Abstract
Purpose
Methicillin resistant Staphylococcus aureus CC15 strains (CC15-MRSA) have only been sporadically described in literature. This study was carried out to describe the genetic make-up for this rare MRSA strain.
Methods
Four CC15-MRSA isolates collected in Riyadh, Saudi Arabia, between 2013 and 2014 were studied. Two isolates were from clinical infection and 2 from retail meat products. Whole genome sequencing was carried out using Illumina HiSeq2500 genome analyzer.
Results
All the CC15-MRSA isolates had the multilocus sequence typing profile ST1535, 13–13-1–1-81-11-13, which is a single locus variant of ST15. Of the 6 contigs related to the SCC element, one comprised a recombinase gene ccrAA, ccrC-PM1, fusC and a helicase, another one included mvaS, dru, mecA and 1 had yobV and Q4LAG7. The SCC element had 5 transposase genes, namely 3 identical paralogs of tnpIS431 and 2 identical paralogs of tnpIS256. Two identical copies of a tnpIS256-based insertion element flank the aacA-aphD gene. Two copies of this insertion element were present with 1 located in the SCC element and another inserted into the sasC gene. A short 3 kb region, which lacks any bacteriophage structural genes and site-specific DNA integrase, was inserted into the hlb gene. The hsdM and the 5’-part of the hsdS gene are replaced by a copy of the hsdM/hsdS paralogs from νSaβ giving rise to a new chimeric paralog of hsdS in νSaα.
Conclusion
CC15-MRSA shows a novel SCCmecV/SCCfus composite element. Its variant of hsdM/hsdS probably facilitated uptake of foreign mobile genetic elements that promoted emergence of CC15-MRSA. Close surveillance is needed to monitor spread and emergence of further CC15 MRSA strains.
Introduction
In recent years, the landscape of the molecular epidemiology of methicillin resistant Staphylococcus aureus (MRSA) has been characterized by the emergence and dissemination of new strains. Clonal complex 15 (CC15) is ubiquitous and widely described in the literature, but these isolates are mostly methicillin susceptible S. aureus (MSSA).Citation1 CC15-MSSA was recently identified as a predominant nasal colonizer in a report from Saudi Arabia.Citation2 Previously, methicillin resistant CC15 strains (CC15-MRSA) have only been sporadically described in literature.Citation3–Citation5 In a large scale genotyping study of MRSA isolates, no CC15-MRSA was identified.Citation1 Two isolates of CC15-MRSA associated with nasal colonization have been reported in Iran and Saudi Arabia.Citation3,Citation5 While whole genome sequencing data are available for CC15-MSSA, there are, to the best of our knowledge, no publications on the genomic data for the rare CC15-MRSA. Recently, we reported the first identification of CC15-MRSA from clinical infections and retail meat products in the Middle East.Citation6,Citation7 In light of the emergence of CC15-MRSA in our setting and to provide much-needed insight into the genetic make-up of this rare MRSA clone, we have carried out whole genome sequencing of these isolates.
Materials and methods
The human isolates were identified as part of a larger MRSA study for which ethical approval was obtained from the Institutional Review Board, King Khalid University Hospital, Riyadh, Kingdom of Saudi Arabia. Patient consent was waived as the study involved use of archived isolates from specimens submitted for routine diagnostic tests and without use of patient identifiers. Four CC15-MRSA isolates collected in Riyadh, Saudi Arabia between 2013 and 2014 were studied. Two isolates (RUH-2 and RUH-71) were from patients with sepsis and wound infection, respectively, while the other 2 (RUH-98 and RUH-99) were from retail camel meat. S. aureus identification and confirmation of methicillin resistance was performed as previously described.Citation6,Citation7 Genomic DNA was extracted using Qiagen DNA isolation kit (Qiagen, Hilden, Germany) in accordance with manufacturer’s instructions. Whole genome sequencing was carried out using the Illumina HiSeq2500 genome analyzer.
