Abstract
The majority of hypertensive patients, especially those with target organ damage, are likely to require multiple-drug therapy in order to reach blood pressure (BP) targets and reduce their risk of adverse vascular outcomes. The rationale for combination therapy with agents that block the renin–angiotensin system (RAS) and a calcium channel blocker (CCB) or diuretic is well founded in growing evidence. Recent published trials have shown that the combination of an RAS suppressor and a dihydropiridinic CCB would offer additional benefits independently of BP reduction. A telmisartan–amlodipine combination has demonstrated significantly greater BP reductions compared with each monotherapy component in the overall population, and in particular in patients with moderate to severe hypertension and high-risk patients. This combination is well tolerated with a safety profile similar to placebo and is consistent with the known safety profile of its monotherapy components.
Introduction
Cardiovascular disease (CVD) places a significant burden on healthcare providers. Despite improvements in morbidity and mortality statistics in some countries over the past few decades, atherosclerotic CVD remains the leading cause of morbid events and mortality worldwide, with the expectation of an increasing prevalence in developing countries.Citation1,Citation2 Worldwide, ischemic heart disease and cerebrovascular disease are projected to account for 24% of total deaths by 2030.Citation1 In the EU alone, where CVD causes 1.5 million deaths each year,Citation3 direct healthcare costs currently amount to €105 billion, with 57% of the total due to inpatient care.Citation4 CVD is also associated with substantial indirect costs due to lost productivity, with an estimated overall annual cost to the EU of around €169 billion.Citation4
High blood pressure (BP) is the single most prevalent risk factor for CVD worldwide. It is responsible for more deaths than any other risk factor.Citation5,Citation6 A meta-analysis has shown that for each increase in systolic BP (SBP) of 20 mm Hg and in diastolic BP (DBP) of 10 mm Hg, there is at least a doubling in the risk of mortality from ischemic heart disease and stroke.Citation7 Attributed to 54% of cerebrovascular disease and 47% of ischemic heart disease worldwide in 2001, high BP was responsible for 7.6 million premature deaths or 13.5% of the global total.Citation5 However, only half of this burden was in patients with clinical hypertension, indicating that lesser degrees of BP elevation remain a cause for concern.Citation5
Patients with elevated BP alone are not considered to be at high risk of a CV event such as myocardial infarction (MI) or stroke. However, the presence of hypertension adds to a patient’s CV risk profile when other risk factors are present.Citation8 “CV high-risk patients” include those patients with atherothrombotic disease, as indicated by a history of MI, stroke, transient ischemic attack, or peripheral arterial disease, together with patients with multiple risk factors and patients with target organ damage associated with type 2 diabetes.Citation9–Citation13 CV high-risk patients are in the middle of the spectrum of risk for CVD progression that is referred to as the “CV continuum”.Citation14,Citation15
Although lifestyle intervention can improve a patient’s CV risk profile and has a fundamental role in the management of all CV high-risk patients,Citation16 pharmacological agents that are able to modify the disease processes involved in CVD and delay its progression may become instrumental in reducing the burden of CVD. In this regard, statins, anti-platelet therapy, hypoglycemic agents in type 2 diabetes, and antihypertensive therapy have all shown a benefit in reducing CV risk.Citation17–Citation20 A continuous and linear relationship has been shown between BP lowering and reduction in CV mortality, with the risk reduction approximately constant for any given BP level down to an SBP of 115 mm Hg and a DBP of 75 mm Hg for all age groups.Citation7,Citation20 Even a small reduction in SBP of 2 mm Hg would predictably lower mortality from ischemic heart disease and stroke by 7% and 10%, respectively.Citation7 A recent study in patients with type 2 diabetes has indicated that aggressive BP lowering of SBP to <120 mm Hg, however, may not confer additional benefit compared with usual control.Citation21 Similar results have been published in African American hypertensive patients.Citation22 A recent reappraisal of the European guidelines for management of hypertension reinforces the scarce evidence to support tighter BP control in order to reduce the risk of CV events in hypertensive patients.Citation23
