Abstract
Despite the efficacy of antibiotics to protect humankind against many deadly pathogens, such as Mycobacterium tuberculosis, nothing can prevent the emergence of drug-resistant strains. Several mechanisms facilitate drug resistance in M. tuberculosis including compensatory evolution, epistasis, clonal interference, cell wall integrity, efflux pumps, and target mimicry. In this study, we present recent findings relevant to these mechanisms, which can enable the discovery of new drug targets and subsequent development of novel drugs for treatment of drug-resistant M. tuberculosis.
Introduction
Tuberculosis (TB) is a significant public health concern with a high disease burden and mortality rate.Citation1 The disease is caused by members of the Mycobacterium tuberculosis complex (MTBC), a group of closely related human-adapted (M. tuberculosis [MTB] and Mycobacterium africanum) and animal-adapted (Mycobacterium bovis, Mycobacterium mungi, Mycobacterium pinnipedii, Mycobacterium microti, and Mycobacterium caprae) strains, as well as smooth tuberculosis bacilli (Mycobacterium canettii).Citation2 Although MTBC species have a remarkable range of mammalian hosts and morphologies, their genomes exhibit ≥99% homology, providing evidence that they evolved from a single ancestor in Africa 70,000 years ago.Citation2–Citation4 However, following advances in agriculture and animal domestication, MTB emerged as a human pathogen that has caused millions of deaths and continues to threaten human health globally.Citation5,Citation6 Successful control and prevention of MTB infection requires tools for a rapid and accurate diagnosis, as well as strategies for effective treatment. Current antibiotics used to treat MTB infection are isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA), and ethambutol (EMB).Citation7,Citation8 Misuse or misadministration of drugs can facilitate the emergence of drug-resistant strains via compensatory evolution, epistasis, and clonal interference phenomena that modulate MTB fitness. These various mechanisms of adaptation have led to the evolution of different drug-resistant levels of MTB strains, including multidrug resistant (MDR; resistance to INH and RIF), extensively drug resistant (XDR; resistance to fluoroquinolones [FQs] and one of the injectable aminoglycosides [AGs]), and totally drug resistant (TDR; resistance to all known drugs).Citation9,Citation10
Therefore, the aim of this review was to highlight recent findings related to mechanisms implicated in the emergence of MTB drug resistance and outline some possible drug targets to contribute to efforts aimed at discovering novel TB treatment.
Development of drug-resistant MTB
MTB diversity and drug resistance
Genomic comparisons have increased our understanding of MTBC diversity. For example, analyses of single nucleotide polymorphisms (SNPs) and the presence or absence of deletion regions (MTB-deleted region 1 or regions of difference) within MTBC genomes have identified several lineages and sub-lineages with distinct characteristics and distributions.Citation2 For instance, lineage 2 (East Asian) and 4 (Euro-American) strains are widely distributed, while lineage 3 (Central Asian and East African Indian) strains are restricted to particular regions in Asia and Africa. These three lineages are characterized by deleted regions and are denoted as “modern lineages.” In contrast, lineage 1 (Indo-Oceanic) strains are common in the Indian Ocean region and the Philippines. This lineage, together with lineages 5 and 6 (West African), and animal lineages comprise the “ancient lineages” and have no deletions characteristic of the modern lineages.Citation2,Citation11 Finally, lineage 7 is considered as an intermediate lineage and has recently reported in Ethiopia.Citation12
Genomic differences among MTBC lineages impact their capability to cause disease and develop drug resistance. For instance, members of the modern lineages are associated with greater disease burden and drug resistance than the ancient lineages, likely due to a high rate of accumulating spontaneous mutations during replication.Citation13,Citation14 Epidemiological studies have illustrated that lineage 2 strains have a higher rate of developing resistance ranging from 1.6 × 10−5 to 5.4 × 10−3 than lineage 1.Citation15
Despite the accumulation of spontaneous mutations among MTBC lineages, their specific (and perhaps unique) drug resistance mechanisms remain unknown. However, increased mutations in DNA repair system and SOS response genes within lineage 2 genomes enhance the possibility of these strains developing resistance and generating mutator phenotype.Citation14,Citation16 Nonetheless, these findings cannot sufficiently explain several enigmas in these strains, such as how the same mutation among MTBC can generate a different level of resistance. Moreover, how do some strains tolerate certain mutations better than others, and why are some strains more strongly associated with infectious transmission and outbreaks? These questions could be resolved by studies of compensatory evolution, epistasis, and clonal interference, as these mechanisms could have large impact on mutation rate acceleration and MTB fitness modulation.Citation17–Citation21
Compensatory evolution and MTB drug resistance
Drug concentration is a primary determinant of resistance-associated mutations during drug therapy.Citation22 Mutations develop when the drug concentration is not optimal, although mutations impose a fitness cost on bacteria that targets genes encoding essential biological functions, often leading to reduced bacterial growth, survival, and virulence.Citation23,Citation24 Contrary to fitness cost concept, several studies have documented that some frequently transmitted MTB strains undergo low-or no-cost mutations but exhibit high level of resistance to drugs.Citation17,Citation19,Citation20 Thus, these strains may harbor resistance mechanisms developed through compensatory evolution, which can modulate MTB fitness ().Citation25,Citation26
Figure 1 Development of drug resistance.
