Abstract
The use of antibiotics in animal production has been associated with the development and spread of antibiotic-resistant organisms including commensals. Coagulase-negative Staphylococcus (CoNS) species, which were until recently considered non-pathogenic, have been associated with opportunistic infections and high resistance to several antibiotics. This study sought to determine the prevalence, identity, and phenotypic resistance of coagulase-negative Staphylococcus spp. isolated from some selected poultry farms and farm workers in the Ashanti, Brong Ahafo, and Greater Accra regions of Ghana. Poultry litter samples and oral swabs of poultry farm workers were collected, from which bacterial species were isolated, identified, and analyzed. Various selective media were used for the presumptive identification of the different species. Confirmation of bacterial identity was done using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry. Antibiotic susceptibility testing of the isolates was performed using the Kirby-Bauer disk diffusion method. Zones of growth inhibition were interpreted based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. Two hundred and fifty-six coagulase-negative Staphylococcus spp., comprising S. sciuri (42.97%), S. lentus (35.94%), S. gallinarum (6.64%), S. xylosus (4.30%), S. haemolyticus (3.91%), S. saprophyticus (1.95%), and S. cohnii (0.39%) were confirmed by MALDI-TOF. CoNS were isolated from samples from the Brong Ahafo (48.83%), Ashanti (33.59%), and Greater Accra (17.78%) regions. Isolates from poultry litter constituted 55.47%, and farm workers 44.53%. All the isolates were susceptible to amoxicillin/clavulanic acid and amikacin. The isolates exhibited high resistance toward tetracycline (57.03%), doxycycline (43.75%), and oxacillin (43.36%). Multi-drug resistance (MDR) was observed in 19.14% of the isolates. MDR was higher in isolates obtained from poultry farm workers (61.22%) than isolates from poultry litter (38.78%). The above findings call for stricter monitoring of antibiotic usage in both animal production and in humans.
Introduction
The bacteria genus Staphylococcus is a Gram-positive, facultative anaerobe, which is round in shape and appears in clusters.Citation1 Staphylococcus spp. are also known to be non-motile, non-sporing, glucose fermenting, and catalase producing.Citation2 Staphylococcus aureus is the most commonly known member of the family, and the most commonly known coagulase-positive strain.Citation1 Members of the Staphylococcus genus that do not produce coagulase are known as coagulase-negative Staphylococci (CoNS). Until recently, CoNS were considered as the non-pathogenic members of the genus and thus were not of much interest to the research community. However, due to their implication in infections in both humans and animals, research interest in CoNS has increased over the past decade.Citation3–Citation5 In addition, CoNS have, over the last decade, developed resistance to multiple antibiotics,Citation4,Citation6,Citation7 making their study worthwhile, especially, since they are known commensals and could be prevalent in most environments.Citation5,Citation8 The economic burden of Staphylococcal infections in animal husbandry includes decreased weight gain, drop in egg production (with respect to poultry), mortality, condemnation at slaughter, and lameness, among others.Citation9,Citation10
In Ghana, a developing country, with a growing population of about 25 million people and a land area of 385,500 square kilometers, agriculture provides employment and serves as the major source of livelihood for over 55% of the population.Citation11 Several individuals engage in livestock production as a means of protein (meat and eggs) supplementation in the home, and also as a source of income.Citation12,Citation13 Commercial poultry production in Ghana is highly concentrated in the Ashanti, Brong Ahafo, and Greater Accra regions and these three regions contribute to almost 70% of commercial poultry production in the country.Citation14,Citation15
However, commercial poultry production in Ghana is challenged with occurrences of bacterial, viral, and parasitic infections.Citation16 This compels poultry farmers to employ several antimicrobial agents, including essential antibiotics, on their farms.Citation13,Citation16 Studies have also shown that the farmers in the said regions employ these antibiotics even in the management of viral infections.Citation13 The misuse of antibiotics in animal production has been closely associated with the development and spread of antibiotic-resistant organisms, including non-pathogenic species and commensals.Citation8,Citation17
Notwithstanding the fact that research interest in CoNS has increased in recent years, there is very little data on their prevalence and resistance profiles in Ghana. Information on CoNS is currently unavailable, especially in areas where misuse of antibiotics could lead to selection pressure, development of resistance, and spread of resistant strains. Hence, the aim of this study was to determine the prevalence and antibiograms of coagulase-negative Staphylococcus spp. isolated from poultry bedding materials (poultry litter) and poultry farm workers in the Ashanti, Brong Ahafo, and Greater Accra regions of Ghana.
