Abstract
Background
Assessing the overall burden of healthcare-associated infections (HAIs) is challenging, but imperative in evaluating the cost-effectiveness of infection control programs. This study aimed to estimate the point prevalence and annual incidence of HAIs in Greece and assess the excess length of stay (LOS) and mortality attributable to HAIs, overall and for main infection sites and tracer antimicrobial resistance (AMR) phenotypes and pathogens.
Patients and methods
This prevalent cohort study used a nationally representative cross-section of 8,247 inpatients in 37 acute care hospitals to record active HAIs of all types at baseline and overall LOS and in-hospital mortality up to 90 days following hospital admission. HAI incidence was estimated using prevalence-to-incidence conversion methods. Excess mortality and LOS were assessed by Cox regression and multistate models correcting for confounding and time-dependent biases.
Results
HAIs were encountered with daily prevalence of 9.1% (95% confidence interval [CI] 7.8%–10.6%). The estimated annual HAI incidence was 5.2% (95% CI 4.4%–5.3%), corresponding to approximately 121,000 (95% CI 103,500–123,700) affected patients each year in the country. Ninety-day mortality risk was increased by 80% in patients with HAI compared to those without HAI (adjusted hazard ratio 1.8; 95% CI 1.3–2.6). Lower respiratory tract infections, bloodstream infections, and multiple concurrent HAIs doubled the risk of death, whereas surgical site and urinary tract infections were not associated with increased mortality. AMR had significant impact on the daily risk of 90-day mortality, which was increased by 90%–110% in patients infected by carbapenem-resistant gram-negative pathogens. HAIs increased LOS for an average of 4.3 (95% CI 2.4–6.2) additional days. Mean excess LOS exceeded 20 days in infections caused by major carbapenem-resistant gram-negative pathogens.
Conclusion
HAIs, alongside with increasing AMR, pose significant burden to the hospital system. Burden estimates obtained in this study will be valuable in future evaluations of infection prevention programs.
Introduction
Healthcare-associated infections (HAIs) represent a major issue for healthcare providers, infection control specialists, public health authorities, and the patients. The most recent estimate of the average daily prevalence of HAIs in acute care hospitals in Europe is 6%, involving approximately 3.2 million affected patients each year.Citation1 The dramatic increase of antimicrobial resistance (AMR) in pathogenic bacteria seen in hospital settings worldwideCitation2 has resulted in more complications to treat HAIs, and associated treatment failure and deaths have risen.Citation3
Even with optimal care, the extent to which HAIs are preventable depends on the setting, type of infection, and baseline infection rates. Systematic reviews of interventions to reduce HAIs have suggested that at least 20% of all HAIs are probably avoidable.Citation4 Preventability proportions may exceed 50% for surgical site and device-associated infections with current evidence-based strategies.Citation5 However, infection prevention programs have an associated cost, which should be compared with the expected benefits to ensure that the most cost-effective measures are implemented.Citation6,Citation7 This requires accurate assessment of the overall burden of HAIs in terms of excess deaths, length of hospitalization, and costs.Citation6,Citation7
The resources and effort required have rarely allowed multicenter studies of the global burden of all types of HAIs to be carried out. Consequently, only a handful of epidemiological studies have attempted to assess the impact of all HAIs on prolongation of length of stay (LOS) and/or mortality in hospital-wide settings.Citation7–Citation11 Moreover, studies attempting to provide this information face considerable challenges. Patients with HAIs are older, suffer from more chronic diseases, and are generally more ill than patients without HAIs;Citation12 consequently, patients with HAIs experience long exposure to the hospital environment before becoming infected. Such confounding effects and time-dependent biases may have been inadequately addressed in previous studies.Citation6,Citation13
The aim of this study was to obtain the first national estimates of the current prevalence and incidence burden of HAIs in acute care hospitals in Greece and assess the excess mortality and LOS attributable to HAIs, overall and separately for main sites of infection and tracer AMR phenotypes and pathogens.