Notes: 1, when MTB acquires mutations during therapy, they reduce MTB fitness (clones A and B). 2, an acquired secondary mutation restores MTB fitness. 3, the epistatic interaction between mutations improves MTB fitness and maintains drug resistance within the specific MTB genetic background. 4, clonal interference determines clone fate via competition, which leads to emergence of the most dominate clone with drug resistance in the population and elimination of the clone with a lower mutational effect.
Abbreviation: MTB, Mycobacterium tuberculosis.
Compensatory evolution is mediated by the acquiring of a second mutation that minimizes the deleterious effect (resistance cost) of the original mutation. This mechanism allows MTB to increase its fitness without losing the resistance phenotype.Citation27 Compensatory evolution can develop from either additional or alternative mutations that occur in intra- or extragenic loci.Citation28
In MTB, compensatory evolution associated with INH resistance occurs when a mutation in the regulatory region of ahpC leads to overexpression of alkyl hydroperoxide reductase (AhpC), which may compensate for the fitness cost of Ser315Thr mutation in katG, normally encodes a catalase-peroxidase, converting INH into a bioactive form.Citation29
Compensatory evolution of RIF resistance has also been reported.Citation30,Citation31 In one study, mutations in rpoB, which encodes the β subunit of RNA polymerase, were detected in 95% of clinical isolates and conferred a high level of RIF resistance, but have also been associated with noticeable fitness cost.Citation17,Citation30,Citation32 However, S531L mutation was seen in most MDR isolates that exhibited a no- or low-cost fitness effect. This phenomenon is explained by the acquisition of compensatory mutations in neighboring rpoA and rpoC genes, which can mitigate the fitness cost of S531L.Citation33–Citation36 Comas et al found that up to 30% of MDR cases in high MTB burden countries carried mutations in rpoA and rpoC, suggesting they may play a role in spreading MDR strains in these countries.Citation33 Interestingly, a recent study revealed the compensatory role of an intragenic V615M mutation, located in rpoB gene, with respect to RIF resistance-associated rpoB mutations. The study showed that V615M mutation can modulate RNA polymerase bridge helix structure and contribute to an increased rate of transcription elongation, thus compensating for defective RNA polymerase activity associated with S531L mutation.Citation37
In addition to compensatory mutations, alternative mechanisms of fitness compensation may exist. For example, Freihofer et alCitation38 found that altered gene regulation can also reduce the deleterious effects of certain genetic mutations. The study found that emergence of the A1408G mutation in the 16S rRNA gene is accompanied by overexpression of tlyA, which encodes a methyltransferase, resulting in methylation of neighboring 16S rRNA position C1409 and increased MTB fitness. The identification of non-mutational mechanisms could provide a new strategy for optimizing current treatment regimens through inhibition of the compensatory event, leading to disruption of the stabilization of drug resistance transmission.
Role of epistasis and genetic background in MTB drug resistance
Epistasis occurs when several mutations interact with each other to express new advantageous traits for an organism and are often necessary for bacteria to modify their fitness cost.Citation39 During epistatic interactions, the effect of multiple mutations is greater or less than the effect of the individual mutation and can lead to either beneficial or deleterious phenotypes.Citation7 Thus, epistasis is classified as 1) positive (antagonistic), 2) negative (synergistic), or 3) sign.Citation40
In positive epistasis, the net fitness of the interactions is higher than expected ().Citation83 A study has reported the role of positive epistasis in drug resistance development in MTB. Borrell et alCitation41 identified a particular combination of mutations in rpoB and gyrA that conferred resistance to RIF and ofloxacin (OFX). The study showed that a gyrA D94G mutation is associated with improving deleterious fitness effects. Thus, gyrA D94G is correlated with positive epistasis in MTB, and it is frequently occurred within XDR strains.
Figure 2 Forms of epistatic interaction between mutations.
Note: (↑) indicated high MTB fitness and (↓) indicated low MTB fitness.
Abbreviation: MTB, Mycobacterium tuberculosis.