Thus, based on BP-lowering effects alone, the treatment of CV high-risk patients with antihypertensive therapy represents an opportunity to modify CV risk and improve patient outcomes. In order to overcome the challenges of achieving BP control in these patients,Citation24,Citation25 the ideal antihypertensive medication should be safe and well tolerated, provide efficacious and long-lasting 24-hour BP reductions as monotherapy with additional efficacy when used in combination, and reduce or slow the pathological processes that lead to target organ damage and CV events.Citation26,Citation27 In this regard, blockade of the renin–angiotensin system (RAS) is a potent therapeutic approach, offering the potential to reduce organ damage not only by reducing BP but also by reducing the inflammatory disease that leads to atherosclerosis.Citation28
The majority of hypertensive patients, especially those with target organ damage, are likely to require multiple-drug therapy in order to reach BP targets and reduce their risk of adverse vascular outcomes.Citation29,Citation30 The rationale for combination therapy with agents that block RAS and a calcium channel blocker (CCB) or diuretic is well founded.Citation30–Citation33 Recent landmark studies, such as ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension) and ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm), have demonstrated the antihypertensive benefits associated with angiotensin-converting enzyme (ACE) inhibitor/CCB combinations.Citation34–Citation36 More recently, the combination of an angiotensin receptor blocker (ARB), such as valsartan or olmesartan, and amlodipine has been introduced and tested in stage 1 and 2 hypertensive patients as well as on those not controlled by monotherapy.Citation37,Citation38
This review will focus on the role of the combination telmisartan–amlodipine, a potent ARB and the most frequently used CCB, to achieve adequate BP targets in hypertensive patients.
Pharmacology, rationale for combination, and pharmacokinetics of fixed-combination telmisartan–amlodipine
Telmisartan is an orally active nonpeptide that lowers BP with once-daily dosing by blocking the type I angiotensin II receptor (AT1 receptor), thus selectively inhibiting the pressor effects of the RAS. Telmisartan was approved by the US Food and Drug Administration (FDA) in November 1998 and by the European Commission in December 1998 for the treatment of hypertension. More recently, in ONTAR-GET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial), telmisartan demonstrated a noninferior capacity than ramipril to prevent CV events in high-risk patients.Citation39 As a consequence of these results, the FDA has approved an expanded indication for telmisartan that can now be used to reduce the risk of MI, stroke, or death from CV causes in patients aged 55 years or older who are intolerant to ACE inhibitors but at high risk for CV events.
Amlodipine is a dihydropyridine CCB that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle cells. It is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in BP. Amlodipine was approved by the FDA in July 1992. The first European approval was in July 1989.
The pharmacokinetics of repeated oral doses of 80 mg telmisartan at steady state alone and in combination with repeated oral doses of amlodipine 10 mg at steady state were studied in a two-way crossover, open, randomized design study (trial 1235.2). This study included 38 healthy male or female Caucasian volunteers aged 18–50 years. A second pharmacokinetic (drug interaction) study (study 502.126) was also conducted in 12 healthy subjects as part of the Micardis® monotherapy development program. This open-label, randomized, two-sequence, two-period crossover study demonstrated that telmisartan 120 mg had no effect on the steady-state pharmacokinetics of amlodipine 10 mg. In summary, there is no clinically significant change in systemic exposure to telmisartan 80 mg on coadministration of amlodipine 10 mg after dosing both medications to steady state, and there is no relevant drug–drug interaction with regard to the effect of amlodipine on telmisartan.