Sequencing reads were assembled de-novo with SPAdes and the final assembly was done with SPAdes version 3.10.1 (http://bioinf.spbau.ru/spades).Citation8 Contigs shorter than 500 nt were dropped. Reads were mapped to the SPAdes contigs but also to the reference sequence (ST15-MSSA strain ST20130938, GenBank: CP012972.1Citation9) with the Burrow– Wheeler aligner “bwa” using the local aligning algorithm “mem” (“bwa” version 0.7.12-r1039, https://github.com/lh3/bwa).Citation10 We also used “bwa-mem” to map the whole SPAdes contigs on the reference sequence (ST15-MSSA strain ST20130938, GenBank: CP012972.1). Read mappings and coverage were visually inspected with “tablet” (“tablet” version 1.14.10.21, https://ics.hutton.ac.uk/tablet/).Citation11 We manually scaffolded and annotated the contigs from isolate RUH-2, which cover the genomic islands of νSaβ and νSaα, a 3 kb element inserted into the hlb gene and the SCC element. We used the GenomeDiagram module from Biopython to draw sketches from the manually annotated sequences.Citation12 The reads and the SPAdes contigs were submitted to NCBI sequence database. The manually scaffolded and annotated regions were submitted to Genbank as short sequences.
Results
For each isolate, a de-novo assembly of the genomic sequence was carried out. The assemblies comprised 73 and 71 contigs for the human isolates RUH-2 and RUH-71, respectively. Isolates RUH-98 and RUH-99 from camel meat had 72 and 66 contigs, respectively. The overall G/C content for the chromosomal contigs was 33%. All the CC15-MRSA isolates had the MLST profile 13–13-1–1-81–11-13. All of the 4 isolates sequenced carried a 30-kb plasmid harboring additional antibiotic resistance genes, namely cadD, cadX, blaI, blaR, blaZ, lnuA, aadD. In addition, isolate (RUH-71, from human wound infection) harbored another putative plasmidic contig encoding tetK. A comparison of the genomic features of the 4 CC15-MRSA isolates reported in this study (RUH-2, RUH71, RUH-98, RUH-99), with CC15-MSSA sequences in the NCBI GenBank (VCU006, MPROS1797, 08–02119, ST20130938, ST20130940, ST20130941) is given in Table S1.
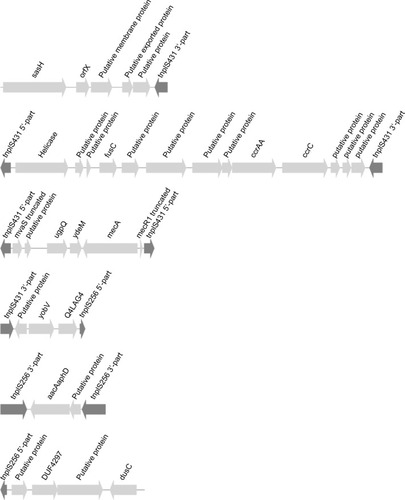
The 6 contigs related to the SCC element () were identical in all isolates. One contig comprised a recombinase gene “ccrAA”, ccrC-PM1, fusC and a helicase; another contig included mvaS, dru, mecA and 1 contig had yobV and Q4LAG7 (putative protein associated with SCCmec V/VT, GenBank AM990992.1: 52112 to 54100) (). The SCC element presumably comprises 5 transposase genes, namely 3 identical paralogs of tnpIS431 (size 675 nt) and 2 identical paralogs of tnpIS256 (size 1173 nt) (). Two identical copies of a tnpIS256-based insertion element flank the bifunctional kanamycin resistance determinant aacA-aphD. Two copies of this insertion element were present in the genome with 1 copy located in the SCC element and another copy inserted into the sasC gene encoding a surface protein.
Figure 1 CC15-MRSA SCC element.
Notes: Six contigs related to the SCC element (MF185204 to MF185209). The first contig comprises the flanking orfX gene, and the last contig the flanking dusC gene. In between, four contigs are shown carrying genes typically found in SCC elements. Contig 2 has the fusidic acid resistance gene fusC and the SCC recombinase genes ccrAA and ccrC. Contig 3 comprises the mecA gene cluster with mecR1 truncated by tnpIS431. Contig 5 constitutes a true insertion element, where the bifunctional kanamycin resistance determinant aacAaphD is flanked by two copies of tnpIS256.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
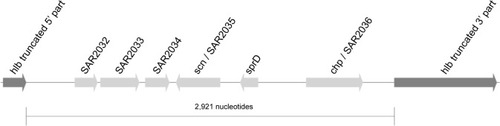
The CC15-MRSA isolates had a short 3 kb region inserted into the hlb gene (). The 3 kb insertion element lacks any bacteriophage structural genes and site-specific DNA integrase. The insertion element comprised 5 genes, including scn (staphylococcal complement inhibitor) and chp (chemotaxis inhibitor), but sak (staphylokinase) was absent ().