Patients and methods
Study design and setting
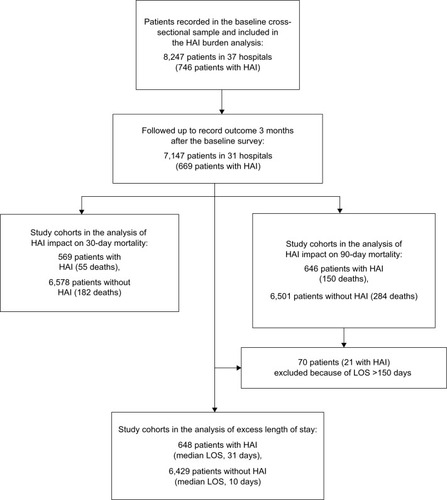
This prevalent cohort study was based on a baseline survey of 8,247 inpatients in 37 hospitals in Greece. The sample was a nationally representative cross-section of all patients hospitalized in acute care hospitals in a single day and was formed as part of the country’s participation in the first pan-European point prevalence survey of HAIs in June 2012.Citation14 Mortality and LOS were ascertained at the time of hospital discharge and up to 90 days after the baseline survey.
The study was approved by the Review Board of Hellenic Center for Disease Control and Prevention. As data collection originated from routine care activities and was included in monitoring activities mandated by national legislation (Ministerial Decisions Y1/4234/13.6.2001 and Y1.114971/18.02.2014), separate approvals by the institutional ethics committees in participating hospitals and patient informed consent were not required. Data were anonymous, kept confidential, and not linked to individuals. Study results are reported according to the STROBE guidelines.
Hospital selection criteria and sample size
We calculated that a total of 40 hospitals (10,506 patients) would be required to estimate an anticipated HAI point prevalence of 7%,Citation12 with precision of ±1% at the national level, based on an average hospital size of 260 beds and a total number of 35,120 beds. We used an estimated design effect of 4.5 to account for clustering at the hospital level.Citation14
We recruited hospitals on a voluntary basis using a purposive sampling method. Three criteria were used to frame the sample: 1) inclusion of at least one district-referral hospital from each of the seven Regional Health Districts in the country, 2) inclusion of at least four general hospitals from each district, and 3) all included hospitals have a fully operational infection control team with prior experience in HAI surveillance. We identified 39 hospitals satisfying the selection criteria, which we invited to participate; two hospitals refused to participate.
The 37 sampled hospitals comprised 27% of all public hospitals in Greece, had 16,164 beds (46% of the country’s total), and had completed 1,068,311 discharges and 4,127,210 patient-days in 2011 (46% and 44% of country’s total, respectively).
Patient selection criteria
All patients admitted to an acute care ward before 8:00 AM and not discharged from the ward at the time of the baseline survey were included in the study cohort. Day-case patients undergoing same-day treatment or surgery, seen in the emergency room or at outpatient departments, and dialysis outpatients were excluded.
Data collection and processing
Data were collected by 115 infection control practitioners across the country who had attended a 2-day online training course based on standardized European Centre for Disease Prevention and Control (ECDC) and national training materials. A help-desk service was provided during data collection by the Infection Control Unit of University Hospital of Heraklion, which served as the coordinating center for this study. All raw data were submitted to the coordinating center through a web-based data entry system and underwent central data management, including data checking for obvious errors and omissions, corrective queries, and statistical analysis.
Data were extracted from review of nursing and medical records and on the basis of information provided by the physicians and nurses in charge of the patients. Data collected for all patients included demographic characteristics, comorbid conditions determined by use of the weighted Charlson comorbidity index,Citation15 severity of underlying disease determined in accordance with the McCabe classification,Citation14 patient specialty, exposure to invasive devices at baseline, and prior surgery in the 30 days preceding the baseline survey. Comorbidities were evaluated at the time of hospital admission. Disease severity was determined in accordance to ECDC guidelines,Citation14 and assessed before infection for patients with an active HAI and at the day of survey for uninfected patients.
