.5±1.9 mmHg, respectively, P=0.009). However, these differences were not apparent when office BP measurements were considered ().Citation51
Table 1 Summary of the most relevant studies comparing the efficacy of hydrochlorothiazide with chlorthalidone in patients with hypertension
In a randomized, double-blind, titrate-to-target BP trial, a fixed combination of azilsartan medoxomil and chlorthalidone was compared with a free combination of azilsartan medoxomil and HCTZ in 609 individuals with stage 2 primary hypertension and a mean baseline clinic BP of 164.6/95.4 mmHg. After 2 weeks of treatment with azilsartan medoxomil 40 mg as monotherapy, 12.5 mg of diuretic for 4 weeks (up to week 6) was added to treatment and then titrated to 25 mg for another 4 weeks (up to week 10) if target BP was not achieved. At week 6, the combination containing chlorthalidone achieved a greater reduction in clinic systolic BP (−35.1 mmHg versus −29.5 mmHg, respectively, mean difference −5.6 mmHg; 95% confidence interval [CI] −8.3 to −2.9; P<0.001), as well as a greater reduction in 24-hour ambulatory BP (mean difference −5.8 mmHg; 95% CI −8.4 to −3.2, P<0.001). As a result, more patients treated in the chlorthalidone group achieved their target BP at week 6 (64.1% versus 45.9%, P<0.001), without a significant increase in drug discontinuations due to adverse events ().Citation52
In a meta-analysis analyzing the dose-response relationship between HCTZ, chlorthalidone, and bendroflumethiazide with regard to BP and serum potassium and urate levels, 26 studies of HCTZ, three of chlorthalidone, and one of bendroflumethiazide were included, providing a total of 4,683 subjects in more than 53 comparison arms. Meta-regression of the effect of thiazides on systolic BP showed different antihypertensive effects, ie, bendroflumethiazide lowered BP more than chlorthalidone, and chlorthalidone lowered BP more than HCTZ. Similar findings were reported for diastolic BP ().Citation53 The results of this study strongly suggest that 25 mg of HCTZ should not be regarded as equivalent to 25 mg of chlorthalidone.Citation53
Another meta-analysis studying the effects of HCTZ and chlorthalidone on systolic BP and potassium levels included 108 clinical trials with HCTZ and 29 with chlorthalidone. Equivalence analysis suggested that the systolic BP reductions achieved with HCTZ and chlorthalidone were not equivalent within the low-dose range currently recommended. In fact, when evaluated on a milligram-per-milligram basis, chlorthalidone generally produced slightly greater reductions in systolic BP. In contrast, within the same dosing range, the mean changes in potassium were similar ().Citation54
In a retrospective study comparing the effects of switching from HCTZ to chlorthalidone in a population from the Veterans Affairs Ann Arbor Healthcare System, in which nearly three quarters of patients were taking three or more antihypertensive agents at the time of the medication change, there was a significant reduction in both systolic BP (−15.8 mmHg; 95% CI −8.9 to −22.6 mmHg, P<0.0001) and diastolic BP (−4.2 mmHg; 95% CI −1.5 to −6.9 mmHg, P=0.0035, ).Citation55 A more recent study showed that, when combined with candesartan 8 mg, chlorthalidone 12.5 mg was as effective as HCTZ 25 mg in reducing central aortic pressure. However, whereas chlorthalidone significantly reduced pulse wave velocity, HCTZ only marginally reduced the augmentation index ().Citation56
These beneficial effects of chlorthalidone over HCTZ with regard to BP levels translate into an improvement in terms of subclinical organ damage. Data from the Multiple Risk Factor Intervention Trial showed that, at the individual level, the Sokolow-Lyon index and left ventricular mass were significantly lower in men receiving chlorthalidone than in those receiving HCTZ at 48 months and 84 months of follow-up.Citation57 It has also been observed that chlorthalidone is significantly more effective than bendroflumethiazide (a thiazide diuretic) in reducing epinephrine-mediated platelet aggregation. Moreover, although both diuretics reduced vascular permeability to albumin, only chlorthalidone increased angiogenesis.Citation58
More importantly, other studies have investigated whether these beneficial properties of chlorthalidone when compared with HCTZ result in better cardiovascular outcomes. A retrospective cohort analysis from the Multiple Risk Factor Intervention Trial showed that, in hypertensive patients at high risk of cardiovascular events, although both drugs reduced cardiovascular events compared with those who took neither drug, chlorthalidone reduced cardiovascular events more effectively than HCTZ ( and ).Citation49
A systematic review of randomized trials in which one arm was based on either HCTZ or chlorthalidone, followed by two types of network meta-analyses, ie, a drug-adjusted analysis and an office systolic BP-adjusted analysis, included three trials based on HCTZ and six based on chlorthalidone. In the drug-adjusted analysis (n=50,946), compared with HCTZ, chlorthalidone reduced the risk of congestive heart failure by 23% (95% CI 2–39, P=0.032), and the risk for all cardiovascular events was 21% (95% CI 12–28, P<0.0001). In the office systolic BP-adjusted analysis (n=78,350), compared with HCTZ, chlorthalidone reduced the risk of cardiovascular events by 18% (95% CI 3–30, P=0.024, and ).Citation59 However, other studies showed that, in older adults, chlorthalidone as typically prescribed was not associated with fewer adverse cardiovascular events or deaths compared with HCTZ ( and ).Citation60