Efficacy studies and comparative trials
The telmisartan–amlodipine clinical development program included a pivotal 8-week, double-blind, randomized, placebo-controlled trial with a factorial study design (including a large ambulatory blood pressure monitoring [ABPM] substudy) to demonstrate the additive nature of combining telmisartan and amlodipine across a wide range of doses of each of the individual components.Citation40 An analysis of the prospectively defined subset of patients with moderate to severe hypertension at baseline has also been published.Citation41 The results of the 24-hour ABPM substudy have also been published.Citation42
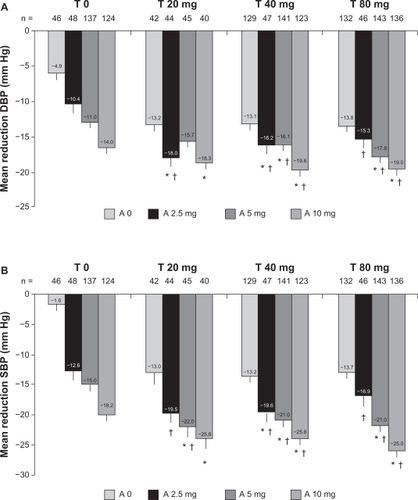
A total of 2607 patients were enrolled in the factorial study between April 2006 and November 2006, and 1461 patients were randomized and treated for up to 8 weeks. A total of 1344 (92%) patients completed the 8-week trial. The efficacy analyses were performed on all patients with a baseline value and at least one efficacy measurement at target dose (n = 1423). Both telmisartan (irrespective of amlodipine dosage; P < 0.0001) and amlodipine (irrespective of telmisartan dosage; P < 0.0001) significantly lowered the in-clinic trough diastolic BP, without evidence of counterproductive telmisartan-by-amlodipine interaction at any dosage (not involving patients treated with placebo; P = 0.1777). As expected, the greatest least-squares mean reductions in in-clinic diastolic and systolic BP were observed with combination therapy compared with respective monotherapies (). The greatest overall reduction in BP was observed with the telmisartan 80 mg plus amlodipine 10 mg combination (mean reduction in systolic BP/diastolic BP: −26.4 mm Hg/−20.1 mm Hg; P < 0.05 vs both monotherapies).Citation40 More than 50% of all patients treated with combination therapy achieved BP control (diastolic BP < 90 mm Hg and systolic BP < 140 mm Hg), with the highest percentages (76.5% [overall control] and 85.3% [diastolic BP control]) being achieved by patients treated with telmisartan 80 mg plus amlodipine 10 mg. Diastolic BP response and systolic BP response was achieved by 91.2% and 90.4% of patients in the telmisartan 80 mg plus amlodipine 10 mg group, respectively.Citation40 This combination was also effective in patients with moderate or severe hypertension. In fact, the greatest reduction in BP (SBP/DBP −26.5 ± 1.2/–21 ± 0.8 mm Hg) was achieved with the highest-dose combination of telmisartan 80 mg plus amlodipine 10 mg and the SBP/DBP response rates >90%. The BP control (<140/90 mm Hg) and DBP control (<90 mm Hg) obtained with this combination was 77% and 85%, respectively.Citation41
Figure 1 Effect of 8 weeks of treatment with telmisartan (T) 0 mg, 20 mg, 40 mg, and 80 mg plus amlodipine (A) 0 mg, 2.5 mg, 5 mg, and 10 mg on the change from baseline in the in-clinic seated trough (A) diastolic blood pressure (DBP) (mm Hg) or (B) systolic blood pressure (SBP) (mm Hg).
