Abstract
Background
Drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) has been associated with loss of viral suppression measured by a rise in HIV-1 RNA levels, a decline in CD4 cell counts, persistence on a failing treatment regimen, and lack of adherence to combination antiretroviral therapy.
Objectives
This study aimed to monitor the prevalence and risk factors associated with drug resistance in Taiwan after failure of first-line therapy.
Materials and methods
Data from the Veterans General Hospital Surveillance and Monitor Network for the period 2009–2014 were analyzed. Plasma samples from patients diagnosed with virologic failure and an HIV-1 RNA viral load >1000 copies/mL were analyzed by the ViroSeq™ HIV-1 genotyping system for drug susceptibility. Hazard ratios (HRs) for drug resistance were calculated using a Cox proportional hazard model.
Results
From 2009 to 2014, 359 patients were tested for resistance. The median CD4 count and viral load (log) were 214 cells/μL (interquartile range [IQR]: 71–367) and 4.5 (IQR: 3.9–5.0), respectively. Subtype B HIV-1 strains were found in 90% of individuals. The resistance rate to any of the three classes of antiretroviral drugs (NRTI, NNRTI, and PI) was 75.5%. The percentage of NRTI, NNRTI, and PI resistance was 58.6%, 61.4%, and 11.4%, respectively. The risk factors for any class of drug resistance included age ≤35 years (adjusted HR: 2.30, CI: 1.48–3.56; p<0.0001), initial NNRTI-based antiretroviral regimens (adjusted HR: 1.70, CI: 1.10–2.63; p=0.018), and current NNRTI-based antiretroviral regimens when treatment failure occurs (odds ratio: 4.04, CI: 2.47–6.59; p<0.001). There was no association between HIV-1 subtype, viral load, and resistance.
Conclusion
This study demonstrated a high level of resistance to NRTI and NNRTI in patients with virologic failure to first-line antiretroviral therapy despite routine viral load monitoring. Educating younger men who have sex with men to maintain good adherence is crucial, as PI use is associated with lower possibility of drug resistance.
Introduction
The introduction of highly active antiretroviral therapy (HAART) has dramatically changed the disease course of HIV infection, making it a chronic and controllable disease.Citation1–Citation4 Suppression of virus replication can reduce disease progression and transmission. Monitoring HIV-1 drug resistance is essential for accessing requirements for new drugs and for stopping the spread of resistances. As such, patients with virologic failure provide a surrogate marker for treatment effectiveness.Citation5 Continuing with failed regimens may lead to more complex mutation patterns, the development of cross-resistance, and the forward transmission of drug-resistant HIV to antiretroviral therapy (ART)-naïve patients.Citation6
The World Health Organization recommends monitoring for acquired HIV drug resistance in individuals receiving ART.Citation7 A recent review reported high drug resistance levels in patients with virologic failure and the most common resistance mutations, M184V and K103N, were found in 65% and 52% of patients, respectively.Citation8 However, the prevalence rate of acquired ART resistance in Asia, where routine viral load monitoring is available, remains unknown.
Genotypic drug resistance testing (GRT) before treatment can influence the rate of acquired drug resistance mutation. In the Swiss HIV Cohort Study, 28.9% of post-ART patients had detectable drug resistance mutation, whereas in the recent ART-initiator group, only 45 of 2092 (1.6%) patients with pretreatment GRT acquired drug resistance mutations on ART.Citation9 Thus, regular surveillance of transmitted drug resistance is important to combat drug resistance.
The prevalence of transmitted HIV resistance had recently increased to 5% in some areas of South Africa, Kenya, and Zambia, and up to 15% in Uganda.Citation10–Citation12 In Asia, this rate was around 4%–12%, including 3.8% in China,Citation13 7.7% in Japan,Citation14 12% in South Korea,Citation15 4.9% in Thailand,Citation16 and 8%–11.1% in northern Taiwan.Citation17–Citation18 However, routine pretreatment GRT in ART-naïve patients is lacking in Taiwan because of financial constraints.