Figure 2 The hlb-3kb-insert in CC15-MRSA.
Notes: The hemolysin beta gene (hlb) is interrupted by a 3 kb insertion element in CC15-MRSA genomes.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
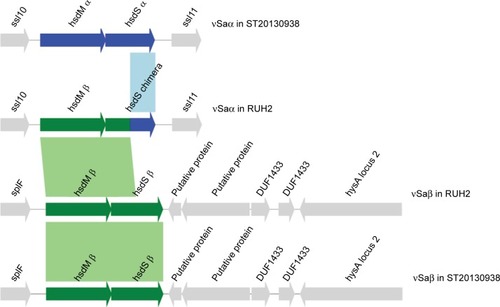
The CC15-MRSA isolates showed a variant of hsdM/hsdS at the major pathogenicity island νSaα compared with the reference CC15-MSSA genome (). The hsdM and the 5′-part of the hsdS gene were replaced by a copy of the hsdM/hsdS paralogs from νSaβ. This gives rise to a new chimeric paralog of hsdS in νSaα (). The chimeric hsdS has an intact reading frame. We can see this recombination in all of the 4 CC15-MRSA isolates. Furthermore, a Sau3AI restriction system is present in all of the CC15 isolates analyzed, while the type IV restriction system SauUSI is absent (Table S1).
Figure 3 hsdM/hsdS recombination in CC15-MRSA.
Notes: The Figure shows the contents of genomic islands νSaα and νSaβ in isolate RUH-2 (ST1535/CC15, MF185202, MF185203) and in ST20130938 (ST15/CC15, Genbank accession CP012972.1). The reference genome CP012972.1 comprises two distinct paralog of hsdM/hsdS in genomic islands alpha and beta. The mapping of the sequencing reads from isolate RUH-2 onto the reference sequence CP012972.1 reveals, that hsdM-alpha and the 5’-end of hsdS- alpha are missing in RUH-2, while the coverage of hsdM-beta and the 5′-end of hsdS-beta is doubled with respect to other chromosomal genes, indicating that this stretch of DNA is duplicated in RUH-2. We extracted the duplicated region of νSaβ from the SPAdes contigs and were able to link it to contigs mapping to νSaα.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
All the CC15-MRSA isolates had the MLST profile 13–13-1–1-81–11-13, which is a single locus variant of ST15. This MLST profile has been assigned to ST1535 (https://pubmlst.org/bigsdb?db=pubmlst_saureus_isolates&page=profiles) and comprises pta-81 instead of pta-12 in canonical ST15. This pta-81 differs from pta-12 by only 1 single-nucleotide polymorphism, which was present in all our isolates. Three of the 4 isolates assigned to ST1535 in the PUBMLST database are MSSA (https://pubmlst.org/bigsdb?db=pubmlst_sau-reus_isolates&page=profiles). The fourth is MRSA isolate MPROS1797, which has a similar SCC element as the CC15 MRSA in this study (https://www.ncbi.nlm.nih.gov/biosample/SAMEA2664415; Table S1). Due to the presence of repeats, the SCC element could not be scaffolded into a single contiguous sequence. The overall constellation of the SCC element as shown in was interpreted as a novel SCCmecV/SCCfus composite element. A very similar element has also been found by microarray hybridization in CC97-MRSA from Saudi Arabia.Citation13 Furthermore, reports from Saudi Arabia have described MRSA isolates from other lineages that also harbored SCCfus in addition to SCCmec IV or V elements.Citation6,Citation13 Insertion elements flanked by 2 antiparallel copies of a transposase are common in bacteria, and often found in association with antibiotic resistance genes. The sasC gene, which is interrupted by insertion of another copy of the tnpIS256-based insertion element, has been linked with biofilm production in S. aureus.Citation14
In S. aureus, an insert in the hlb gene is typically a prophage comprising several structural genes encoding the capsule, head and tail of the phage alongside an integrase at the terminus. It also frequently carries virulence associated genes like sea, sep (N315), see, chp, sak and scn in various combinations.Citation15 The Riyadh CC15-MRSA isolates (as well as other published CC15 genomes) had a short 3 kb region inserted into the hlb gene (). The absence of bacteriophage structural genes and site-specific DNA integrase suggests that it is no longer mobile but blocking the hlb insertion site. This insertion element seems to be a remnant of a bacteriophage as it is homologous to the terminus of the hlb converting phage in MRSA252 (Genbank accession BX571856.1, genes SAR2032 to SAR2036). In MRSA252, the hlb converting phage has a size of about 44 kb. However, we did not find any resemblance between the putative SCC elements in our CC15-MRSA isolates and the SCC element in MRSA252. MRSA252 has a full mec gene cluster comprising mecA/mecR1/mecI/mecR2, while the mec cluster is truncated in mecR1 in the CC15-MRSA isolates. MRSA252 has cassette recombinase ccrA-2/ccrB-2, while we find ccrC in the CC15-MRSA isolates. The sak gene is associated with tissue invasion.Citation16,Citation17 Its absence could be the reason why most CC15 isolates are associated with carriage rather than invasive infection.