Active HAIs at baseline were identified using the ECDC case definitions.Citation14 Data collected for each HAI included date of onset, site, microorganisms, and AMR status. For purposes of data analysis, HAIs were categorized into lower respiratory tract infections (LRTIs) including pneumonias, bloodstream infections (BSIs) including catheter-related infections, urinary tract infections (UTIs), surgical site infections (SSIs), systemic infections including clinical sepsis, and other infections. Patients with multiple concurrent HAIs were analyzed as a separate group. AMR was assessed based on antibiotic susceptibility data that were available at the time of the baseline survey. Selected tracer phenotypes were recorded, including methicillin-resistant Staphylococcus aureus (MRSA); vancomycin-resistant Enterococcus species (VRE); third-generation cephalosporins and carbapenems for Enterobacteriaceae; and carbapenems for Acinetobacter baumannii and Pseudomonas aeruginosa. Intermediate sensitive strains were recorded as resistant.
Baseline data collection was completed in a single day at the ward/unit level and within a period of 3–4 weeks at the hospital level. Patient outcome was recorded as in-hospital death, discharge alive, or hospital stay 3 months following the baseline survey. Six hospitals did not provide outcome data as patient follow-up was an optional part of the study protocol.
Statistical analysis
HAI point prevalence was calculated as the number of infected patients divided by the total number of patients, overall and separately for each site of infection, resistance phenotype, and pathogen. Population estimates of prevalence proportions were calculated with 95% confidence intervals (CI) and were compared across different patient groups using chi-square tests accounting for the stratified clustered design (by region and hospital) of the baseline survey.
The annual incidence burden of HAIs was quantified by estimating the number of patients expected to acquire a HAI in a year in acute care hospitals in the country, overall and separately by type of HAI and AMR phenotype. This was calculated by multiplying the point prevalence estimate by the national total of acute care patient-days in the country (9,312,024 patient-days in 2011) and dividing the product by the average duration of infection. The calculation has been described by Freeman and Hutchison,Citation16 and forms the basis of the Rhame and Sudderth prevalence-to-incidence conversion.Citation17 The estimated number of patients acquiring a HAI in a year was divided by the national total of discharges (2,344,999 discharges in 2011) to obtain annual cumulative incidence proportions for each type of HAI and resistance phenotype. Duration of infection was estimated as the interval between date of onset of infection and date of baseline survey, excluding HAIs present at admission.Citation1,Citation18 Median values were used to account for the high degree of right skewness in the distribution of infection durations, and associated CIs were calculated using bootstrapping.
Multivariable Cox proportional hazards models were used to compare the daily risk (hazard) of death between patients with HAIs and uninfected patients in terms of adjusted hazard ratios (HRs) and associated 95% CIs. The day of admission was used as the time variable, and times to death within 30 and 90 days of hospital admission were the outcome variables in the models. Occurrence of HAI was treated as a time-dependent exposure to account for the indirect effect on mortality of a potentially extended LOS due to HAI. Main infection types and AMR phenotypes were examined separately. Baseline covariates were adjusted for in the models with the assumption that patient characteristics at admission remained unaltered throughout a patient’s stay. The models accounted for clustering at the hospital level and stratification at the regional level.
Differences in hospital LOS between patients with HAI and those without were estimated by a multistate model using the “Empirical Transition Matrix” package (version 0.6–2) in R, version 3.3.0.Citation19 The model comprised two transient states (hospital admission and HAI) and two absorbing states (discharge alive and in-hospital death). HAI was considered an intermediate state between admission and discharge or death in order to mitigate the potential for time-dependent bias, which has been shown to overestimate the extra LOS.Citation13,Citation20 This time-adjusted estimate of excess LOS cannot be adjusted for other confounders. Associated CIs were calculated using bootstrapping.