Informed strategies to prevent the development of virologic failure to first-line ART and the emergence of HIV resistance are, therefore, crucial. Although studies have been conducted on the prevalence of HIV-transmitted drug resistance in northern Taiwan,Citation17,Citation18 data on the impact of epidemiologic information and drug resistance after failure to ART are unknown. This study aims to establish the prevalence of genotypic drug resistance after failure of anti-HIV therapy and to establish the clinical and viral characteristics that might be predictive of drug resistance.
Materials and methods
Ethical statement
The institutional review board of the Kaohsiung Veterans General Hospital in Taiwan approved this study (VGHKS98-CT1-08 and VGHKS13-CT4-12). The protocol complied with all ethical considerations involving human subjects, and all information obtained followed standard clinical guidelines. All of the study participants provided written informed consent.
Study design and participants
Samples covering the period 2009–2014 were obtained from a clinical HIV cohort on HIV drug resistance in adult patients at the Veterans General Hospital Surveillance and Monitor Network (VGHSMN), which consisted of branches in Taipei, Taichung, and Kaohsiung, as well as 10 veterans and other satellite hospitals. The inclusion criteria were: 1) HIV-1-infected adult aged ≥20 years; 2) HAART initiation between 1 January 2009 and 31 December 2014; and 3) treatment failure with HIV-1 viral load ≥1000 copies/mL. A standardized questionnaire was also used to collect demographic data that included age, sex, risk factors for HIV infection, CD4 count, viral load, and duration and name of ART used.
Patients with a previous history of ART exposure for prevention of mother to child transmission or for post-exposure prophylaxis were excluded. When the patients returned to the clinics, laboratory exams for CD4 cell counts (FACS Flow; Becton Dickinson and Company, Franklin Lakes, NJ, USA) and plasma viral load (Cobas Amplicor HIV-1 monitor test, version 1.5; Roche Diagnostics Corporation, Indianapolis, IN, USA) were performed. First-line ART was provided for free in designated hospital nationwide by infectious diseases physicians and supported by the HIV case managers. Treatment switches were guided by clinical, immunologic, and virologic criteria, as determined by the physician in charge.
Standard follow-up of HIV-infected patients on ART consisted of outpatient visits every 3 months, with physical examinations, CD4+ T cell count, viral load, hematology, and biochemistry. However, HIV-1 GRT was not routinely performed for patients with virologic failure. Blood samples for such patients could be sent to the Center for Diseases Control for GRT once in every 3 years or to the reference laboratory if the patient was in the VGHSMN cohort.
Genotypic drug resistance testing
Resistance testing was performed on plasma samples using the ViroSeq™ HIV-1 Genotyping System version v2.8, according to the manufacturer’s instructions (Celera, Alameda, CA, USA). Antiretroviral resistance mutations were defined using the 2015 International Antiviral Society–USA (IAS–USA) HIV drug resistance algorithm,Citation19 while drug resistances were compared using the HIVdb program of the Stanford University HIV Drug Resistance Database. Patients were then classified as low-level, intermediate, or high-level resistance, as defined accordingly.
Statistics analysis
The Mann–Whitney U test was used to compare the median values of continuous variables between groups (resistance and wild virus), while the Fisher’s exact test was used to compare categorical variables between the two groups. Kaplan–Meier curves were estimated to determine the association between duration of current ART use and the development of drug resistance. A Cox proportional hazard model was used to calculate the hazard ratio (HR) for drug resistance. A two-sided p<0.05 was considered statistically significant. Statistical calculations were performed using the SPSS program version 12.0 (IBM Corporation, Armonk, NY, USA).
Results
From 2009 to 2014, a total of 359 patients had GRT and sequences were successfully obtained from 290 (81%). Sixty-nine did not report resistance due to low viral load (n=13), poor sample quality (n=1), and cancellation by the requesting physician due to viral re-suppression after education (n=55). The demographic data between patients with successful GRT and those without did not show any difference. Among the 359 patients, 93.6% (n=336) were male and 69.4% (n=247) were aged 20–39 years. Moreover, 73.4% (n=256) were men who had sex with men (MSM).
Upon virologic failure, the median CD4 cell count was 214 (interquartile range [IQR]: 71–367) cells/μL and the viral load was 4.5 log (IQR: 3.9–5.0). Most (90%) of the 290 patients with available GRT report had HIV subtype B, 9% had CRF01_AE, and 1% had subtype C ().