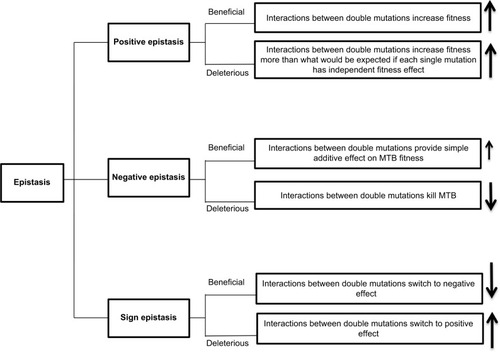
Contrary to positive epistasis, negative epistasis is characterized by fitness lower than expected after mutation interactions. In bacteria, interactions between beneficial mutations provide a simple additive effect on fitness, while interactions between deleterious mutations are lethal ().Citation83 However, in sign epistasis, the fitness of mutations depends on the genetic background of the bacteria. These mutations can be deleterious, beneficial, or neutral. Reciprocal sign epistasis is an extreme form of the interactions in which beneficial mutations together exert a negative effect or when deleterious mutations become positive ().Citation40,Citation42 The interaction between compensatory and drug resistance mutations is an example of sign epistasis.Citation43 When compensatory mutations occur in a susceptible genetic background, they become deleterious. Thus, the acquisition of resistance mutations promotes the epistatic interaction between compensatory and acquired resistance mutations, which results in the emergence and maintenance of the resistance phenotype in that particular bacterial genetic background.Citation44
In addition, increasing evidence supports the role of sign epistasis in MTBC diversity.Citation43 This is mainly due to the epistatic interactions between the mutations within the MTBC genetic background, the acquired resistance mutations, and the compensatory mutations.Citation45 Fenner et alCitation46 found that a mutation in either katG or inhA confers different levels of resistance. Lineage 2 strains carry mutations in both katG (high INH resistance) and inhA (low INH resistance) genes and show different levels of drug resistance compared to lineage 1 bacteria, in which only the inhA mutation has been identified. Similarly, when bacteria from different lineages were exposed to the same dose of RIF, they exhibited different fitness costs and resistance levels.Citation17 These data support the role of epistasis interactions between MTBC genetic background and acquired mutations that confer various levels of resistance across MTBC lineages.
Dynamics of clonal interference in MTB
Depending on the bacterial population size, mutation rate (U), and distribution of fitness effects, various mutations can simultaneously develop in a single population.Citation47 In this situation, clonal interference may occur and significantly impact resistance development in the population. When two distinct resistance mutations develop independently within distinctive bacterial individuals, they compete with each other. Thus, a clone with a greater mutation effect outcompetes a clone with smaller mutation effects, which is then eliminated from the population ().Citation48
The intra-host evolution of MTB provides a straightforward model for understanding clonal interference in vivo. Some studies have reported competition between MTB clones in a single patient sample. Sun et alCitation47 examined seven isolates from three patients; the first patient was free from MTB drug resistance, but after 19 months of treatment, four independent mutations were detected: three mutations in katG and one mutation in the regulatory region of inhA. After 5 months, most of the mutations reverted, and only one mutation in katG was detected. The second patient harbored MTB with a mutation in rpoB (L533P) but was still sensitive to RIF. After 18 months, the L533P mutation was replaced with a second mutation in rpoB (H526Y), leading to RIF-resistant MTB. The third patient was a relapsed case of MTB with two unfixed mutations of ethA (L35R and A341E) after 11 months of treatment that showed no change in EMB resistance status. These observations illustrate how the competition and interchange between resistance-related mutations can lead to MDR. Similarly, Eldholm et alCitation49 followed an XDR-TB patient for 3.5 years and performed genome sequencing of nine isolates from the same patient. They observed a high level of heterogeneity in the isolate population: 35 mutations were identified, including 20 transient and 15 fixed mutations. Eventually, 12 mutations were determined to be drug resistance related, although only seven of these mutations reached fixation stage. This observation indicates that the competition between high and low effective resistance mutations lead to high resistance.
Alternative mechanisms implicated in drug resistance
MTB shows intrinsic resistance to different drugs through various mechanisms, including cell wall or membrane impermeability and efflux pump action. Mutations can enhance intrinsic resistance, generate new proteins that inactivate the drug or block interactions with its target, or alter the target to prevent its recognition by the drugs ().Citation50
Figure 3 MTB can exhibit resistance to drugs via: 1, intrinsically decreased permeability of the cell wall; 2, acquisition of mutations that block drug entry; 3, extrusion drugs via efflux pumps; 4, modification of the drug or its target, or 5, target mimicry.
Note: Possible drug targets are indicated by red circles.
Abbreviations: INH, isoniazid; MTB, Mycobacterium tuberculosis; TB, tuberculosis.