As a result, the question concerning whether chlorthalidone is better than HCTZ at reducing cardiovascular events in hypertensive patients remains unresolved. Head-to-head trials have shown that chlorthalidone is more effective than HCTZ in reducing BP levels, particularly during the nighttime due to the longer duration of action of chlorthalidone, and in decreasing left ventricular hypertrophy. Moreover, although no head-to-head outcomes trials comparing the efficacy of chlorthalidone and HCTZ are available, and data regarding this issue is provided from post hoc analyses, the majority of the studies have shown superiority for chlorthalidone in reducing cardiovascular events, probably not only due to the higher antihypertensive efficacy of chlorthalidone but also as a result of its pleiotropic effects.Citation61
Despite the guidelines, such as those of the National Institute for Health and Clinical Excellence, recommending that when a diuretic is prescribed, a thiazide-like diuretic, such as chlorthalidone or indapamide, should be preferred over bendroflumethiazide or HCTZ,Citation62 the fact is that prescriptions for HCTZ outnumber those for chlorthalidone by more than 20-fold.Citation59
Efficacy and safety of indapamide
Several studies have analyzed the efficacy and safety of indapamide as add-on therapy, particularly with perindopril and delapril. A 9-month study comparing the efficacy and tolerability of three different strategies for the treatment of hypertension, ie, a low-dose combination (perindopril 2 mg and indapamide 0.625 mg with the possibility to increase to 4 and 1.25 mg, respectively), sequential monotherapy (treatment initiated with atenolol 50 mg, replaced if necessary by losartan 50 mg, and then by amlodipine 5 mg), and stepped-care (valsartan 40 mg, then 80 mg, and finally if required the addition of HCTZ 12.5 mg), included 533 patients with uncomplicated essential hypertension. At the end of the study, 62% of patients in the low-dose combination group achieved their target BP, compared with 49% in the sequential monotherapy group (P=0.02) and 47% in the stepped-care group (P=0.005). This better BP control was not associated with an increase in side effects.Citation63
In a 3-month, open-label, observational study, outpatients with hypertension who did not attain their target BP with antihypertensive treatment were included if their treating physician switched them to fixed-dose perindopril 10 mg/indapamide 2.5 mg according to the clinical criteria of the physicians. Nearly 9,300 patients were enrolled. At the end of the study, 72.7% of patients had achieved their BP goal. Reductions in total cholesterol, low-density lipoprotein cholesterol, triglycerides, fasting glucose, and uric acid levels were clinically significant, without changes in sodium or potassium levels. These changes in the metabolic profile were likely due to withdrawal of previous treatment with thiazides and beta-blockers.Citation64
In a 6-month, prospective, open-label clinical study performed in 397 patients with hypertension and type 2 diabetes, a fixed-dose combination of perindopril/indapamide (from 5/1.25 mg to 10/2.5 mg if BP targets were not attained) was prescribed (started, switched, or added to previous therapy). At the end of the study, 84% of patients taking perindopril/indapamide 5/1.25 mg alone and 90% of patients taking perindopril/indapamide 10/2.5 mg alone showed normalization of their BP levels, with good tolerability. Microalbuminuria decreased in 75% of patients with microalbuminuria.Citation65
In an other study performed in more than 2,300 hypertensive patients being seen in daily clinical practice, 69% of whom had been unsuccessfully treated with other antihypertensive agents, 4.6% of whom had not tolerated previous treatments, and 26.8% of whom were newly diagnosed with hypertension, 87.1% achieved their target BP after 3 months of treatment with perindopril/indapamide (2.5/0.625 mg or 5/1.25 mg uptitrated to 10/2.5 mg at any time during the study if required). BP reductions were similar, irrespective of the presence of diabetes mellitus, metabolic syndrome, or left ventricular hypertrophy. Moreover, no significant changes in laboratory parameters were observed and patient quality of life was improved.Citation66
In a systematic review of the efficacy and safety of a perindopril/indapamide 2 mg/0.625 mg combination as first-line treatment for hypertension, a total of 11 trials with 5,936 individuals (five studies versus placebo and six studies versus routine antihypertensive agents) were included. Compared with placebo, the combination of perindopril/indapamide effectively reduced BP levels (systolic BP −9.03 mmHg, P<0.01; diastolic BP −5.09 mmHg, P<0.01). Similarly, compared with routine antihypertensive agents, the combination of perindopril/indapamide was more effective in reducing BP (systolic BP −3.72 mmHg, P=0.03; diastolic BP −1.71 mmHg, P<0.01). Adverse events and withdrawal rates were similar between the perindopril-indapamide group and the placebo or routine antihypertensive drug groups.Citation67
Regarding organ damage, the ADVANCE (Action in Diabetes and Vascular Disease) Echocardiography Substudy showed that although the perindopril-indapamide combination did not improve left ventricular diastolic function in patients with diabetes to a greater extent than placebo, this combination significantly reduced BP and left ventricular mass.Citation68