Table 1 Demographic data among HIV-1 infected patients with treatment failure (N=359)
The median duration of HAART use was 24 months (IQR: 9–50.5 months). The median duration of current HAART upon presentation for GRT was 9 months (IQR: 4–20.3 months). Upon presentation for GRT, 49.8% (158/317) of the patients were under non-nucleoside reverse transcriptase inhibitor (NNRTI)-based treatment. The most commonly used nucleoside reverse transcriptase inhibitor (NRTI) backbone included zidovudine/lamivudine (41%), abacavir/lamivudine (36.8%), and tenofovir/lamivudine (9.4%). The most commonly used NNRTIs were nevirapine (53.7%, 87/162) and efavirenz (46.3%, 75/162). Booster protease inhibitor (PI) was used in 109 (66.9%, 109/163) patients with lopinavir/ritonavir (47%, 77/163), atazanavir/ritonavir (13%, 21/163), darunavir/ritonavir (6%, 10/163), and tipranavir/ritonavir (0.6%, 1/163). Unboosted PI (atazanavir) was used in 54 (33.1%) patients.
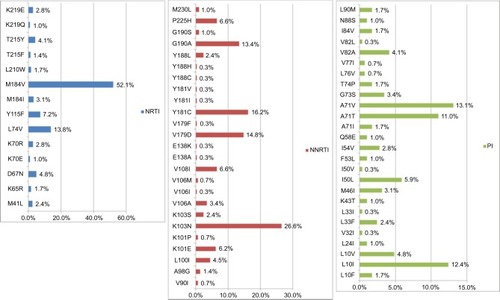
Of the 290 patients with GRT, 75.5% (n=219) had drug resistance to any of the three classes of antiretroviral drugs, including 58.6% showing resistance to NRTI, 61.4% to NNRTI, and 11.4% to PI (). The NNRTI cross-resistance was common in 51.7% of patients who were also resistant to rilpivirine, although they had no previous exposure to this new second-generation NNRTI (). The prevalence of IAS–USA drug resistance associated mutations in NRTI, NNRTI, and PI was 62.1%, 72.1%, and 37.2%, respectively (). The most common NRTI drug resistance associated mutations were M184V (52.1%), L74V (13.8%), and Y115F (7.2%). For NNRTI, the most common drug resistance associated mutations were K103N (26.6%), Y181C (16.2%), V179D (14.8%), and G190A (13.4%), while for PI, these were A71V (13.1%), L10I (12.4%), A71T (11.0%), and I50L (5.9%), as shown in .
Figure 1 Percentage of IAS–USA HIV drug resistance associated mutations and drug resistance by HIVdb program of the Stanford University among 290 HIV-1 infected patients with virologic failure, 2009–2014.
Note: A high of 75.5% of patients had drug resistance to any of the three classes of ART and 86.6% of the patients harbored any of the drug resistance associated mutations.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
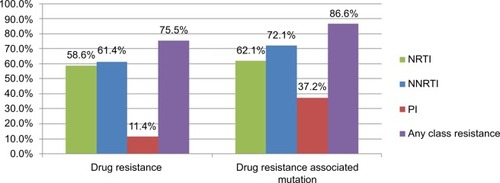
Figure 2 The prevalence of drug resistance to NRTI, NNRTI, and PI among 290 HIV-1 infected patients with virologic failure.
Note: Only 5.9% of the patients had drug resistance to tenofovir. The NNRTI cross-resistance was common and 51.7% of patients were also resistant to rilpivirine although they had no previous exposure to this new second-generation NNRTI.
Abbreviations: HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
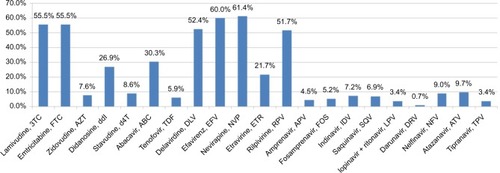
Figure 3 Percentage of ISA–USA HIV drug resistance associated mutations in NRTI, NNRTI, and PI among 290 HIV-1 infected patients with virologic failure.