Materials and methods
Ethical clearance and approval
Ethical clearance for the study was obtained from the Committee on Human Research Publications and Ethics (CHRPE), Kwame Nkrumah University of Science and Technology (KNUST), Kumasi. In addition, written consent was obtained from all participants.
Selection of farms
A total of 400 poultry farms were randomly selected from the three regions as previously described by Boamah et al.Citation13 Sixty-one percent of the farms were located in the Ashanti region, 28.5% in the Brong Ahafo region, and 10.5% in the Greater Accra region. Eighty-two (20.5%) of the farms were small scale, 254 (63.5%) medium, and 64 (16%) large, as categorized by the Food and Agriculture Organisation (FAO).Citation12
Sampling from poultry bedding and poultry workers
One gram of poultry bedding material (litter) was aseptically collected from three different points in a pen, in sterile sample containers. In a situation where a farm had less than five pens, samples were collected from at least three different pens, and from farms with more than five pens, samples were collected from at least five different pens. Oral swabs from farm workers who had worked on the farm for at least 1 month, and those directly involved in the day-to-day management of birds in the farm were also taken. The samples were appropriately labeled and transported to the laboratory in a cold box.
Isolation of CoNS species
Five and ten milliliters of trypticase soy broth (TSB) (Thermo Fisher Scientific, Waltham, MA, USA) were added to the samples from humans and poultry litter respectively, and then incubated at 37°C for 24 h. CoNS were isolated from the media by methods described by ReynoldsCitation18 and Kateete et al.,Citation19 with slight modifications. Five hundred microliters suspension of TSB was aseptically transferred into 10 mL Mueller-Hinton broth (Thermo Fisher Scientific), supplemented with 6.5% NaCl, and incubated at 37°C for 24 h. After incubation, 10 µL was spread on 20 mL mannitol salt agar (MSA) (Thermo Fisher Scientific), and incubated at 37°C for 24 h. Well-isolated colonies on MSA medium were aseptically streaked onto 20 mL plates of Staphylococcus-aureus-select (SaSelect), (Bio-Rad Laboratories Inc., Hercules, CA, USA) and incubated at 37°C for 24 h. Bluish, purple-like, and whitish colonies grown on SaSelect were further streaked on 20 mL nutrient agar (Thermo Fisher Scientific) enriched with 5% sheep blood and then incubated at 37°C for 24 h. Identity of non-hemolytic colonies was then confirmed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry.
Confirmation of isolates by MALDI-TOF mass spectrometry
Twenty-four hour cultures of non-hemolytic colonies on blood agar plates were used. Isolated colonies were uniformly spread on the MALDI-TOF slide to form a thin film and then covered with 1 µL of ready-to-use α-cyano-4-hydroxycinnamic acid and allowed to air-dry. Escherichia coli 25922 was used as the control strain. The MALDI-TOF slide was loaded into the VITEK MS (BioMérieux Corporate, Paris, France) for identification. The identity of the isolate was determined using Saramis® program (BioMérieux Corporate). Isolates with identities >99% were confirmed as CoNS. S. aureus 25923, E. coli ATCC 25922 (American Type Culture Collection, Manassas, VA, USA), and all other strains of CoNS were stored at −80°C until needed.
Antibiotic susceptibility testing
Antibiotic sensitivity of the Staphylococcus spp. was determined using the disk diffusion method described by EUCASTCitation20 and Bauer et al.Citation21 Twenty-four hour colonies of Staphylococci growing on blood agar plates were used for the susceptibility testing. To ensure a good representation of the isolates in the culture, approximately five to seven well-separated colonies were picked and suspended in 5 mL sterile distilled water and vortexed at high speed until the suspension was uniform. The turbidity of the suspension was determined using a nephelometer already calibrated to 0.5 McFarland. The turbidity of the suspensions were then adjusted appropriately to 0.5 McFarland with the addition of more colonies or sterile distilled water.