Moreover, it was reported that approximately 85% of physicians considered the efficacy and tolerability of perindopril/indapamide 2/0.625 mg in hypertensive patients with diabetes seen in daily clinical practice to be “good” or “very good” and that 93% of patients were “satisfied” or “very satisfied” with this therapy.Citation69
On the other hand, it has been shown that the combination of perindopril and indapamide has additional beneficial effects. Improvements in vascular function have been demonstrated in hypertensive patients by a reduction in wave reflection, lowering of peripheral arterial stiffness, and improvement in endothelial function,Citation70 and reductions in BP and left ventricular mass index in hypertensive patients with left ventricular hypertrophy, along with improvements in resting and hyperemic myocardial blood flow.Citation71 Experimental data in rats have shown that the improvements in coronary flow observed with this combination are due to reverse remodeling of intramural coronary arterioles and improved microvascular function.Citation71 Moreover, it has been reported that indapamide decreases BP, left ventricular hypertrophy, and the collagen ratio.Citation72
Perhaps more importantly, clinical trials have investigated the benefits of the combination of perindopril and indapamide with regard to cardiovascular outcomes. PROGRESS (Perindopril pROtection aGainst REcurrent Stroke Study) was performed to assess the effects of perindopril (4 mg daily), with the addition of indapamide at the discretion of treating physicians, in patients with a history of stroke or transient ischemic attack, irrespective of the presence of hypertension. A total of 6,105 individuals were randomized to active treatment or placebo. After 4 years of follow-up, active treatment was associated with a 28% reduction in the risk of stroke (43% in those treated with the combined therapy), and a 26% reduction in the risk of total major vascular events ().Citation73
Figure 2 Effects of perindopril and indapamide combination on cardiovascular outcomes.
Abbreviations: CV, cardiovascular; VE, vascular events; PROGRESS, Perindopril pROtection aGainst REcurrent Stroke Study; ADVANCE, Action in Diabetes and Vascular Disease study; HYVET, the Hypertension in the Very Elderly Trial.
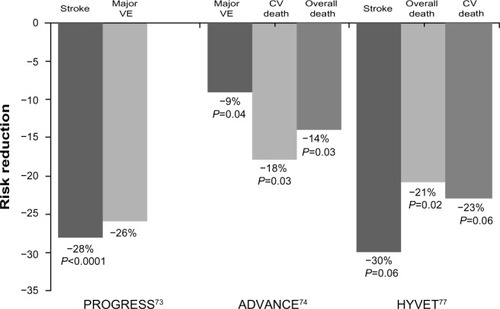
In ADVANCE, the effects of a combination of perindopril and indapamide on serious vascular events were investigated in 11,140 patients with type 2 diabetes, irrespective of their initial BP levels or use of antihypertensive drugs. Patients were randomized to active treatment or placebo in addition to current therapy. After a mean follow-up of 4.3 years, the perindopril/indapamide combination was associated with a 9% reduction in the risk of major macrovascular or microvascular events (hazards ratio [HR] 0.91; 95% CI 0.83–1.00, P=0.04), an 18% reduction in the risk of death from cardiovascular disease (HR 0.82; 95% CI 0.68–0.98, P=0.03), and a 14% reduction in risk of death from any cause (HR 0.86; 95% CI 0.75–0.98, P=0.03, ).Citation74 A substudy of ADVANCE showed that active treatment with a combination of perindopril and indapamide reduced BP levels safely and reduced the risk of major clinical outcomes in patients with type 2 diabetes and aged over 75 years.Citation75 Similarly, the beneficial effects of the perindopril/indapamide combination on cardiovascular and renal outcomes and death were consistent across all stages of chronic kidney disease at baseline.Citation76
In HYVET (the Hypertension in the Very Elderly Trial), 3,845 patients (aged ≥80 years) with hypertension and a sustained systolic BP ≥160 mmHg were randomized to indapamide (sustained-release, 1.5 mg) or placebo. Perindopril (2 or 4 mg) or placebo was added when required to achieve a BP goal <150/80 mmHg. After a mean follow-up of 1.8 years, active treatment was associated with a 30% reduction in the risk of fatal or nonfatal stroke (P=0.06), a 39% reduction in the risk of death from stroke (P=0.05), a 21% reduction in the risk of death from any cause (P=0.02), a 23% reduction in the risk of death from cardiovascular causes (P=0.06), and a 64% reduction in the risk of heart failure (P<0.001, ).Citation77
In the 1-year, open-label active treatment extension of HYVET, patients on active treatment continued taking the active drug, and those initially assigned to placebo received active BP-lowering treatment. Those patients initially assigned to active treatment had less total mortality (HR 0.48; 95% CI 0.26–0.87, P=0.02) and cardiovascular mortality (HR 0.19; 95% CI 0.04–0.87, P=0.03).Citation78
On the other hand, other studies have analyzed the antihypertensive efficacy of a combination of indapamide and delapril in hypertensive patients.Citation79–Citation82 In DIMS II (Delapril-Indapamide Multicenter Study II), approximately 800 patients with uncomplicated mild to moderate hypertension were randomized to receive delapril/indapamide or captopril/HCTZ for 6 months. At the end of the study, more patients treated with delapril/indapamide responded to treatment (92.6% versus 85.2%, P<0.001). Side effects occurred in 7.6% and 8.1% of patients, respectively.Citation80 Moreover, in elderly patients aged 65–85 years with a sitting BP of 160–200/95–115 mmHg, the combination of delapril 30 mg plus indapamide 1.25 mg once daily effectively reduced BP levels as well as left ventricular mass index.Citation81 Similarly, treatment with this combination was associated with a significant increase in glomerular filtration rate.Citation82
Hydrochlorothiazide versus indapamide: what is the evidence?