Notes: The most common NRTI drug resistance associated mutations were M184V (52.1%) and L74V (13.8%). For NNRTI, the most common drug resistance associated mutations were K103N (26.6%) and Y181C, while for PI, these were A71V (13.1%) and L10I (12.4%).
Abbreviations: HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Risk factors associated with the presence of drug-resistant strains in a single variance analysis were younger age (p=0.045), MSM (adjusted HR [aHR]: 2.171, 95% CI: 1.210–3.894; p=0.011), and NNRTI-based HARRT at failure (aHR: 4.706, 95% CI: 2.331–9.499; p<0.0001), as shown in . Risk factors for any class drug resistance in multivariate analysis included age ≤35 years (aHR: 2.30, CI: 1.48–3.56; p<0.0001), initial NNRTI-based antiretroviral (ARV) regimens (aHR: 1.70, CI: 1.10–2.63; p=0.018), and currently used NNRTI-based ARV when there is the occurrence of virological failure (odds ratio: 4.04, CI: 2.47–6.59; p<0.001), as shown in . There were no associations among HIV-1 subtype, viral load, and resistance ().
Table 2 Risk factors associated with HIV-1 drug resistance in univariate analysis
Table 3 Risk factors associated with drug resistance in Cox regression model
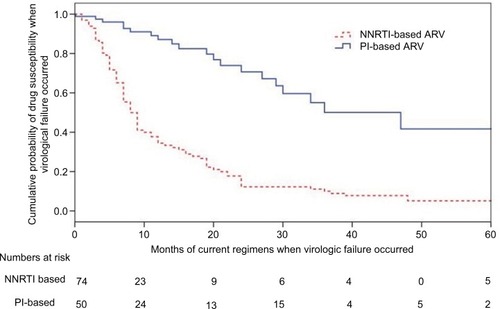
Figure 4 Kaplan–Meier curves for probabilities of developing drug resistances in patients after failure of their current regimen (p<0.0001, log rank test).
Notes: Patients with NNRTI-based regimen were more likely to develop virologic failure, compared to those on PI-based regimens (odds ratio: 4.04, CI: 2.47–6.59; p<0.001). The numbers used in the category at risk were 234. However, 290 samples were successfully tested for resistance. The detailed information for the drug prescription was not available in 56 subjects.
Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Discussion
This study illustrates the prevalence of HIV drug resistance among HIV-infected patients with virologic failure after first-line ART in Taiwan. In particular, this study highlights the high rate of drug resistance (75.5%) and the association of younger age (<35 years), even with widely available routine viral load monitoring. More importantly, the drug resistance to tenofovir and PI is low, compared to that of NNRTIs.
The findings here are similar to the resistance data from those patients in Africa who have suffered from early failures to the first-line treatment, showing that 70% with more than one drug resistance mutation after 12 months of treatment.Citation10,Citation20 Compared to other studies, the patients here had been a long duration on ART, with a median of 24 months.Citation20 Thus, the high prevalence of resistance in this study is likely due to a limited availability of resistance testing, leading to prolonged failure of ART and an accumulation of resistance mutations.
Most of the NRTI backbone used in this study included lamivudine and zidovudine: 52% (n=152), in the initial regimen when starting ART and 41% (n=135) in the current regimen when virologic failure occurred, explaining the predominance of M184V mutations and thymidine-associated mutations at the point of failure. In this study, the K65R mutation (1.7%) was rare, probably because tenofovir had been introduced in Taiwan in 2011 and had a restricted use in the national HIV treatment guideline initially. These results suggest that tenofovir remains a good option for second-line therapy.
The prevalence of resistance to NNRTIs was high, which was 60% for efavirenz and 61.4% for nevirapine. These were similar to the results of studies reported from Africa, where a high rate of resistance to first-generation NNRTIs was observed.Citation10,Citation20 In the present cohort, initial and current NNRTI-based ARV was associated with the development of drug resistance and only 78.3% and 48.3% of the individual genotypes predicted full susceptibility to etravirine and rilpivirine, respectively. None of the patients here had a previous exposure to rilpivirine or etravirine. These results also impacted on the second-generation NNRTI drug choices in patients with treatment failure. Studies show that nevirapine selects for the Y181C and G190A mutations and leads to reduced rilpivirine or etravirine susceptibility.Citation21,Citation22 Efavirenz failure is more likely to be associated with K103N mutation and has little impact on etravirine susceptibility.Citation21,Citation22 The median duration for the development of NNRTI resistance was 9 months, reflecting the low genetic barrier nature of NNRTI and poor drug compliance in the younger age group.