The largest reductions in 24-hour mean BP were observed with the combination of telmisartan 80 mg and amlodipine 10 mg when compared with their respective monotherapies (P < 0.0001 in each comparison): telmisartan 80 mg and amlodipine 10 mg (–22.4/–14.6 mm Hg), telmisartan 80 mg (–11.0/–6.9 mm Hg), and amlodipine 10 mg (–11.9/–6.9 mm Hg). Greater BP reductions were also observed for the combinations of lower doses of telmisartan (40 mg) and amlodipine (5 mg) in combination compared with the components.Citation42
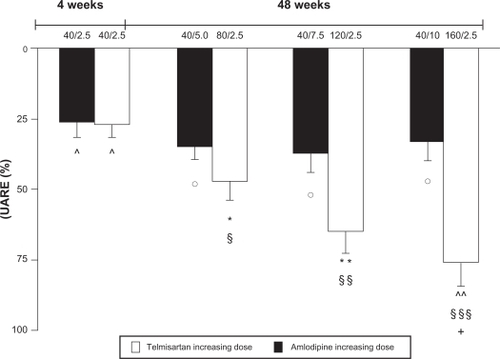
Fogari et alCitation43 evaluated the effect of a combination therapy with telmisartan and amlodipine on urinary albumin excretion rate (UAER) in hypertensive patients with type 2 diabetes and microalbuminuria and examined whether two different dose regimens (high-dose telmisartan/low-dose amlodipine and vice versa) offered different benefits in terms of reduction of proteinuria. After a 2-week placebo washout period, during which antihypertensive but not oral antidiabetic drugs were discontinued, patients fulfilling the inclusion criteria were treated with the telmisartan (40 mg)/ amlodipine (2.5 mg) combination. After 4 weeks, patients whose BP was not controlled (BP > 130/80 mm Hg) were enrolled in the study and randomized to two different dose titration regimens: one based on increasing doses of telmisartan (40 mg every 4 weeks until 160 mg) and a fixed 2.5 mg dose of amlodipine (group T), the other based on an increasing dose of amlodipine (2.5 mg every 4 weeks until 10 mg) and a fixed 40 mg dose of telmisartan (group A). After 16 weeks, the nonresponder patients were given 0.1 mg/day transdermic clonidine.Citation43 High-dose telmisartan/low-dose amlodipine and low-dose telmisartan/high-dose amlodipine combinations produced similar reductions in systolic and diastolic BP values, with no significant differences between the two regimens at any time of the study. With increasing doses of telmisartan (from 40 mg to 80 mg, 120 mg, and 160 mg), systolic/diastolic BP values were reduced from baseline by 16/10 mm Hg (P < 0.01 vs baseline), 24/21 mm Hg, 23/21 mm Hg, and 24/21 mm Hg (all P < 0.001 vs baseline), respectively. With increasing doses of amlodipine (from 2.5 mg to 5 mg, 7.5 mg, and 10 mg), systolic/diastolic BP values were reduced from baseline by 16/10 mm Hg (P < 0.01 vs baseline), 25/22 mm Hg, 25/21 mm Hg, and 25/22 mm Hg (all P < 0.001 vs baseline), respectively. The UAER was significantly decreased from baseline by both combination regimens, but such a decrease was significantly more marked in the T group (). Reductions of UAER from baseline were of 34.6 mg/24 hours (P < 0.05 vs baseline), 62.9 mg/24 hours (P < 0.01 vs baseline and P < 0.05 vs A group), 86.5 mg/24 hours (P < 0.001 vs baseline and P < 0.01 vs A group) and 102 mg/24 hours (P < 0.0001 vs baseline and P < 0.001 vs A group) for telmisartan 40 mg, 80 mg, 120 mg, and 160 mg/amlodipine 2.5 mg daily, respectively. Reductions of UAER from baseline were of 35.1 mg/24 hours (P < 0.05 vs baseline), 46.2 mg/24 hours (P < 0.03 vs baseline), 50.3 mg/24 hours (P < 0.03 vs baseline), and 45 mg/24 hours (P < 0.03 vs baseline) for amlodipine 2.5 mg, 5 mg, 7.5 mg, and 10 mg/telmisartan 40 mg daily, respectively.Citation43
Safety and tolerability
In the factorial study, a total of 545 (37.3%) patients reported at least one adverse event during the 8-week study.Citation40 When analyzed by treatment groupings, the percentage of patients reporting adverse events on specific treatment was comparable: placebo (39.1%, n = 18), telmisartan monotherapy (36.8%, n = 113), amlodipine monotherapy (36.1%, n = 115), and combination therapy (37.9%, n = 299) groups. The most commonly reported adverse events were headache (5.4%, n = 79) and peripheral edema (4.4%, n = 65). Headache was more frequent in the placebo group (10.9%, n = 5) compared with the telmisartan monotherapy (5.9%, n = 18), amlodipine monotherapy (6.0%, n = 19), and combination therapy (4.7%, n = 37) groups. The incidence of peripheral edema was highest in the amlodipine 10 mg group (17.8%, n = 23); however, this rate was lower when amlodipine was used in combination with telmisartan: 11.4% (telmisartan 20 mg/amlodipine 10 mg), 6.2% (telmisartan 40 mg/amlodipine 10 mg), and 11.3% (telmisartan 80 mg/amlodipine 10 mg) ().