Two distinct copies of the hsdM and hsdS genes are present in most genomes of S. aureus. These genes encode components of a type I restriction-modification (R-M) system (hsdM encodes a DNA methylase, hsdS encodes the specificity determinate). One pair of hsdM/hsdS is typically located in the genomic island νSaα between the superantigen-like genes ssl10 and ssl11. A second pair of hsdM/hsdS genes resides in genomic island νSaβ. The CC15-MRSA isolates showed a different variant of hsdM/hsdS at the major pathogenicity island νSaα compared with the reference CC15-MSSA genome. This has arisen presumably due to intrachromosomal recombination, resulting in a repertoire of hsdM/hsdS restriction enzymes that deviate from the CC15 parent. This might have played a role in acquiring an SCC element as bacteria use the type I R-M system to control uptake of foreign DNA. In the type I R-M system, the hsdM/hsdS gene products are required in this process.Citation18 Usually, the composition of the genomic island νSaα and νSaβ is highly conserved within clonal complexes. It has been shown that the type I R-M system has facilitated the evolution of distinct S. aureus lineages and controls the horizontal transfer of mobile genetic elements.Citation18 The Sau3A is a type II system that digests DNA at GATC sites and that was first described by Seeber et al.Citation19 Interestingly, Sau3AI is present in all CC15 isolates, while SauUSI is absent (Table S1). This is rather unusual as Sau3AI is uncommon in S. aureus. Indeed, most S. aureus isolates harbor the SauUSI (type IV) at the DNA locus for Sau3AI.Citation19,Citation20 Therefore, based on our findings, we suggest that changes in the hsdM/hsdS system and the type II R-M locus facilitated uptake of foreign mobile genetic elements, that is, of SCCmec/SCCfus by the ancestral CC15-MSSA promoting emergence of CC15-MRSA.
The limitation of our work is that the gaps between the contigs, which are presumably caused by repeated sequence elements, could not be resolved since the average fragment size of the Illumina library was only about 250 nt. Also, we were unable to determine the spa type reliably from our assembly since spa is a highly repetitive locus of a variable number of imperfect repeats. This genomic arrangement typically provokes artifacts in read assembly.
Accession numbers
The raw read sequences have been deposited in the Sequence Read Archive database (Bioproject PRJNA386092) with accession numbers: SAMN06925301, SAMN06925302, SAMN06925303, SAMN06925304.
De-novo assembled contigs have been deposited at DDBJ/ENA/GenBank under the accession NHZU00000,000, NHZV00000000, NHZW00000000, NHZX00000000. The version described in this paper is version NHZU01000000, NHZV01000000, NHZW01000000, NHZX01000000.