Results
Studied population
The study cohort consisted of 8,247 inpatients at baseline, who had a median age of 63 years (interquartile range [IQR], 38–76 years) and 54.4% were males. Most patients had an emergency admission (74.7%) and were located in tertiary care hospitals (63.6%). The median Charlson comorbidity index was 1 (IQR, 0–2), and 26.8% of the patients had a rapidly or ultimately fatal underlying disease. At the time of the baseline survey, 80.8% were exposed to one or more invasive devices and 28.4% had undergone recent surgery. Outcome data were obtained for 7,147/8,247 (87%) patients in 31/37 (84%) hospitals (). Any-cause mortality was 3.3% at 30 days and 6.1% at 90 days following hospital admission. The median LOS was 11 (IQR, 6–23) days.
Characteristics of HAIs
A total of 746 patients with an active HAI were identified, of whom 71 (9.5%) patients had two concurrent infections and three (0.4%) patients had three infections. Among the 820 recorded episodes of HAI, the most frequent type was LRTI (2.7 infections per 100 patients; 26.7% of all infections), followed by BSIs (2.1 infections per 100 patients, 20.7% of infections), UTIs (1.7 infections per 100 patients; 17.0% of infections), SSIs (3.8 infections per 100 operated patients; 10.9% of infections), and systemic infections (0.7 infections per 100 patients; 6.7% of infections). The “other” category accounted for an additional 18.0% of HAIs (1.8 infections per 100 patients). A total of 222 (27.1%) HAIs were present at hospital admission, originating from the same or other hospital. The median time from hospital admission to onset of infection was 11 (IQR, 6–25) days. The median duration of infection was 7.0 (95% CI, 7.0–8.0) days.
A total of 564 microorganisms were recorded in 449 (54.8%) of 820 episodes of HAI. The pathogens isolated most frequently included Klebsiella species (17.6%), P. aeruginosa (16.8%), Acinetobacter species (16.8%), Staphylococcus species (9.2%, including 2.7% S. aureus), Enterococcus species (8.9%), and Escherichia coli (8.3%). Of the 424 isolates with available antibiogram data, 204 (48.1%) had a tracer AMR phenotype. Fifty percent (8/16) of S. aureus isolates were MRSA; 12.5% (6/48) of enterococci were VRE; and 39.9% (48/183) of Enterobacteriaceae, 83% (73/88) of A. baumannii, and 49.4% (44/89) of P. aeruginosa were resistant to carbapenems.
HAI prevalence and incidence burden
The overall point prevalence of patients with HAI was 9.1% (95% CI 7.8–10.6). shows HAI prevalence according to baseline patient characteristics. HAI prevalence was higher in males than in females, varied by patient specialty and increased with age, severity of underlying disease, comorbidity index, and number of invasive devices. Higher HAI prevalence was also observed for patients who had undergone recent surgery and those with an emergency admission. The highest overall prevalence proportions were observed in intensive care patients (32.7%, 95% CI 27.4–38.4), patients admitted with a rapidly fatal disease (30.5%, 95% CI 25.0–36.7), and those exposed to 3–4 invasive devices (47.9%, 95% CI 42.3–53.5).
Table 1 Comparison of patients with and without HAI and population estimates of HAI prevalence according to baseline patient characteristics
The total number of patients with at least one HAI on any given day in the country was estimated at 2,323 patients (95% CI 1,985–2,712). The estimated annual incidence of patients acquiring at least one HAI in a year was 5.2% (95% CI 4.4%–5.3%), corresponding to an absolute number of 121,142 patients (95% CI 103,522–123,738) acquiring a HAI per year in the country. National estimates of prevalence and incidence burden according to type of infection and AMR phenotype are shown in .