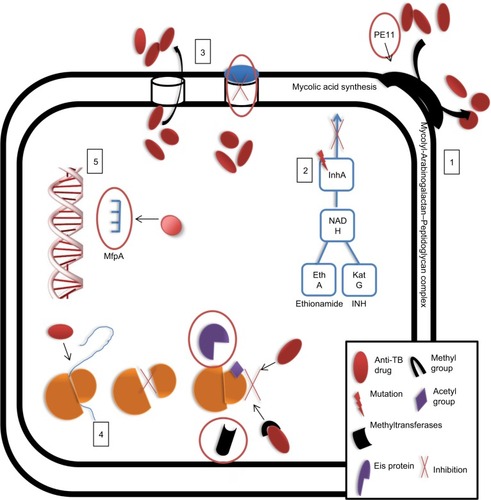
Impermeability of the MTB cell wall and drug resistance
The MTB cell wall structure is unique due to mycolylarabinogalactan–peptidoglycan complexes and free glycolipids (e.g., trehalose dimycolate, PPE family proteins, and phthiocerol dimycocerosates), forming a hydrophobic barrier that limits the entrance of various drugs.Citation51 Several studies have identified the essential genes, enzymatic activities, and cellular components that increase resistance levels by decreasing cell wall permeability. For example, the expression of monooxygenase (mymA) operon is regulated by virS, which maintains mycolic acid composition and enhances cell wall integrity.Citation52 Mutations in virS-mymA lead to increased cell wall permeability and diffusion of INH, RIF, PZA, and ciprofloxacin inside the cells.Citation53–Citation56
The PE11 protein of MTB plays a role in cell wall maintenance and is a putative lipase/esterase involved in cell wall remodeling.Citation57 Expression of PE11 in M. smegmatis mcCitation2155 modulates cell wall morphology, composition, aggregation, and pellicle formation and mediates resistance to INH, RIF, EMB, vancomycin, and ampicillin. These findings suggest that upregulation of PE11 in MTB reduces penetration of antibiotics during active TB.Citation58
Moreover, MTB can preserve cell wall integrity through acquired mutations that lead to overexpression of genes encoding enzymes involved in cell wall synthesis. For example, pro-drugs, INH and ethionamide, which share a similar mechanism of action, are converted into their bioactive forms (isonicotinic-acyl radicals and 2-ethyl-4-amidopyridine, respectively) by katG and ethA gene products. These bioactive forms react with nicotinamide adenine dinucleotide to form nicotinamide adenine dinucleotide adducts that bind to enoyl-acyl carrier protein reductase (encoded by inhA), a key enzyme involved in fatty acid synthase II system, and inhibit mycolic acid synthesis.Citation50,Citation59 Therefore, mutations in inhA prevent binding of INH and ethionamide to their targets and confer resistance to these drugs.Citation60 Furthermore, arabinosyl transferases (which link peptidoglycan with an outer mycolic acid layer to form the mycolyl-arabinogalactan–peptidoglycan complex) may be a target for EMB, a drug that inhibits arabinosyl transferase activity and causes increased cell wall permeability.Citation61 When emb genes acquire mutations, overexpression of emb genes and increased EMB proteins can overcome certain levels of the drug. A specific mutation in embB at codon 306 may correlate with EMB resistance, and mutations in cell wall synthesis-associated genes aftA and ubiA lead to overexpression of embC and EmbCAB substrates and subsequent resistance to EMB.Citation62–Citation64 In addition, novel mutations at embA G43C and G554N and at embB S412P have found to confer a high level of resistance to EMB among MTB isolates.Citation65,Citation66
Overall, these findings highlight the role of MTB cell wall maintenance in intrinsic and acquired drug resistance. Thus, identification of novel proteins (e.g., PE11) that increase the cell wall integrity could facilitate their use as promising drug targets for MTB treatment.