Patient-focused perspectives such as quality of life, patient satisfaction/acceptability, adherence, and uptake
Hypertension is a chronic disease for which there is no cure. Antihypertensive medications will lower BP in patients for as long as patients comply with therapy. Poor patient adherence with health-related advice, medication, tests, and clinic appointments has been reported as the most common cause of nonresponse to medication.Citation44 Poor adherence to medication regimens is a major contributor to the gap between practice guidelines and clinical outcome in CVD.Citation45–Citation47 Poor persistence may be affected by many patient-related factors. Poor motivation to accept treatment can lead to a cycle of treatment failure. Patients and physicians frequently accept failure of initial treatment and lack the motivation to continue treating the condition. Patients may embark on a series of treatment steps, each viewed as a failure, further undermining motivation and fulfilling negative expectations, resulting in poor BP control and an increased risk of mortality and morbidity for the patient. In consequence, poor compliance and persistence with medication by patients results in poor clinical outcomes. Given the scale of the problem and its effect on everyday practice, it is essential to develop and implement strategies to improve compliance.
Persistence rates with currently available classes of antihypertensive medications vary widely. Patients prescribed ARBs as their initial medication are more likely to persist with treatment than patients on other classes of antihypertensive.Citation48 Moreover, it has been confirmed that the treatment discontinuation rate differs between antihypertensive drug classes. The treatment discontinuation rate is lowest in patients in whom the drug initially prescribed was an ARB, and became progressively greater with the initial administration of an ACE inhibitor, a CCB, an α-blocker, a β-blocker, and a diuretic.Citation49
The use of multiple medications and complexity of treatment regimen are two of the major determinants of poor medication adherence. A survey conducted by the National Council on Patient Information and Education showed that one-third of patients receive at least two prescriptions and 10% of patients receive four or more prescriptions after a visit to a primary care physician.Citation50 In a systematic review of the impact of dose regimen on medication compliance, it has been shown that dose taking was inversely related to the prescribed number of doses per day. Compliance was significantly higher for once daily versus three times daily (P = 0.008), once daily versus four times daily (P < 0.001), and twice daily versus four times daily (P = 0.001) regimens.Citation51 Adherence to antihypertensive agents varies inversely with dosing frequency,Citation52 and nonadherent patients tend to have higher BP than adherents.Citation53 As adherence rates are inversely related to the number of drugs given, it would be expected that patients would be more adherent when they take a combination of drugs as a single tablet than if they are given the same drugs as two separate pills, even when dosed once daily.Citation54,Citation55 In the 2009 reappraisal of European guidelines on hypertension management, the European Society of Hypertension Task Force notes: “Whenever possible, use of single pill (or single pill) combinations should be preferred, because simplification of treatment carries advantages for compliance to treatment”.Citation23
Conclusion
Adequate BP control and reduction of CV events are particularly effective with the combination of antihypertensive agents, including an ACE inhibitor or an ARB. Recently, the combination of an ACE inhibitor or ARB plus a CCB appears to be rational and effective. These combinations can thus be recommended for priority use. Telmisartan–amlodipine combination has demonstrated significantly larger DBP and SBP reductions compared with each monotherapy component in the overall population, and in particular in patients with moderate to severe hypertension. This combination is well tolerated with a safety profile similar to placebo and is consistent with the known safety profile of its monotherapy components.