In this study, only 11.4% of patients developed PI resistance. The emergence of PI resistance at the time of virologic failure is uncommon in PI-naïve patients who experience virologic failure during their PI regimen. Moreover, the PI mutations can be located outside the pol gene, thereby underestimating the prevalence of PI resistance.Citation23–Citation25 In previous studies in Taiwan, the transmitted drug resistance to PI was extremely low (<4%).Citation17,Citation18 The PIs used in this study with failure were mostly lopinavir/ritonavir (60/125, 48%) and unboosted atazanavir (42/125, 33.6%). Up to 88.6% of patients did not have PI resistance on treatment failure and there was no association between unboosted PI use and the development of resistance. This might be due to the poor access to GRT and poor drug adherence.
Rates of PI resistance at the time of second-line failure are high in resource-limited settings and are associated with the duration of exposure to previous drug regimens and poor adherence.Citation26 A high prevalence of PI resistance was reported in four studies, two of which studied patients from Asia, especially from India (n=45, 73%)Citation27 and Vietnam (n=231, 59%).Citation28 The other two studies were from Mali (n=93, 25%)Citation29 and Nigeria (n=61, 62%).Citation30 Except for the study from India, where indinavir/ritonavir and atazanavir/ritonavir were used, the studies in these other countries used lopinavir/ritonavir for second-line therapy, similar to this study.
In this study, younger age (<35 years) is associated with the development of resistance. Several large cohort studies also report that older age is associated with better treatment outcomes,Citation31,Citation32 although better adherence does not necessarily explain the lower risk of failure in this group. No data about the level of adherence was available in this study. However, 24.5% of patients who experienced virologic failure had the wild-type virus, suggesting that adherence was an important factor in virologic failure.
The resistance rates to any of the three classes of antiretroviral drugs (NRTI, NNRTI, and PI) were up to 75.5%, despite the viral load measurements taken every 3–4 months. This rate was similar to those of developing countries in which viral load monitoring was not routinely available. In an HIV clinical trial in the USA where viral load was measured regularly every 3 months, the frequency of resistance in the study subjects receiving optimal first-line regimens was 62% at virologic failure.Citation33 This was similar to the 70% drug resistance rate in the Pharm Access African Studies to Evaluate Resistance, where HIV plasma viral load was measured after 12 months of a first-line regimen.Citation10 In the ANRS (National Agency for AIDS Research) 1268 study, the overall prevalence of major drug resistance mutations after virologic failure without any RNA monitoring was 71% and 86% among those assessed after 1 and 2 years, respectively, consistent with the evidence of a modest increase in resistance without monitoring among recipients of first-line treatment in Africa.Citation34–Citation36
It is very difficult to ascribe rates of virologic failure in resource-limited settings to a lack of viral load monitoring. Patient treatment adherence, HAART regimens used, accessibility of genotypic drug resistance, duration of failing regimen before resistance testing, even challenges in providing sample transport, laboratory and clinical infrastructure, and trained personnel,Citation10 and competing for treatment resources – all play significant roles that weaken the efficiency of routine viral load monitoring in the prevalence of drug resistance.
Limitations
There was no data available on the baseline prevalence of HIV-transmitted drug resistance for the study population. Nonetheless, the prevalence of transmitted antiretroviral resistance was reported to be around 8%–10% in Taiwan. The duration of virologic failure under treatment was not precisely determined in all of the patients because the information was lacking in some of the patients. In patients failing ART, GRT was found to have a significant benefit on the virologic response when choosing the salvage regimens.Citation37–Citation39 But the treatment outcomes for patients with virologic failures switching to another regimen were not available, making it impossible to evaluate the clinical impact of GRT. There was also no information on adherence to the ART regimen, making interpretation of the effect of age on the development of resistance more complicated. However, several large cohort studies reported that older age was associated with better treatment outcomes, although better adherence did not necessarily explain the lower risk of treatment failure in this group. Lastly, the results were from the VGHSMN cohort. Thus, it would be interesting to extend this work to the other parts of Taiwan.