A sterile cotton swab was soaked in the inoculum and rotated twice against the inner side of the test tube to remove excess liquid. The swab was used to streak the entire surface of 20 mL MHA plate whilst rotating the plate at an angle of 60° with repeated streaking (three times in total). With the aid of a disk dispenser, antibiotic disks (benzyl penicillin 1 unit; ampicillin 10 µg; oxacillin 1 µg; cefoxitin 30 µg; trimethoprim/sulfamethoxazole 25 µg; ciprofloxacin 5 µg; norfloxacin 10 µg; gentamicin 10 µg; erythromycin 15 µg; tetracycline 30 µg; doxycycline 30 µg; chloramphenicol 30 µg) purchased from Thermo Fisher Scientific, were placed on the surface of the MHA. The plates were incubated at 37°C for 20 h. Zones of growth inhibition (mm) were measured and recorded. Susceptibility of the isolates to different antibiotics was determined and classified according to EUCAST.Citation20
Results
Distribution of different Staphylococcus spp. in the selected farms
Of the 368 presumptive Staphylococcal colonies isolated using selective media, 256 (69.57%) were confirmed by MALDI-TOF mass spectrometry as coagulase-negative Staphylococcus spp. One hundred and fourteen (44.53%) were from farm workers whilst isolates from the poultry bedding material constituted 55.47%. Seven different CoNS species were identified and these include S. sciuri (42.97%), S. lentus (35.94%), S. gallinarum (6.64%), S. xylosus (4.30%), S. haemolyticus (3.91%), S. saprophyticus (1.95%), and S. cohnii (0.39%) ().
Table 1 Distribution of isolated coagulase-negative Staphylococci among the different sources
Antibiotic sensitivity profiles of isolated CoNS
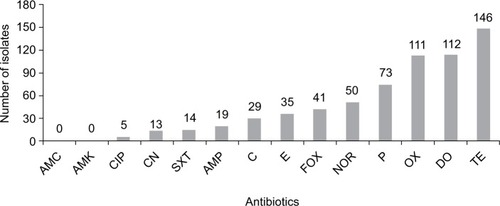
All the isolates (256) were susceptible to amoxicillin/clavulanic acid and amikacin. Fifty-seven percent (57.03%) of the isolates were resistant to tetracycline, 43.75% to doxycycline, 43.36% to oxacillin, and 28.52% to penicillin. The isolates showed low resistance to ampicillin (7.42%), trimethoprim/sulfamethoxazole (5.47%), gentamycin (5.00%), and ciprofloxacin (1.95%) ().
Figure 1 Resistance of CoNS isolates to selected antibiotics.
Notes: AMC, amoxicillin/clavulanic acid; AMK, amikacin; CIP, ciprofloxacin 5 µg; CN, gentamicin 10 µg; SXT, trimethoprim/sulfamethoxazole 25 µg; AMP, ampicillin 10 µg; C, chloramphenicol 30 µg; E, erythromycin 15 µg; FOX, cefoxitin 30 µg; NOR, norfloxacin 10 µg; P, benzyl penicillin 1 unit; OX, oxacillin 1 µg; DO, doxycycline 30 µg; TE, tetracycline 30 µg.
Abbreviation: CoNS, coagulase-negative Staphylococcus.
Distribution of resistant coagulase-negative Staphylococcus spp
Each of the seven different coagulase-negative Staphylococcus spp. exhibited some level of resistance toward penicillin, oxacillin, cefoxitin, and tetracycline. All the different species were most susceptible toward ciprofloxacin. Isolates from all the three regions showed varying levels of resistance to the different antibiotics, with the exception of isolates from the Ashanti region which were totally susceptible to ciprofloxacin. Both sets of isolates from farm workers and poultry litter exhibited various levels of resistance to the antibiotics. However, isolates from farm workers’ samples in the Brong Ahafo and Ashanti regions and isolates from litter samples in the Ashanti regions did not exhibit any resistance toward ciprofloxacin. Also, none of the isolates obtained from litter samples in the Greater Accra region was resistant to chloramphenicol ().