Disclosure
The authors report no conflicts of interest in this work.
References
- MathersCDLoncarDProjects of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- EzzatiMEstimates of global and regional potential health gains from reducing multiple major risk factorsLancet2003362938027128012892956
- PetersenSPetoVRaynerMEuropean Cardiovascular Disease StatisticsLondon, UKBritish Heart Foundation2005
- LealJLuengo-FernándezRGrayAEconomic burden of cardiovascular diseases in the enlarged European UnionEur Heart J200627131610161916495286
- LawesCMVander HoomSRodgersAInternational Society of Hypertension. Global burden of blood pressure-related disease, 2001Lancet200837196231513151818456100
- LopezADMathersCDEzzatiMGlobal and regional burden of disease and risk factors, 2001: systematic analysis of population health dataLancet200636795241747175716731270
- LewingtonSClarkeRQizilbashNProspective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- ManciaGDe BackerGDominiczakA2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- StegPGBhattDLWilsonPWOne-year cardiovascular event rates in outpatients with atherothrombosisJAMA2007297111197120617374814
- CapewellSMurphyNFMacIntyreKShort-term and long-term outcomes in 133,429 emergency patients admitted with angina or myocardial infarction in Scotland, 1990–2000: population-based cohort studyHeart200692111563157016775090
- DhamoonMSSciaccaRRRundekTRecurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan StudyNeurology200666564164616534100
- Cea-CalvoLConthePGómez-Fernández-PérezCRICARHD InvestigatorsTarget organ damage and cardiovascular complications in patients with hypertension and type 2 diabetes in Spain: a cross-sectional studyCardiovasc Diabetol200652317083718
- YusufSHawkenSOunpuuSEffect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control studyLancet2004364943893795215364185
- DzauVBraunwaldEResolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statementAm Heart J19911214 Pt 1124412632008853
- DzauVJAntmanEMBlackHRThe cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease)Circulation2006114252850287017179034
- BakrisGBöhmMDagenaisGCardiovascular protection for all individuals at high risk: evidence-based best practiceClin Res Cardiol2008971071372518726243
- Cholesterol Treatment Trialists’ (CTT) CollaborationEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statinsLancet200536694931267127816214597
- Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patientsBMJ20023247329718811786451
- DuckworthWAbrairaCMoritzTGlucose control and vascular complications in veterans with type 2 diabetesN Engl J Med2009360212913919092145
- StaessenJALiYThijsLWangJGBlood pressure reduction and cardiovascular prevention: an update including the 2003–2004 secondary prevention trialsHypertens Res200528538540716156503
- The ACCORD Study GroupEffects of intensive blood-pressure control in type 2 diabetes mellitusN Engl J Med2010362171575158520228401
- AppelLJWrightJTJrGreeneTAASK Collaborative Research GroupIntensive blood-pressure control in hypertensive chronic kidney diseaseN Engl J Med20103631091892920818902
- ManciaGLaurentSAgabiti-RoseiEEuropean Society of HypertensionReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentJ Hypertens200927112121215819838131
- Wolf-MaierKCooperRSKramerHHypertension treatment and control in five European countries, Canada, and the United StatesHypertension2004431101714638619
- WongNDLopezVAL’ItalienGInadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003–2004Arch Intern Med2007167222431243618071164
- Mustone AlexanderLDesirable therapeutic characteristics of an optimal antihypertensive agentDrugs20066691239125216827600
- MulcahyDCircadian variation in cardiovascular eventsBlood Press Monit199831293410212329
- SchmiederREHilgersKFSchlaichMPSchmidtBMRenin-angiotensin system and cardiovascular riskLancet200736995681208121917416265
- WangYRAlexanderGCStaffordRSOutpatient hypertension treatment, treatment intensification, and control in Western Europe and the United StatesArch Intern Med2007167214114717242314