As with chlorthalidone, several studies have determined the relative antihypertensive efficacy and relative cardiovascular risk reduction for HCTZ and indapamide. It has been observed in hypertensive patients aged 65–80 years that, indapamide sustained-release was an effective and well tolerated antihypertensive therapy over a 12-month period, even after a switch from amlodipine or HCTZ ().Citation83 In a small randomized clinical trial of patients with I–II degree high and very high risk hypertension, after 6 months of treatment, the fixed combination of perindopril/indapamide 4/1.25 mg was superior to a combination of captopril/HCTZ 50/25 mg ().Citation84
Table 2 Summary of most relevant studies comparing the efficacy of hydrochlorothiazide with indapamide in patients with hypertension
In a Russian study, administration of perindopril arginine/indapamide (10 mg/2.5 mg) instead of ACEi or ARB plus HCTZ in more than 2,100 patients with inadequately controlled hypertension significantly reduced BP levels from 177/99 mmHg to 149/89 mmHg after 2 weeks of treatment, and to 130/80 mmHg after 3 months of treatment, with good tolerance of medication ().Citation85
In a randomized 12-week study, fixed combinations of delapril (30 mg) plus indapamide (2.5 mg) and fosinopril (20 mg) plus HCTZ (12.5 mg) were compared in 171 patients with mild to moderate hypertension; the proportion of patients who achieved normal BP, defined as a sitting diastolic BP ≤90 mmHg, was similar between the groups (87.4% versus 81%, respectively) and also with regard to those who responded to therapy, defined as a sitting diastolic BP reduction of 10 mmHg or diastolic BP ≤90 mmHg (92% versus 86.9%, respectively). Both combinations were well tolerated ().Citation86
A meta-analysis comparing the efficacy and safety of a combination of delapril and indapamide with different ACEi/HCTZ combinations in patients with mild to moderate hypertension included four head-to-head randomized controlled trials (n=643 and 629, respectively). The proportion of patients who achieved normal BP values or were responders was higher with the delapril/indapamide combination (P=0.024 and P=0.002, respectively). Moreover, the number of withdrawals due to drug-related side effects was lower with the delapril/indapamide combination (2.3% versus 4.8%, respectively, P=0.018, ).Citation87
Not only the antihypertensive efficacy of indapamide and HCTZ has been compared. In a small study that specifically compared the metabolic and endothelial effects of indapamide retard with those of HCTZ, patients with hypertension received either indapamide retard (1.5 mg/day) or HCTZ (25 mg/day) for 12 weeks. At the end of the study, both drugs reduced BP levels to a similar extent. However, whereas indapamide retard was metabolically neutral, the patients who received HCTZ showed a significant increase in triglycerides (+15.3%, P<0.05) and glucose levels (+12.2%, P<0.05). Moreover, there was a tendency for endothelium-dependent vasodilation to improve with indapamide and become worse with HCTZ.Citation88 Finally, in an experimental study performed in rats, treatment with losartan was associated with antiatherogenic activity, reflected by lipid-lowering and an antioxidant effect in erythrocytes. However, whereas this activity was abolished by addition of HCTZ to losartan, it remained unchanged when indapamide was added. Moreover, in contrast with indapamide, treatment with HCTZ was associated with hypokalemia.Citation89
Conclusion and place in therapy
When considering antihypertensive drugs as monotherapy, although the JNC 7 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure)Citation90 indicated that five classes should be considered as initial therapy and recommended thiazide-type diuretics as initial therapy for most patients, the JNC 8 recommends selection between four specific medication classes (ACEi, ARB, calcium channel blockers, and diuretics).Citation16 Moreover, the 2013 European guidelines reconfirm that all major classes of antihypertensive agents (diuretics, beta-blockers, calcium channel blockers, ACEi, and ARB) are suitable for the initiation and maintenance of antihypertensive therapy.Citation17 As a result, it is very likely that diuretics will no longer be recommended as the only first option in monotherapy. Of note, when a diuretic is used for the treatment of hypertension, thiazide and thiazide-like diuretics are mainly prescribed. Loop diuretics are not recommended for the treatment of hypertension, except in the event of advanced renal impairment, and are commonly prescribed when heart failure is also present.