Action of efflux pumps and drug resistance
Efflux pumps are natural drug barriers widely distributed in both prokaryotic and eukaryotic cell walls. These pumps maintain cellular hemostasis and regulate exchange of nutrients across the cell membrane.Citation67 They are classified into six major families based on their energy source, size, and substrates. These include the ATP-binding cassette (ABC), small multidrug resistance (SMR), resistance nodulation division (RND), major facilitator superfamily (MFS), multidrug toxic compound extrusion (MATE), and drug metabolite transporter (DMT) superfamily.Citation68 All but except MATE and DMT efflux pumps are specific to MTB strains.Citation69 Drug resistance mediated by efflux pumps depends on their basal expression and by drug-induced gene expression or overexpression that result from acquired mutations.Citation70
Recently, several studies have used whole-genome sequencing to identify relevant mutations in efflux pump-associated genes that confer resistance. Li et alCitation70 examined the expression level of efflux pump genes within MDR isolates and found that at least one efflux pump was overexpressed in eight out of nine isolates, suggesting that this system contributed to the development of resistance to multiple drugs. Interestingly, one MDR isolate carried a mutation in rpoB that conferred RIF resistance but intact katG, inhA, and oxyR-ahpC, indicating that efflux pumps, rather than mutations alone, were responsible for the INH resistance. An additional study showed that mutations within the Rv0678-encoded transcription repressor of MmpL5 (RND family) led to overexpression of the MmpL5 pump and resistance to clofazimine.Citation71 Similarly, Kanji et alCitation72,Citation73 examined SNPs associated with Rv2688c, Rv0194, Rv2936 (drrA), Rv2937 (drrB), and Rv1634, which encode pumps from the ABC and MFS transporter families, within XDR strains. The study showed significant levels of gene expression compared to susceptible and H37Rv strains, thereby demonstrating the importance of efflux pumps in drug resistance development in XDR isolates. Another study explored the expression of Rv2686c, Rv2687c, Rv2688c, Rv0933c, and Rv1258c within MDR and XDR isolates and found high expression of the Rv0933c-encoded PstB pump (ABC transporter family) in response to FQ treatment, suggesting that the expression of pstB is associated with resistance to FQ. In addition, MFS Tap efflux pumps showed a high level of expression, suggesting a correlation with kanamycin resistance.Citation74 Expression of Rv1258c-encoded Tap pumps is regulated by transcription activator WhiB7, which is more highly expressed following a point mutation in the 5′ untranslated region of whiB7.Citation75–Citation78
These findings confirm that efflux pumps strongly contribute to developing drug resistance in MTB, thus highlighting efflux pumps as potential candidate targets for novel MTB drugs. Diverse synthetic and plant-derived molecules that act as efflux pump inhibitors (EPIs) have recently been identified, all of which exert different levels of efflux pump inhibition in MTB.Citation79 When these inhibitors bind to efflux pumps, they increase drug retention inside the cytoplasm, restore the drug’s activity, and prevent the selection of resistant mutants.Citation79 These results occur when EPIs act as a single drug such as an SQ109 inhibitor, or enhances the efficacy of certain drug combinations such as timcodar.Citation80,Citation81 More recently, Kumar et alCitation82 found that synthesis and design of hybrid EPIs by fusion of Verapamil™ with phenothiazines enhances inhibition of efflux pumps and may enable identification of novel molecules with a high efficacy for killing MTB.
Modification of drugs and their respective targets
An additional mechanism implicated in MTB drug resistance is the modification of either the drug or its target through specific enzymes. These enzymes are often enable acetylation or methylation of the drug or its target to prevent recognition and interaction between the two. One study found that MTB and M. bovis carry erm37, which encodes an rRNA methyltransferase that blocks interactions between macrolides and the ribosome.Citation83 MTB also expresses enhanced intracellular survival (EIS) proteins, which are homologs of AG acetyltransferases.Citation84 These proteins can acetylate multiple sites of AGs, resulting in their inactivation.Citation85 Houghton et alCitation87 found that EIS proteins can also protect MTB against capreomycin.Citation86 A more recent study conducted by Warrier et al determined that MTB can methylate drugs via N-methylation, such as inactivation of the “14” drug at the N-5 position via a methyltransferase encoded by Rv0560c.Citation93
Target mimicry and drug resistance