Table 2 Distribution of resistant coagulase-negative Staphylococcus spp. among selected antibiotics
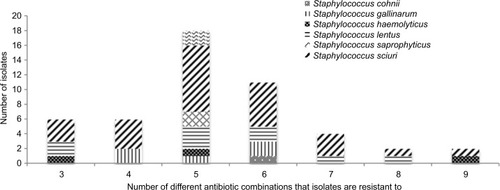
Multi-drug resistant CoNS
Forty-nine (19.14%) of the isolates exhibited multi-drug resistance (MDR) (simultaneous resistance to three or more antibiotics) ().Citation22 Thirty isolates (61.22%) from farm workers were MDR isolates whilst 19 (38.78%) MDR isolates were from poultry litter. Twenty-three (46.94%) of the MDR strains were from samples from the Ashanti region, 15 (30.61%) from the Brong Ahafo region, and 11 (22.45%) from the Greater Accra region (). Among the Staphylococcus spp., 27 (55.1%) of the MDR isolates were S. sciuri, 9 (18.37%) S. lentus, 5 (10.20%) S. gallinarum, 3 (6.12%) S. haemolyticus, 2 (4.08%) isolates each were S. saprophyticus and S. xylosus, and 1 (2.04%) S. cohnii. Farm workers contributed 30 (61.22%) of the MDR Staphylococcal isolates whilst poultry litter had 19 (38.78%) of the MDR isolates ().
Table 3 Antibiograms of isolated CoNS from different sources in the selected regions
Table 4 Distribution of multi-drug resistant CoNS strains against selected antibiotics
Discussion
The increasing rates of antibiotic resistance and MDR among pathogenic, non-pathogenic, commensals, and opportunistic bacteria call for increased report on the distribution (prevalence) of these organisms and their antibiotic profiles.Citation23 Many Staphylococcus spp. are part of the normal bacterial flora in humans and animals.Citation18 Several of these commensal and non-pathogenic Staphylococci have been implicated in infections.Citation5 Many of such species have also been reported as multi-drug resistant, which has resulted in increased cost of treating infections and increased disease burden in both humans and animal husbandry.Citation3,Citation23
Seven different coagulase-negative Staphylococcus spp. including S. sciuri, S. lentus, S. gallinarum, S. xylosus, S. haemolyticus, S. saprophyticus, and S. cohnii were identified in both farm workers and poultry litter samples. The presence of different Staphylococcus spp. among poultry litter and farm workers has been reported by Simjee et al.Citation24 and Vadari et al.Citation25 The latter identified 19 different Staphylococcus spp. from poultry litter using molecular techniques (16s rDNA). The species included all the species identified in this study, with the exception of S. gallinarum. The low number of species identified in this study compared to that of Vadari et al.Citation25 could be due to the identification method used. Molecular techniques detect the presence of both viable and non-viable organisms, whilst only viable organisms are identified using morphological and biochemical means.
In the report by Simjee et al.,Citation24 38% of the coagulase-negative Staphylococcus spp. were S. sciuri, whereas S. lentus and S. xylosus constituted 21% and 14%, respectively. The prevalence of S. sciuri among the farms in this study was 44%, whilst S. lentus and S. xylosus were 37% and 4%, respectively. Staphylococcus simulans was not found in this current study but was detected by Simjee et al.Citation24 On the other hand, Staphylococcus spp. including S. cohnii, S. saprophyticus, and S. gallinarum were not identified and reported by Simjee et al.Citation24 The differences in the Staphylococcus spp. identified could be due to the different bacterial flora within the study areas.Citation26
There have been reports of increasing resistance of Staphylococcus spp. to several essential antibiotics over the past 30 yearsCitation26–Citation28 in different countries including Ghana.Citation29 In this study, coagulase-negative Staphylococcus spp. equally showed a high level of resistance mostly to tetracycline (57%), doxycycline (44%), and penicillin (28%). However, the resistance observed toward penicillin was less than the number Lerbech et al.Citation29 reported, with 98% CoNS isolates from humans being resistant to penicillin. The high resistance of CoNS to penicillin reported by Lerbech et al.Citation29 could be due to the fact that all the isolates were obtained from patients, and such isolates have been found to be highly resistant to beta-lactams.Citation30 Resistance of the isolates to tetracycline (57%) was almost similar to the 63% reported by Lerbech et al.Citation29
All the isolates from this study were susceptible to amoxicillin/clavulanic acid and amikacin. This may be due to the fact that these two antibiotics are not part of the antimicrobial agents routinely employed in poultry production in Ghana,Citation31 hence, amoxicillin/clavulanic acid and amikacin preparations remain reserved antibiotics used for the treatment of Staphylococcus spp. infections.Citation32
Forty-nine (19.14%) of the CoNS isolated were multi-drug resistant with the highest resistance to tetracycline (59.18%), doxycycline (59.18%), and erythromycin (57.14%). Suleiman et al.Citation33 reported 65% MDR Staphylococci isolates in a similar study in Nigeria. The high level of MDR Staphylococci could be due to the misuse of antibiotics in the poultry industry in many developing countries, including GhanaCitation13 and Nigeria.Citation34 Coagulase-negative Staphylococci are also known to form biofilms and this reduces the effect of antimicrobial agents against them.Citation26,Citation27
The majority (61%) of the MDR CoNS isolates were isolated from humans including farm workers, owners, and managers, whereas the bedding material had 39% of the MDR isolates. These findings could be due to the fact that farm workers are more exposed to MDR Staphylococcus strains because of the direct contact or interactions between humans and animals. These findings confirm the reports by Lerbech et al.,Citation29 Feglo et al.,Citation35 Obeng-Nkrumah et al.,Citation36 and Newman et al.,Citation37 which showed a high level of antibiotic resistance among humans in Ghana.