Conclusion
This study demonstrates a high level of resistance to NRTI and NNRTI among patients who experienced virologic failure to first-line antiretroviral therapy even with routine viral load monitoring. It is crucial to educate younger MSM individuals to maintain good adherence to their treatment regimen. The use of PI is also associated with lower possibility of drug resistance.
Acknowledgments
The authors thank Dr Wing-Wai Wong, Department of Infectious Diseases, Taipei Veterans General Hospital and Dr Yu-Huei, Lin, Department of Infectious Diseases, Taichung Veterans General Hospital, Taiwan for their help. This study was funded by the Medical Foundation in Memory of Dr Deh-Lin Cheng (MF-DLC100053S5C2Y1) and by the Veterans General Hospitals and University System of Taiwan Joint Research Program Grant (VGHUST105-G3-2-1).
Disclosure
The authors report no conflicts of interest in this work.
References
- SterneJAHernanMALedergerberBEggerMSwiss HIV Cohort StudyLong-term effectiveness of potent anti-retroviral therapy in preventing AIDS and death: a prospective cohort studyLancet2005366948337838416054937
- BorJHerbstAJNewellMLBärnighausenTIncreases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatmentScience2013339612296196523430655
- JinYLiuZWangXA systematic review of cohort studies of the quality of life in HIV/AIDS patients after anti-retroviral therapyInt J STD AIDS2014251177177724598977
- KumarasamyNSolomonSChaguturuSKThe changing natural history of HIV disease: before and after the introduction of generic anti-retroviral therapy in southern IndiaClin Infect Dis200541101525152816231268
- KatzensteinDAHIV RNA and genotype in resource-limited settings: can we do better?Clin Infect Dis201458111011224076967
- RupérezMPouCMaculuveSDeterminants of virological failure and anti-retroviral drug resistance in MozambiqueJ Antimicrob Chemother20157092639264726084302
- World Health OrganizationWHO Global Strategy for the Surveillance and Monitoring of HIV Drug Resistance2012 Available from: http://apps.who.int/iris/bitstream/10665/77349/1/9789241504768_eng.pdfAccessed September 11, 2015
- HosseinipourMCGuptaRKVan ZylGEronJJNachegaJBEmergence of HIV drug resistance during first- and second-line anti-retroviral therapy in resource limited settingsJ Infect Dis2013207Suppl 2S49S5623687289
- ScherrerAUvon WylVYangWLand the Swiss HIV Cohort StudyEmergence of acquired HIV-1 drug resistance has almost been stopped in Switzerland – a 15 year prospective cohort analysisClin Infect Dis201662101310131726962075
- HamersRLSigaloffKCWensingAMPharmAccess African Studies to Evaluate Resistance (PASER)Patterns of HIV-1 drug resistance after first-line anti-retroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategiesClin Infect Dis201254111660166922474222
- HuntGMLedwabaJBassonAESurveillance of transmitted HIV-1 drug resistance in Gauteng and KwaZulu-Natal Provinces, South Africa, 2005–2009Clin Infect Dis201254Suppl 4S334S33822544199
- WoodEMontanerJSTime to get serious about HIV anti-retroviral resistanceLancet Infect Dis2011111072372421958572
- LiaoLXingHShangHThe prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in ChinaJ Acquir Immune Defic Syndr201053Suppl 1S10S1420104099
- HattoriJShiinoTGatanagaHTrends in transmitted drug-resistant HIV-1 and demographic characteristics of newly diagnosed patients: nationwide surveillance from 2003 to 2008 in JapanAntiviral Res2010881727920692295
- KimMHSongJEAhnJYHIV anti-retroviral resistance mutations among anti-retroviral treatment-naive and -experienced patients in South KoreaAIDS Res Hum Retroviruses2013291617162023952717
- SungkanuparphSSukasemCKiertiburanakulSPasomsubEChantratitaWEmergence of HIV-1 drug resistance mutations among anti-retroviral-naïve HIV-1-infected patients after rapid scaling up of anti-retroviral therapy in ThailandJ Int AIDS Soc20121511222410286