The addition of diuretics to an ACEi or ARB for reducing BP to recommended targets is an adequate choice in patients with hypertension. Thiazides, mainly HCTZ and thiazide-type diuretics, such as chlorthalidone and indapamide, have been widely used for this purpose. However, not all diuretics seem to be equal, as evident in this review. Thus, given that the plasma elimination half-lives of chlorthalidone and indapamide are longer than that of HCTZ, better antihypertensive efficacy over 24 hours may be assured using these agents. In fact, the majority of studies have shown greater BP reductions with chlorthalidone or indapamide than with HCTZ.Citation50,Citation91
Moreover, chlorthalidone and indapamide have shown some clinically relevant additional benefits. Chlorthalidone can reduce platelet aggregation and vascular permeability, stimulate angiogenesis, and improve oxidative status, endothelial function, and antiplatelet activity.Citation41,Citation92 Moreover, compared with placebo, chlorthalidone has only small effects on fasting glucose and total cholesterol,Citation48 and compared with HCTZ, chlorthalidone is associated with significantly lower total cholesterol and low-density lipoprotein cholesterol levels.Citation49 Similarly, indapamide has demonstrated no negative impact on glucose or lipid metabolism.Citation93
However, more importantly, although addition of HCTZ to renin angiotensin system inhibitors has been shown to effectively reduce BP levels, and decreasing BP to recommended targets improves the cardiovascular prognosis, a reduction in outcomes using low doses of HCTZ as add-on therapy has not yet been demonstrated.Citation20 In contrast, although head-to-head clinical outcomes trials comparing the effects of indapamide or chlorthalidone with HCTZ are not available, indirect comparisons and post hoc analyses suggest that the use of chlorthalidone or indapamide is associated with a reduction in cardiovascular events.Citation42,Citation73,Citation74,Citation77 On the other hand, the benefits of indapamide with regard to cardiovascular outcomes have been shown only when indapamide is combined with perindopril, but not with other antihypertensive drugs.
Finally, hypokalemia is a potential side effect of thiazide and thiazide-like diuretics that may decrease the beneficial effects of these drugs in patients with hypertension. However, the risk of hypokalemia at the doses usually prescribed for this purpose is low. Moreover, combination with renin angiotensin system inhibitors may reduce this potential limitation.Citation18–Citation21 Despite that, addition of potassium supplements or potassium-sparing diuretics, including aldosterone receptor blockers (such as spironolactone and eplerenone) or epithelial sodium channel blockers (such as amiloride and triamterene) can sometimes be necessary, depending on the clinical characteristics of the patient.Citation94 Combination with an aldosterone receptor blocker may be particularly beneficial in hypertensive patients with heart failure.Citation95–Citation97
Despite these limitations, the evidence suggests that the use of a thiazide-like diuretic, such as chlorthalidone or indapamide, appears to be a preferable option over HCTZ when combined therapy with a renin angiotensin system inhibitor is required. However, the diuretic used most often as add-on therapy in clinical practice is HCTZ.Citation98
Disclosure
The authors have no conflicts of interests directly related to this work.
References
- BarriosVEscobarCIs a new crash coming?J Hypertens Open Access20121e105
- LawesCMVander HoornSRodgersAInternational Society of HypertensionGlobal burden of blood-pressure-related disease, 2001Lancet200837196231513151818456100
- PereiraMLunetNAzevedoABarrosHDifferences in prevalence, awareness, treatment and control of hypertension between developing and developed countriesJ Hypertens200927596397519402221
- StephensMMFoxBAMaxwellLTherapeutic options for the treatment of hypertension in children and adolescentsClin Med Insights Circ Respir Pulm Med20126132522408373
- WijeysunderaHCMachadoMFarahatiFAssociation of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005JAMA2010303181841184720460623
- Flores-MateoGGrauMO’FlahertyMAnalyzing the coronary heart disease mortality decline in a Mediterranean population: Spain 1988–2005Rev Esp Cardiol20116411988996
- ManciaGMesserliFBakrisGBlood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril StudyHypertension200750229930517606861
- EganBMZhaoYAxonRNUS Trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008JAMA2010303202043205020501926
- McAlisterFAWilkinsKJoffresMChanges in the rates of awareness, treatment and control of hypertension in Canada over the past two decadesCMAJ201118391007101321576297
- FasceECamposIIbáñezPTrends in prevalence, awareness, treatment and control of hypertension in urban communities in ChileJ Hypertens20072591807181117762644
- CífkováRSkodováZBruthansJLongitudinal trends in cardiovascular mortality and blood pressure levels, prevalence, awareness, treatment, and control of hypertension in the Czech population from 1985 to 2007/2008J Hypertens201028112196220320651603