Conclusion
Coagulase-negative Staphylococci (256 isolates) from seven different species were identified and they exhibited varying levels of resistance to the selected antibiotics, with 49 multi-drug resistant isolates, and over 60% of these MDR Staphylococcus strains were from human samples. These call for increased surveillance measures and monitoring of antibiotic use in both animal husbandry and in humans in Ghana.
Acknowledgments
The authors are grateful to L’Oreal/African Network of Scientific and Technological Institutions (ANSTI)/United Nations Educational, Scientific and Cultural Organization (UNESCO)/Fellowship for Women in Science (FWIS) and the Danish International Development Agency (DANIDA)/Building Stronger Universities (BSU) for grants and fellowships to VEB.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarrowGIFelthamRKCowan and Steel’s Manual for the Identification of Medical Bacteria3rd edCambridge University Press2009
- HarleyJPPrescottLMLaboratoy Exercises in Microbiology5th edNew YorkThe McGraw-Hill Companies, New York2002
- BoerlinPKuhnertPHussyDSchaellibaumMMethods for identification of Staphylococcus aureus isolates in cases of bovine mastitisJ Clin Microbiol200341276777112574280
- KoksalFYasarHSamastiMAntibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in TurkeyMicrobiol Res2009164440441017475456
- MayLKleinEYRothmanRELaxminarayanRTrends in antibiotic resistance in coagulase-negative staphylococci in the United States, 1999 to 2012Antimicrob Agents Chemother20145831404140924342646
- MaXXWangEHLiuYLuoEJAntibiotic susceptibility of coagulase-negative staphylococci (CoNS): emergence of teicoplanin-non-susceptible CoNS strains with inducible resistance to vancomycinJ Med Microbiol201160Pt 111661166821799199
- QuYDaleyAJIstivanTSGarlandSMDeightonMAAntibiotic susceptibility of coagulase-negative staphylococci isolated from very low birth weight babies: comprehensive comparisons of bacteria at different stages of biofilm formationAnn Clin Microbiol Antimicrob201091620504376
- CarletJJarlierVHarbarthSVossAGoossensHPittetDParticipants of the 3rd World Healthcare-Associated Infections ForumReady for a world without antibiotics? The Pensières Antibiotic Resistance Call to ActionAntimicrob Resist Infect Control2012111122958833
- AndreasenCBOverview of Staphylococcosis in PoultryMerck Vet Man2013
- GiguèreSPrescottJFDowlingPMAntimicrobial Therapy in Veterinary Medicine5th edWiley Blackwell2013
- Ghana Statistical Service2010Population and Housing Census2012 Available from: http://www.statsghana.gov.gh/docfiles/2010phc/Census2010_Summary_report_of_final_results.pdfAccessed April 29, 2015
- Food and Agriculture Organization of the United NationsFAO Animal Production and Health DivisionPoultry Sector Ghana; FAO Animal Production and Health; Livestock Country Reviews2014 Available from: http://www.fao.org/docrep/019/i3663e/i3663e.pdfAccesssed May 20, 2015
- BoamahVEAgyareCOdoiHDalsgaardAAntibiotic practices and factors influencing the use of antibiotics in selected poultry farms in GhanaJ Antimicrob Agents201622120
- SumbergJAwoMFiankorDDKwadzoGT-MThompsonJGhana’s Poultry Sector: Limited Data, Conflicting Narratives, Competing Visions2013 Available from: https://steps-centre.org/wp-content/uploads/Ghana-Poultry-online.pdfAccessed June 6, 2015
- Food and Agriculture Organization of the United NationsFAO Animal Production and Health DivisionPoultry sector country reviewGhana2008 Available from: ftp://ftp.fao.org/docrep/fao/011/ai354e/ai354e00.pdfAccessed April 29, 2015