- LaiCCHungCCChenMYTrends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line anti-retroviral therapy in TaiwanJ Antimicrob Chemother20126751254126022302562
- LaiCCLiuWCFangCTTransmitted drug resistance of HIV-1 strains among individuals attending voluntary counselling and testing in TaiwanJ Antimicrob Chemother201671122623426404079
- WensingAMCalvezVGünthardHF2015 Update of the Drug resistance Mutations in HIV-1Top Antivir Med201523413214126713503
- UgbenaRAberle-GrasseJDialloKVirological response and HIV drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in NigeriaClin Infect Dis201254Suppl 4S375S38022544206
- TambuyzerLNijsSDaemsBPicchioGVingerhoetsJEffect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirineJ Acquir Immune Defic Syndr2011581182221637112
- VingerhoetsJNijsSTambuyzerLHoogstoelAAndersonDPicchioGSimilar predictions of etravirine sensitivity regardless of genotypic testing method used: comparison of available scoring systemsAntivir Ther20121781571157922869341
- NijhuisMvan MaarseveenNMLastereSA novel substrate-based HIV-1 protease inhibitor drug resistance mechanismPLoS Med200741e3617227139
- LarrouyLChazallonCLandmanRand the ANRS 127 Study GroupGag mutations can impact virological response to dual-boosted protease inhibitor combinations in anti-retroviral-naïve HIV-infected patientsAntimicrob Agents Chemother20105472910291920439606
- FunAWensingAMVerheyenJNijhuisMHuman immunodeficiency virus gag and protease: partners in resistanceRetrovirology201296322867298
- AjoseOMookerjeeSMillsEJBoulleAFordNTreatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysisAIDS201226892993822313953
- SaravananSVidyaMBalakrishnanPViremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern IndiaClin Infect Dis2012547995100022323567
- ThaoVPQuangVMDayJNHigh prevalence of PI resistance in patients failing second-line ART in VietnamJ Antimicrob Chemother201671376277426661398
- MaigaAIFofanaDBCisseMCharacterization of HIV-1 anti-retroviral drug resistance after second-line treatment failure in Mali, a limited-resources settingJ Antimicrob Chemother201267122943294822888273
- RawizzaHEChaplinBMeloniSTAccumulation of protease mutations among patients failing second-line anti-retroviral therapy and response to salvage therapy in NigeriaPLoS One20138e7358224069209
- WeintrobACFiebergAMAganBKIncreasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAARTJ Acquir Immune Defic Syndr2008491404718667932
- GrabarSKousignianISobelAImmunologic and clinical responses to highly active anti-retroviral therapy over 50 years of age. Results from the French Hospital Database on HIVAIDS200418152029203815577624
- MollanKDaarESSaxPEand the AIDS Clinical Trials Group Study A5202 TeamHIV-1 amino acid changes among participants with virologic failure: associations with first-line efavirenz or atazanavir plus ritonavir and disease statusJ Infect Dis2012206121920193023148287
- BartlettJARibaudoHJWallisCLLopinavir/ritonavir mono-therapy after virologic failure of first-line anti-retroviral therapy in resource-limited settingsAIDS201226111345135422441252
- OrrellCWalenskyRPLosinaEHIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community anti-retroviral therapy programmeAntivir Ther20091452353119578237
- SigaloffKCRamatsebeTVianaRde WitTFWallisCLStevensWSAccumulation of HIV drug resistance mutations in patients failing first-line anti-retroviral treatment in South AfricaAIDS Res Hum Retroviruses201228217117521819219
- DurantJClevenberghPHalfonPDrug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trialLancet199935391712195219910392984
- BaxterJDMayersDLWentworthDNA randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDSAIDS2000149F83F9310894268
- FalloonJTime to genotype for selection of antiretroviral regimens in previously treated patients?Lancet199935391712173217410392975