- LlisterriJLRodriguez-RocaGCEscobarCWorking Group of Arterial Hypertension of the Spanish Society of Primary Care Physicians (Group HTASEMERGEN)PRESCAP 2010 investigatorsTreatment and blood pressure control in Spain during 2002–2010J Hypertens201230122425243122990354
- BarriosVEscobarCLetter from Barrios and Escobar regarding article, “trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010”Circulation201312724e85923775199
- EscobarCEcharriRBarriosVEmerging drug combinations to optimize renovascular protection and blood pressure goalsInt J Nephrol Renovasc Dis20125698022536084
- EscobarCBarriosVCombined therapy in the treatment of hypertensionFundam Clin Pharmacol20102413819682088
- JamesPAOparilSCarterBL2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- ManciaGFagardRNarkiewiczK2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)Eur Heart J201334282159221923771844
- MotwaniJGCombining renin-angiotensin-aldosterone system blockade with diuretic therapy for treatment of hypertensionJ Renin Angiotensin Aldosterone Syst200232727812228846
- BarriosVEscobarCOlmesartan medoxomil plus hydrochlorothiazide for treating hypertensionExpert Opin Pharmacother20089112913618076344
- BarriosVEscobarCAzilsartan medoxomil in the treatment of hypertension: the definitive angiotensin receptor blocker?Expert Opin Pharmacother201314162249226124070321
- BarriosVEscobarCComplementary mechanisms of action and rationale for the fixed combination of perindopril and indapamide in treating hypertension – update on clinical utilityIntegr Blood Press Control20103111921949617
- CarterBLErnstMECohenJDHydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeabilityHypertension20044314914638621
- GiudicelliJFRicherCMatteiAPharmacokinetics and biological effects of captopril and hydrochlorothiazide after acute and chronic administration either alone or in combination in hypertensive patientsBr J Clin Pharmacol198723Suppl 151S63S3034318
- WeirSJDimmittDCLanmanRCMorrillMBGeisingDHSteady-state pharmacokinetics of diltiazem and hydrochlorothiazide administered alone and in combinationBiopharm Drug Dispos19981963653719737817
- AllenJHMcKenneyJMStrattonMALinkKAntihypertensive effect of hydrochlorothiazide administered once or twice dailyClin Pharm1982132392436764388
- KohvakkaASaloHGordinAEisaloAAntihypertensive and biochemical effects of different doses of hydrochlorothiazide alone or in combination with triamtereneActa Med Scand198621943813863521208
- CushmanWCKhatriIMatersonBJTreatment of hypertension in the elderly, III: response of isolated systolic hypertension to various doses of hydrochlorothiazide: results of a Department of Veterans Affairs cooperative study. Department of Veterans Affairs Cooperative Study Group on Antihypertensive AgentsArch Intern Med199115110195419601929683
- JounelaAJLiljaMLummeJRelation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effectsBlood Press1994342312357994447
- MatersonBJOsterJRMichaelUFDose response to chlorthalidone in patients with mild hypertension: efficacy of a lower doseClin Pharmacol Ther1978242192198354839
- MorledgeJHEttingerBArandaJIsolated systolic hypertension in the elderly: a placebo controlled, dose-response evaluation of chlorthalidoneJ Am Geriatr Soc19863431992063512670
- SchiaviPJochemsenRGuezDPharmacokinetics of sustained and immediate release formulations of indapamide after single and repeated oral administration in healthy volunteersFundam Clin Pharmacol200014213914610796061
- RobinsonDMWellingtonKIndapamide sustained release: a review of its use in the treatment of hypertensionDrugs200666225727116451099
- GuerreroPFuchsFDMoreiraLMBlood pressure-lowering efficacy of amiloride versus enalapril as add-on drugs in patients with uncontrolled blood pressure receiving hydrochlorothiazideClin Exp Hypertens200830755356418855259
- NeutelJMSaundersEBakrisGLThe efficacy and safety of low- and high-dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trialJ Clin Hypertens (Greenwich)200571057858616227760
- KarlsonBWZetterstrandSOlofssonBElmfeldtDA dose-response analysis of candesartan-hydrochlorothiazide combination therapy in patients with hypertensionBlood Press200918314915619462314
- EdesIBurgessLParowWCombination therapy with candesartan cilexetil 32 mg and hydrochlorothiazide 25 mg provides the full additive antihypertensive effect of the components: a randomized, double-blind, parallel-group study in primary careClin Drug Investig2009295293304
- BönnerGCalderBDzyakGAntihypertensive efficacy and tolerability of candesartan-hydrochlorothiazide 32/12.5 mg and 32/25 mg in patients not optimally controlled with candesartan monotherapyBlood Press Suppl20082223019203019
- BarriosVEscobarCCandesartan in the treatment of hypertension: what have we learnt in the last decade?Expert Opin Drug Saf201110695796821848481
- BarriosVBoccanelliAEwaldSEfficacy and tolerability of olmesartan medoxomil in patients with mild to moderate essential hypertension: the OLMEBEST studyClin Drug Investig2007278545558