Target mimicry is a novel mechanism developed by MDR strains to detoxify FQ drugs, which target DNA gyrase. When FQs bind to DNA gyrase, they inhibit DNA replication, repair, and transcription.Citation88 A previous study showed that FQ resistance develops through the Mycobacteria FQ resistance protein A (MfpA).Citation89 MfpA can resemble the shape, size, and surface of the DNA double helix, suggesting that MfpA mimics the structure of MTB DNA.Citation90,Citation91 Once MfpA binds to DNA gyrase, the protein prevents FQ binding and protects MTB from the drug’s action.Citation92
Conclusion
The ongoing evolution of resistance mechanisms among MTB population is a serious concern. In this review, we highlighted the major mechanisms that lead to drug resistance. These mechanisms include compensatory evolution, epistasis, clonal interference, decreased cell wall permeability, overexpression of efflux pumps, drug/target modification, and target mimicry. These mechanisms allow MTB to modulate their fitness, enhance their transmissibility, and stabilize the resistance phenotype within their population. Understanding of these mechanisms enable researchers to identify novel drug targets in order to develop effective drugs.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationGlobal tuberculosis report 2016GenevaWorld Health Organization2016 Available from: http://www.who.int/tb/publications/global_report/en/Accessed October 4, 2017
- BritesDGagneuxSCo-evolution of Mycobacterium tuberculosis and Homo sapiensImmunol Rev2015264162425703549
- GutierrezMCBrisseSBroschRAncient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosisPLoS Pathog20051119
- HershbergRLipatovMSmallPMHigh functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demographyPLoS Biol20086120060311
- ComasICoscollaMLuoTOut-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humansNat Genet201345101176118223995134
- DeloguGSaliMFaddaGThe biology of Mycobacterium tuberculosis infectionMediterr J Hematol Infect Dis201351070
- FonsecaJDKnightGMMcHughTDThe complex evolution of antibiotic resistance in Mycobacterium tuberculosisInt J Infect Dis2015329410025809763
- CombsDLO’BrienRJGeiterLJUSPHS tuberculosis short-course chemotherapy trial 21: effectiveness, toxicity, and acceptability. The report of final resultsAnn Intern Med199011263974062155569
- EspinalMAKimSJSuarezPGStandard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countriesJAMA2000283192537254510815117
- GuntherGMultidrug-resistant and extensively drug-resistant tuberculosis: a review of current concepts and future challengesClin Med2014143279285
- CoscollaMGagneuxSConsequences of genomic diversity in Mycobacterium tuberculosisSemin Immunol201426643144425453224
- CollFMcNerneyRGuerra-AssuncaoJAA robust SNP barcode for typing Mycobacterium tuberculosis complex strainsNat Commun20145481225176035
- BorrellSGagneuxSInfectiousness, reproductive fitness and evolution of drug-resistant Mycobacterium tuberculosisInt J Tuberc Lung Dis200913121456146619919762
- McGrathMGey van PittiusNCvan HeldenPDWarrenRMWarnerDFMutation rate and the emergence of drug resistance in Mycobacterium tuberculosisJ Antimicrob Chemother201469229230224072169
- MerkerMKohlTARoetzerAWhole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patientsPLoS One2013812e8255124324807
- Dos VultosTMestreORauzierJEvolution and diversity of clonal bacteria: the paradigm of Mycobacterium tuberculosisPLoS One2008320001538
- GagneuxSLongCDSmallPMVanTSchoolnikGKBohannanBJThe competitive cost of antibiotic resistance in Mycobacterium tuberculosisScience200631257821944194616809538
- BillingtonOJMcHughTDGillespieSHPhysiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosisAntimicrob Agents Chemother19994381866186910428904
- DaviesAPBillingtonOJBannisterBAWeirWRMcHughTDGillespieSHComparison of fitness of two isolates of Mycobacterium tuberculosis, one of which had developed multi-drug resistance during the course of treatmentJ Infect200041218418711023769
- SanderPSpringerBPrammanananTFitness cost of chromosomal drug resistance-conferring mutationsAntimicrob Agents Chemother20024651204121111959546
- BottgerECSpringerBPletschetteMSanderPFitness of antibiotic-resistant microorganisms and compensatory mutationsNat Med1998412134313449846553
- AnderssonDIHughesDPersistence of antibiotic resistance in bacterial populationsFEMS Microbiol Rev201135590191121707669
- SprattBGAntibiotic resistance: counting the costCurr Biol1996610121912218939559
- OlofssonSKCarsOOptimizing drug exposure to minimize selection of antibiotic resistanceClin Infect Dis2007145S129S136
- GagneuxSBurgosMVDeRiemerKImpact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosisPLoS Pathog20062616
- van DoornHRde HaasPEKremerKVandenbroucke-GraulsCMBorgdorffMWvan SoolingenDPublic health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino-acid position 315 of katG: a decade of experience in The NetherlandsClin Microbiol Infect200612876977516842572
- AnderssonDIHughesDAntibiotic resistance and its cost: is it possible to reverse resistance?Nat Rev Microbiol20108426027120208551
- AnderssonDILevinBRThe biological cost of antibiotic resistanceCurr Opin Microbiol19992548949310508723
- ShermanDRMdluliKHickeyMJCompensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosisScience19962725268164116438658136
- RamaswamySMusserJMMolecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 updateTuber Lung Dis199879132910645439
- SomoskoviAParsonsLMSalfingerMThe molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosisRespir Res20012316416811686881
- MariamDHMengistuYHoffnerSEAnderssonDIEffect of rpoB mutations conferring rifampin resistance on fitness of Mycobacterium tuberculosisAntimicrob Agents Chemother20044841289129415047531
- ComasIBorrellSRoetzerAWhole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genesNat Genet201144110611022179134
- YangCLuoTShenXTransmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigationLancet Infect Dis201717327528427919643
- de VosMMullerBBorrellSPutative compensatory mutations in the rpoC gene of rifampin-resistant Mycobacterium tuberculosis are associated with ongoing transmissionAntimicrob Agents Chemother201357282783223208709
- BrandisGPietschFAlemayehuRHughesDComprehensive phenotypic characterization of rifampicin resistance mutations in Salmonella provides insight into the evolution of resistance in Mycobacterium tuberculosisJ Antimicrob Chemother201570368068525362573
- MeftahiNNamouchiAMhenniBBrandisGHughesDMardassiHEvidence for the critical role of a secondary site rpoB mutation in the compensatory evolution and successful transmission of an MDR tuberculosis outbreak strainJ Antimicrob Chemother201671232433226538504
- FreihoferPAkbergenovRTeoYJuskevicieneRAnderssonDIBottgerECNonmutational compensation of the fitness cost of antibiotic resistance in mycobacteria by overexpression of tlyA rRNA methylaseRNA201622121836184327698071
- PhillipsPCEpistasis–the essential role of gene interactions in the structure and evolution of genetic systemsNat Rev Genet200891185586718852697
- WongAEpistasis and the evolution of antimicrobial resistanceFront Microbiol2017824628261193
- BorrellSTeoYGiardinaFEpistasis between antibiotic resistance mutations drives the evolution of extensively drug-resistant tuberculosisEvol Med Public Health201316574
- TrindadeSSousaAXavierKBDionisioFFerreiraMGGordoIPositive epistasis drives the acquisition of multidrug resistancePLoS Genet20095724
- BorrellSGagneuxSStrain diversity, epistasis and the evolution of drug resistance in Mycobacterium tuberculosisClin Microbiol Infect201117681582021682802
- SchragSJPerrotVLevinBRAdaptation to the fitness costs of antibiotic resistance in Escherichia coliProc Biol Sci19972641386128712919332013
- MullerBBorrellSRoseGGagneuxSThe heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosisTrends Genet201329316016923245857
- FennerLEggerMBodmerTEffect of mutation and genetic background on drug resistance in Mycobacterium tuberculosisAntimicrob Agents Chemother20125663047305322470121
- SunGLuoTYangCDynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patientsJ Infect Dis2012206111724173322984115
- MullerHJSome genetic aspects of sexAm Nat193266703118138
- EldholmVNorheimGvon der LippeBEvolution of extensively drug-resistant Mycobacterium tuberculosis from a susceptible ancestor in a single patientGenome Biol2014151149025418686
- GreenKDGarneau-TsodikovaSResistance in tuberculosis: what do we know and where can we go?Front Microbiol2013420823888158
- FavrotLRonningDRTargeting the mycobacterial envelope for tuberculosis drug developmentExpert Rev Anti Infect Ther20121091023103623106277
- ForrelladMAKleppLIGioffreAVirulence factors of the Mycobacterium tuberculosis complexVirulence20134136623076359
- SinghAGuptaRVishwakarmaRARequirement of the mymA operon for appropriate cell wall ultrastructure and persistence of Mycobacterium tuberculosis in the spleens of guinea pigsJ Bacteriol2005187124173418615937179
- SinghAJainSGuptaSDasTTyagiAKmymA operon of Mycobacterium tuberculosis: its regulation and importance in the cell envelopeFEMS Microbiol Lett20032271536314568148
- GaoLYLavalFLawsonEHRequirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: implications for therapyMol Microbiol20034961547156312950920
- PhilalayJSPalermoCOHaugeKARustadTRCangelosiGAGenes required for intrinsic multidrug resistance in Mycobacterium aviumAntimicrob Agents Chemother20044893412341815328105
- SinghGSinghGJadejaDKaurJLipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model systemCrit Rev Microbiol201036325926920500016
- SinghPRaoRNReddyJRPE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulenceSci Rep201662162426902658
- MarrakchiHLaneelleMADaffeMMycolic acids: structures, biosynthesis, and beyondChem Biol2014211678524374164
- BanerjeeADubnauEQuemardAinhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosisScience199426351442272308284673
- TelentiAPhilippWJSreevatsanSThe emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutolNat Med1997355675709142129
- AlcaideFPfyfferGETelentiARole of embB in natural and acquired resistance to ethambutol in mycobacteriaAntimicrob Agents Chemother19974110227022739333060
- SafiHSayersBHazbonMHAllandDTransfer of embB codon 306 mutations into clinical Mycobacterium tuberculosis strains alters susceptibility to ethambutol, isoniazid, and rifampinAntimicrob Agents Chemother20085262027203418378710
- SafiHLingarajuSAminAEvolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-beta-D-arabinose biosynthetic and utilization pathway genesNat Genet201345101190119723995136
- ZhaoLLSunQLiuHCAnalysis of embCAB mutations associated with ethambutol resistance in multidrug-resistant Mycobacterium tuberculosis isolates from ChinaAntimicrob Agents Chemother20155942045205025605360
- XuYJiaHHuangHSunZZhangZMutations Found in embCAB, embR, and ubiA genes of ethambutol-sensitive and -resistant Mycobacterium tuberculosis clinical isolates from ChinaBiomed Res Int20159517061031
- NguyenLAntibiotic resistance mechanisms in M. tuberculosis: an updateArch Toxicol20169071585160427161440
- SunJDengZYanABacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitationsBiochem Biophys Res Commun2014453225426724878531
- LiuHXieJPComparative genomics of Mycobacterium tuberculosis drug efflux pumps and their transcriptional regulatorsCrit Rev Eukaryot Gene Expr201424216318024940769
- LiGZhangJGuoQEfflux pump gene expression in multidrug-resistant Mycobacterium tuberculosis clinical isolatesPLoS One2015102e011901325695504
- ZhangSChenJCuiPShiWZhangWZhangYIdentification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosisJ Antimicrob Chemother20157092507251026045528
- KanjiAHasanRZaverAAlternate efflux pump mechanism may contribute to drug resistance in extensively drug-resistant isolates of Mycobacterium tuberculosisInt J Mycobacteriol20165suppl 1S97S9828043640
- KanjiAHasanRZhangYIncreased expression of efflux pump genes in extensively drug-resistant isolates of Mycobacterium tuberculosisInt J Mycobacteriol20165suppl 1S15028043519
- OhTSKimYJKangHYKimCKChoSYLeeHJRNA expression analysis of efflux pump genes in clinical isolates of multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in South KoreaInfection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases201749111115
- ReevesAZCampbellPJSultanaRAminoglycoside cross-resistance in Mycobacterium tuberculosis due to mutations in the 5′ untranslated region of whiB7Antimicrob Agents Chemother20135741857186523380727
- AinsaJABlokpoelMCOtalIYoungDBDe SmetKAMartinCMolecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosisJ Bacteriol199818022583658439811639
- BurianJRamon-GarciaSSweetGGomez-VelascoAAv-GayYThompsonCJThe mycobacterial transcriptional regulator whiB7 gene links redox homeostasis and intrinsic antibiotic resistanceJ Biol Chem2012287129931022069311
- MorrisRPNguyenLGatfieldJAncestral antibiotic resistance in Mycobacterium tuberculosisProc Natl Acad Sci U S A200510234122001220516103351
- SongLWuXDevelopment of efflux pump inhibitors in antituberculosis therapyInt J Antimicrob Agents201647642142927211826
- de KnegtGJvan der MeijdenAde VogelCPAarnoutseREde SteenwinkelJEActivity of moxifloxacin and linezolid against Mycobacterium tuberculosis in combination with potentiator drugs verapamil, timcodar, colistin and SQ109Int J Antimicrob Agents201749330230728162983
- GrossmanTHShoenCMJonesSMJonesPLCynamonMHLocherCPThe efflux pump inhibitor timcodar improves the potency of antimycobacterial agentsAntimicrob Agents Chemother20155931534154125534740
- KumarMSinghKNaranKDesign, synthesis, and evaluation of novel hybrid efflux pump inhibitors for use against Mycobacterium tuberculosisACS infectious diseases201621071472527737555
- BuriankovaKDoucet-PopulaireFDorsonOMolecular basis of intrinsic macrolide resistance in the Mycobacterium tuberculosis complexAntimicrob Agents Chemother200448114315014693532
- ZaunbrecherMASikesRDJrMetchockBShinnickTMPoseyJEOverexpression of the chromosomally encoded aminoglycoside acetyltransferase Eis confers kanamycin resistance in Mycobacterium tuberculosisProc Natl Acad Sci U S A200910647200042000919906990
- ChenWBiswasTPorterVRTsodikovOVGarneau-TsodikovaSUnusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TBProc Natl Acad Sci U S A2011108249804980821628583
- HoughtonJLGreenKDPricerREMayhoubASGarneau-TsodikovaSUnexpected N-acetylation of capreomycin by mycobacterial Eis enzymesJ Antimicrob Chemother201368480080523233486
- MaloneKMGordonSVAntibiotic methylation: a new mechanism of antimicrobial resistanceTrends Microbiol2016241077177227593675
- AndrioleVTThe quinolones: past, present, and futureClin Infect Dis20051541S113S119
- MonteroCMateuGRodriguezRTakiffHIntrinsic resistance of Mycobacterium smegmatis to fluoroquinolones may be influenced by new pentapeptide protein MfpAAntimicrob Agents Chemother200145123387339211709313
- FerberDBiochemistry. Protein that mimics DNA helps tuberculosis bacteria resist antibioticsScience20053085727139315933168
- HegdeSSVettingMWRoderickSLA fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNAScience200530857271480148315933203
- VettingMWHegdeSSFajardoJEPentapeptide repeat proteinsBiochemistry200645111016388575
- WarrierTKapilashramiKArgyrouAN-methylation of a bactericidal compound as a resistance mechanism in Mycobacterium tuberculosisProc Natl Acad Sci USA201611331E4523E453027432954