The manually scaffolded sequences for hlb_3kb_insert, νSaα, νSaβ and the 6 SCC element contigs have been submitted to the GenBank under the following accession numbers: MF185201, MF185202, MF185203, MF185204, MF185205, MF185206, MF185207, MF185208, MF185209
Conclusion
We provide the molecular characterization of a MRSA strain from a common lineage that until recently gave rise only to very few MRSA. The findings indicate that CC15-MRSA has a novel SCCmecV/SCCfus composite element. Changes in the hsdM/hsdS system and the type II R-M locus probably played role in the emergence of this rare MRSA strain. Close surveillance is needed, especially with regard to spread among humans and livestock in the Middle East and emergence of further CC15 MRSA strains.
Acknowledgments
This work was presented in part at the 27th European Congress of Clinical Microbiology and Infectious Diseases (22–25 April 2017, Vienna, Austria).
Supplementary material
Table S1 Comparison of CC15-MRSA and CC15-MSSA
Disclosure
The authors report no conflicts of interest in this work.
References
- MoneckeSCoombsGShoreACA field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureusPLoS One201164e1793621494333
- SarkarARajiAGaraweenGAntimicrobial resistance and virulence markers in methicillin sensitive Staphylococcus aureus isolates associated with nasal colonizationMicrob Pathog20169381226796298
- Abou ShadyHMBakrAEHashadMEAlzohairyMAStaphylococcus aureus nasal carriage among outpatients attending primary health care centers: a comparative study of two cities in Saudi Arabia and EgyptBraz J Infect Dis2015191687625523075
- CampanileFBongiornoDBorboneSStefaniSHospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) in ItalyAnn Clin Microbiol Antimicrob200982219552801
- Japoni-NejadARezazadehMKazemianHFardmousaviNvan BelkumAGhaznavi-RadEMolecular characterization of the first community-acquired methicillin-resistant Staphylococcus aureus strains from Central IranInt J Infect Dis20131711e949e95423706379
- SenokAEhrichtRMoneckeSAl-SaedanRSomilyAMolecular characterization of methicillin-resistant Staphylococcus aureus in nosocomial infections in a tertiary-care facility: emergence of new clonal complexes in Saudi ArabiaNew Microbes New Infect201614131827621823
- RajiMAGaraweenGEhrichtRMoneckeSShiblAMSenokAGenetic Characterization of Staphylococcus aureus Isolated from Retail Meat in Riyadh, Saudi ArabiaFront Microbiol2016791127375611
- BankevichANurkSAntipovDSPAdes: a new genome assembly algorithm and its applications to single-cell sequencingJ Comput Biol201219545547722506599
- Trouillet-AssantSLelievreLMartins-SimoesPAdaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patientsCell Microbiol201618101405141426918656
- LiHAligning sequence reads, clone sequences and assembly contigs with BWA-MEM2013 arXiv:1303.3997v2. Available from: https://arxiv.org/abs/1303.3997Accessed August 24, 2017
- MilneIStephenGBayerMUsing Tablet for visual exploration of second-generation sequencing dataBrief Bioinform201314219320222445902
- PritchardLWhiteJABirchPRTothIKGenomeDiagram: a python package for the visualization of large-scale genomic dataBioinformatics200622561661716377612
- MoneckeSSkakniLHasanRCharacterization of MRSA strains isolated from patients in a hospital in Riyadh, Kingdom of Saudi ArabiaBMC Microbiol20121214622823982
- SpezialePPietrocolaGFosterTJGeogheganJAProtein-based biofilm matrices in StaphylococciFront Cell Infect Microbiol2014417125540773
- van WamelWJRooijakkersSHRuykenMvan KesselKPvan StrijpJAThe innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophagesJ Bacteriol200618841310131516452413
- MoneckeSLuedickeCSlickersPEhrichtRMolecular epidemiology of Staphylococcus aureus in asymptomatic carriersEur J Clin Microbiol Infect Dis20092891159116519434432
- Wieckowska-SzakielMSadowskaBRozalskaBStaphylokinase production by clinical Staphylococcus aureus strainsPol J Microbiol20075629710217650679
- WaldronDELindsayJASau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineagesJ Bacteriol2006188155578558516855248
- SeeberSKesslerCGotzFCloning, expression and characterization of the Sau3AI restriction and modification genes in Staphylococcus carnosus TM300Gene199094137432227451
- XuSYCorvagliaARChanSHZhengYLinderPA type IV modification-dependent restriction enzyme SauUSI from Staphylococcus aureus subsp. aureus USA300Nucleic Acids Res201139135597561021421560