- SicaDBakrisGLWhiteWBBlood pressure lowering efficacy of the fixed dose combination of azilsartan and chlorthalidone: a factorial studyJ Clin Hypertens (Greenwich)201214528429222533654
- RoushGCBuddharajuVErnstMEHolfordTRChlorthalidone: mechanisms of action and effect on cardiovascular eventsCurr Hypertens Rep201315551452123839110
- SHEP Cooperative Research GroupPrevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP)JAMA199126524325532642046107
- CurbJDPresselSLCutlerJAEffect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research GroupJAMA199627623188618928968014
- KostisJBDavisBRCutlerJPrevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research GroupJAMA199727832122169218667
- KostisJBCabreraJChengJQAssociation between chlorthalidone treatment of systolic hypertension and long-term survivalJAMA2011306232588259322187278
- OparilSDavisBRCushmanWCALLHAT Collaborative Research GroupMortality and morbidity during and after Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial: results by sexHypertension201361597798623529173
- RahmanMPresselSDavisBRRenal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)Arch Intern Med2005165893694615851647
- SavagePJPresselSLCurbJDInfluence of long-term, low-dose, diuretic-based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: The Systolic Hypertension in the Elderly Program. SHEP Cooperative Research GroupArch Intern Med19981587417519554680
- DorschMPGillespieBWEricksonSRBleskeBEWederABChlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysisHypertension201157468969421383313
- BasileJNBlochMJDetermining the relative antihypertensive potency and relative cardiovascular risk reduction associated with different thiazide and thiazide-type diureticsJ Clin Hypertens (Greenwich)201315635936123730981
- ErnstMECarterBLGoerdtCJComparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressureHypertension200647335235816432050
- BakrisGLSicaDWhiteWBAntihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomilAm J Med201212512122922939358
- PeterzanMAHardyRChaturvediNHughesADMeta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urateHypertension20125961104110922547443
- ErnstMECarterBLZhengSGrimmRHJrMeta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassiumAm J Hypertens201023444044620111008
- MatthewsKABrennerMJBrennerACEvaluation of the efficacy and safety of a hydrochlorothiazide to chlorthalidone medication change in veterans with hypertensionClin Ther20133591423143023993697
- KwonBJJangSWChoiKYComparison of the efficacy between hydrochlorothiazide and chlorthalidone on central aortic pressure when added on to candesartan in treatment-naïve patients of hypertensionHypertens Res2013361798423034468
- ErnstMENeatonJDGrimmRHJrMultiple Risk Factor Intervention Trial Research GroupLong-term effects of chlorthalidone versus hydrochlorothiazide on electrocardiographic left ventricular hypertrophy in the Multiple Risk Factor Intervention TrialHypertension20115861001100722025372
- WoodmanRBrownCLocketteWChlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesisHypertension201056346347020625077
- RoushGCHolfordTRGuddatiAKChlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analysesHypertension20125961110111722526259
- DhallaIAGomesTYaoZNaggeJChlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort studyAnn Intern Med2013158644745523552325
- RoushGCBuddharajuVErnstMEIs chlorthalidone better than hydrochlorothiazide in reducing cardiovascular events in hypertensives?Curr Opin Cardiol201328442643223736816
- National Institute for Health and Clinical ExcellenceHypertension. The clinical management of primary hypertension in adults82011 Available from: http://www.nice.org.uk/guidance/CG127Accessed April 3, 2014
- MouradJJWaeberBZannadFLavilleMDuruGAndréjakMInvestigators of the STRATHE TrialComparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approachJ Hypertens200422122379238615614033
- FarsangCPicasso InvestigatorsBlood pressure and metabolic efficacy of fixed-dose combination of perindopril and indapamide in everyday practiceBlood Press201322Suppl 131023163322
- NetchessovaTAShepelkevichAPGorbatTVNIKA Study GroupEfficacy of single-pill perindopril/indapamide in patients with hypertension and type 2 diabetesHigh Blood Press Cardiovasc Prev2014211636924357222
- PellaDEfficacy and safety of treatment of hypertensive patients with fixed combination perindopril/indapamide up to 10/2.5 mg: results of the FALCO FORTE programmeHigh Blood Press Cardiovasc Prev201118310711321950782
- KangSWuYFAnNRenMA systematic review and meta-analysis of the efficacy and safety of a fixed, low-dose perindopril-indapamide combination as first-line treatment of hypertensionClin Ther200426225727015038948
- ADVANCE Echocardiography Substudy InvestigatorsADVANCE Collaborative GroupEffects of perindopril-indapamide on left ventricular diastolic function and mass in patients with type 2 diabetes: the ADVANCE Echocardiography SubstudyJ Hypertens20112971439144721610514
- BarriosVEscobarCDivisonJAMedialdeaFLow-dose fixed combination of perindopril plus indapamide in the diabetic hypertensive populationExpert Rev Cardiovasc Ther2008681063106918793109