- TedescoMANataleFCalabroREffects of monotherapy and combination therapy on blood pressure control and target organ damage: a randomized prospective intervention study in a large population of hypertensive patientsJ Clin Hypertens200689634641
- WeirMRTargeting mechanisms of hypertensive vascular disease with dual calcium channel and renin-angiotensin system blockadeJ Hum Hypertens2007211077077917597800
- EganBMCombination therapy with an angiotensin converting enzyme inhibitor and a calcium channel blockerJ Clin Hypertens2007910783789
- EpsteinMBakrisGNewer approaches to antihypertensive therapy. Use of fixed-dose combination therapyArch Intern Med199615617196919788823150
- WeberMABakrisGLDahlofBBaseline characteristics in the Avoiding Cardiovascular Events Through Combination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular riskBlood Press2007161131917453747
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding per-indopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- KjeldsenSEJamersonKABakrisGLPredictors of blood pressure response to intensified and fixed combination treatment of hypertension: the ACCOMPLISH studyBlood Press200817171718568687
- AllemannYFraileBLambertMEfficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure After Single Therapy (EX-FAST) studyJ Clin Hypertens2008103185194
- ChrysantSGMelinoMKarkiSThe combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety studyClin Ther200830458760418498909
- ONTARGET InvestigatorsYusufSTeoKKPogueJTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- LittlejohnTW3rdMajulCROlveraRResults of treatment with telmisartan-amlodipine in hypertensive patientsJ Clin Hypertens (Greenwich)200911420721319614805
- LittlejohnTW3rdMajulCROlveraRTelmisartan plus amlodipine in patients with moderate or severe hypertension: results from a subgroup analysis of a randomized, placebo-controlled, parallel-group, 4 × 4 factorial studyPostgrad Med2009121251419332958
- WhiteWBLittlejohnTWMajulCREffects of telmisartan and amlodipine in combination on ambulatory blood pressure in stages 1–2 hypertensionBlood Press Monitor2010154205212
- FogariRDerosaGZoppiAEffect of telmisartan-amlodipine combination at different doses on urinary albumin excretion in hypertensive diabetic patients with microalbuminuriaAm J Hypertens200720441742217386350
- MurphyJCosterGIssues in patient complianceDrugs19975467978009421690
- MichalsenAKonigGThimmeWPreventable causative factors leading to hospital admission with decompensated heart failureHeart19988054374419930040
- GehiAKAliSNaBSelf-reported medication adherence and CV events in patients with stable coronary heart disease: the heart and soul studyArch Inter Med20071671617981803
- SokolMCMcGuiganKAVerbruggeRRImpact of medication adherence on hospitalization risk and healthcare costMed Care200543651752015908845
- EspostiLDDi MartinoMSaragoniSPharmacoeconomics of antihypertensive drug treatment: an analysis of how long patients remain on various antihypertensive therapiesJ Clin Hypertens2004627684
- CorraoGZambonAParodiADiscontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in ItalyJ Hypertens200826481982418327094
- DeziiCMMedication noncompliance: what is the problem?Manag Care200099 Suppl71211729418
- ClaxtonAJCramerJAPierceCA systematic review of the associations between dose regimens and medication complianceClin Ther20012381296131011558866
- SicaDSingle pill combination antihypertensive drugs. Do they have a role in rational therapy?Drugs199448116247525192
- BramleyTJGerbinoPPNightengaleBSFrech-TamasFRelationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizationsJ Manag Care Pharm200612323924516623608
- BangaloreSKamalakkannanGParkarSFixed-dose combinations improve medication compliance: a meta-analysisAm J Med2007120871371917679131
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysisHypertension201055239940720026768
![Figure 3 Incidence of peripheral edema (%) in the amlodipine 10 mg (A10) group compared with combinations (telmisartan 40 mg [T40] or 80 mg [T80] plus amlodipine 5 mg [A5] or 10 mg).](/cms/asset/2494ba08-9ba7-4441-ad8f-30c6d62b92cc/dibp_a_9934_f0003_b.jpg)