- Annan-PrahAAgbemafleEAsarePTAkorliSYAntibiotic use, abuse and their public health implication: the contributory role of management flaws in the poultry industry in two agro-ecological zones in GhanaJournal of Veterinary Advances201224199208
- CoglianiCGoossensHGrekoCRestricting antimicrobial use in food animals: lessons from EuropeMicrobe201166274279
- ReynoldsJGenus Staphylococcus: Identification of SpeciesNew York, NYVCH Publisher, Inc2011
- KateeteDPKimaniCNKatabaziFAIdentification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase testAnn Clin Microbiol Antimicrob201092320707914
- EUCASTThe European Committee on Antimicrobial Susceptibility Testing Available from: www.eucast.orgAccessed May 5, 2017
- BauerAKirbyWSherrisJTurkMAntibiotic susceptibility testing by a standard single disc diffusion methodAm J Clin Pathol19664544934965325707
- SaanaSBAduFAgyareCGbedemaSYBoamahVEGeorgeDFAntibiotic resistance patterns of strains of Staphylococcus aureus isolated from patients in three hospitals in Kumasi, GhanaAfrican Journal of Bacteriology Research2013533540
- World Health Organization (WHO)Antimicrobial resistance: global report on surveillance 20142014 Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/Accessed April 29, 2016
- SimjeeSMcDermottPWhiteDGAntimicrobial susceptibility and distribution of antimicrobial-resistance genes among enterococcus and coagulase-negative Staphylococcus isolates recovered from poultry litterAvian Dis200751488489218251398
- VadariYMasonBPDoernerKCIsolation from poultry litter and characterization [corrected] of Staphylococcus spp. capable of growth in high phosphate conditions [corrected]Lett Appl Microbiol2006431647016834723
- JohnJFHarvinAMHistory and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agentsTher Clin Risk Manag2007361143115218516271
- Hamilton-MillerJMIliffeAAntimicrobial Resistance in coagulase-negative staphylococciJ Med Microbiol19851922172263845121
- YurdakulNEErginkayaZÜnalEAntibiotic resistance of enterococci, coagulase negative staphylococci and staphylococcus aureus isolated from chicken meatCzech Journal of Food Sciences20133111419
- LerbechAMOpintanJABekoeSOAntibiotic exposure in a low-income country: screening urine samples for presence of antibiotics and antibiotic resistance in coagulase negative staphylococcal contaminantsPLoS One2014912e11305525462162
- SharmaVJindalNDeviPPrevalence of methicillin resistant coagulase negative staphylococci in a tertiary care hospitalIran J Microbiol20102418518822347570
- TurksonPKUse of drugs and antibiotics in poultry production in GhanaGhana Journal of Agricultural Science2008411
- Ministry of Health (GNDP) GhanaStandard Treatment Guidelines6th edYamens Press LtdAccra2010
- SuleimanAZariaLTGremaHAAhmaduPAntimicrobial resistant coagulase positive Staphylococcus aureus from chickens in Maiduguri, NigeriaSokoto Journal of Veterinary Sciences20131115155
- MaindaGBessellPBMumaJBPrevalence and patterns of antimicrobial resistance among Escherichia coli isolated from Zambian dairy cattle across different production systemsSci Rep201551243926211388
- FegloPAdu-SarkodieYAyisiLEmergence of a novel extended-spectrum-β-Lactamase (ESBL)-producing, fluoroquinolone-resistant clone of extraintestinal pathogenic Escherichia coli in Kumasi, GhanaJ Clin Microbiol201351272873023241377
- Obeng-NkrumahNTwum-DansoKKrogfeltKANewmanMJHigh levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistanceAm J Trop Med Hyg201389596096424043693
- NewmanMJFrimpongEDonkorESOpintanJAAsamoah-AduAResistance to antimicrobial drugs in GhanaInfect Drug Resist2011421522022259250