- ProtasovKVSinkevichDAReshinaIVZhizhkoNVLogovikovaSIGolubevaLVVascular effects of perindopril arginine and indapamide fixed combination in patients with arterial hypertensionKardiologiia2012529814 Russian23098541
- NegliaDFommeiEVarela-CarverAPerindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertensionJ Hypertens20112936437221045728
- HlavačkováLVrankováSJanegaPPecháňováOBabálPThe effect of indapamide on development of myocardial hypertrophy and fibrosis in L-NAME-induced hypertension in ratPhysiol Res20116084585221995907
- PROGRESS Collaborative GroupRandomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attackLancet200135892871033104111589932
- PatelAADVANCE Collaborative GroupEffects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trialLancet2007370959082984017765963
- NinomiyaTZoungasSNealBADVANCE Collaborative GroupEfficacy and safety of routine blood pressure lowering in older patients with diabetes: results from the ADVANCE trialJ Hypertens20102861141114920486273
- HeerspinkHJNinomiyaTPerkovicVADVANCE Collaborative GroupEffects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney diseaseEur Heart J201031232888289620501479
- BeckettNSPetersRFletcherAEHYVET Study GroupTreatment of hypertension in patients 80 years of age or olderN Engl J Med2008358181887189818378519
- BeckettNPetersRTuomilehtoJHYVET Study GroupImmediate and late benefits of treating very elderly people with hypertension: results from active treatment extension to Hypertension in the Very Elderly randomised controlled trialBMJ2011344d754122218098
- González-JuanateyJRCorderoABenefits of delapril in hypertensive patients along the cardiovascular continuumExpert Rev Cardiovasc Ther201311327128123469905
- RoseiEARizzoniDDIMS II(Delapril-Indapamide Multicenter Study II)Evaluation of the efficacy and tolerability of the combination delapril plus indapamide in the treatment of mild to moderate essential hypertension: a randomised, multicentre, controlled studyJ Hum Hypertens200317213914612574793
- AcanforaDLowenthalDTFurgiGEffects of delapril in combination with indapamide on blood pressure and left ventricular mass in elderly hypertensive patientsAm J Ther199851172310099033
- AcanforaDLowenthalDTFurgiGThe effects of delapril in combination with indapamide on glomerular filtration rate in elderly hypertensive patientsAm J Ther1997411–1240540810423638
- LeonettiGEmeriauJPKnaufHPujadasJOCalvo-GomezCAbateGEuropean Study InvestigatorsEvaluation of long-term efficacy and acceptability of indapamide SR in elderly hypertensive patientsCurr Med Res Opin2005211374615881474
- NedogodaSVMarchenkoIVChaliabiTATsomaVVBrelUAProkhorovaEAComparative efficacy of fixed dose combinations of perindopril with indapamide and captopril with hydrochlorothiazide in patients with high risk hypertensionKardiologiia200545112426 Russian16353060
- KarpovIAThe FORTISSIMO program: advantages of fixed full dose combination of perindopril arginine and indapamide in the treatment of poorly controlled arterial hypertensionKardiologiia20135333743 Russian23548425
- CremonesiGCavalieriLCikesIFixed combinations of delapril plus indapamide vs fosinopril plus hydrochlorothiazide in mild to moderate essential hypertensionAdv Ther200219312913712201354
- CircelliMNicoliniGEganCGCremonesiGEfficacy and safety of delapril/indapamide compared to different ACE-inhibitor/hydrochlorothiazide combinations: a meta-analysisInt J Gen Med2012572573423049265
- SemenkinAAZhivilovaLAGolevtsovaZSComparative assessment of hypotensive, metabolic, and endothelial effects of indapamide-retard and hydrochlorothiazide in patients with essential hypertensionKardiologiia20064653539 Russian16858352
- IslamMZRahmanMSComparative study of hydrochlorothiazide and indapamide on the anti-atherogenic potential of losartan in cholesterol fed ratBangladesh Med Res Counc Bull2010361141921280553
- ChobanianAVBakrisGLBlackHRNational Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- CarterBLGuidelines for use of diuretics: a view from a member of JNC 7J Clin Hypertens (Greenwich)201214527327622533652
- KountzDSGoldmanAMikhailJEzerMChlorthalidone: the forgotten diureticPostgrad Med20121241606622314115
- WaeberBRotaruCFeihlFPosition of indapamide, a diuretic with vasorelaxant activities, in antihypertensive therapyExpert Opin Pharmacother201213101515152622725706
- PadillaMCArmas-HernándezMJHernándezRHIsrailiZHValascoMUpdate of diuretics in the treatment of hypertensionAm J Ther200714215416017414583
- PittBZannadFRemmeWJThe effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study InvestigatorsN Engl J Med19993411070971710471456
- PittBRemmeWZannadFEplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med2003348141309132112668699
- ZannadFMcMurrayJJKrumHEplerenone in patients with systolic heart failure and mild symptomsN Engl J Med20113641112121073363
- ErnstMELundBCRenewed interest in chlorthalidone: evidence from the Veterans Health AdministrationJ Clin Hypertens (Greenwich)2010121292793421122058