Table 2 National estimates of prevalence and incidence burden according to type of HAI and antimicrobial resistance status in Greek acute care hospitals
Impact of HAI on inpatient mortality
shows cumulative proportions and HRs for 30-day and 90-day mortality according to patient characteristics. No major difference in mortality was observed according to sex and hospital type. Patients in internal medicine wards and intensive care units had significantly higher mortality compared to surgical patients, whereas no deaths were recorded in pediatric, gynecology, and obstetrics departments. Patients with emergency admission had higher mortality than those with elective admission. Mortality increased with age, underlying disease severity, comorbidity index, and exposure to invasive devices. Mortality was substantially higher for patients with HAI at baseline (9.7% and 23.2% at 30 and 90 days, respectively) than those without HAI (2.8% and 4.4% at 30 and 90 days, respectively).
Table 3 Univariate comparisons of all-cause in-hospital mortality within 30 and 90 days following hospital admission according to baseline patient characteristics in 7,147 acute care patients
Following adjustment for confounding and time-dependent bias in Cox regression, presence of HAI continued to show elevated risk of mortality at 30 days (HR=1.3, 95% CI 0.7–2.4, p=0.363) and 90 days (HR=1.8, 95% CI 1.3–2.6, p=0.001). shows case-fatality rates and adjusted HRs according to type of infection, resistance phenotype, and pathogen. Compared to uninfected patients, increased mortality risk was observed for patients with LRTIs, BSIs, and multiple concurrent infections. By contrast, no increase in the risk of death was observed in patients with UTIs, SSIs, systemic infections, or other infections. Mortality risk was also increased in patients infected by third-generation cephalosporin-resistant Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and carbapenem-resistant A. baumannii. No significant impact on mortality was seen in patients infected with MRSA or VRE.
Table 4 Clinical impact of HAIs according to infection type and antimicrobial resistance status
Excess length of hospital stay associated with HAI
Using a multistate model, presence of HAI was found to significantly increase LOS for a mean of 4.3 (95% CI 2.4–6.2) additional days. The time-adjusted mean difference in LOS between patients with HAI and those without HAI varied substantially according to the type of infection and ranged from −2.8 (95% CI −6.6–1.0) days in UTIs to 10.5 (95% CI 5.3–15.8) days in BSIs. High excess LOS estimates were obtained in patients with multiple concurrent infections (16.6 days; 95% CI 8.9–24.3) and those infected by an antimicrobial-resistant pathogen (16.9 days; 95% CI 12.9–20.9). Mean excess LOS peaked in patients infected by carbapenem-resistant Enterobacteriaceae, P. aeruginosa, or A. baumannii ().
Discussion
In this first attempt to assess the overall burden of HAIs at the national level in Greece, we estimated that HAIs are encountered with an average daily prevalence of 9.1% (95% CI 7.8%–10.6%) in acute care hospitals in the country, and at an annual incidence rate of 5.2% (95% CI 4.4%–5.3%) involving approximately 121,000 affected patients each year in the country. We found that the daily risk of hospital death within 90 days of admission was increased by 80% in patients with a HAI compared to those without HAI (HR 1.8, 95% CI 1.3–2.6). Presence of HAI was seen to significantly increase hospital LOS for an average of 4.3 (95% CI 2.4–6.2) additional days. Our site-specific results suggested that LRTIs, BSIs, and multiple concurrent HAIs double the risk of death in hospitalized patients, whereas SSIs and UTIs are not associated with increased mortality. AMR was shown to have a significant effect on the risk of in-hospital mortality, which was particularly high in patients infected by carbapenem-resistant gram-negative pathogens. Infections caused by pathogens with tracer AMR phenotypes were shown to independently increase hospitalization by more than 2 weeks (mean excess of 16.9 days, 95% CI 12.9–20.9 days).
Few comparable studies of the global burden of HAIs have been performed to date, mostly at the regional or single-center level,Citation8,Citation9,Citation11,Citation12 and even fewer at the national level.Citation7,Citation10 Most multicenter studies assessing the impact of HAIs have been primarily conducted in intensive care units,Citation21,Citation22 or have focused on a single type of HAI and/or resistance phenotype.Citation23,Citation24 This is not surprising given the amount of resources and effort required to conduct multicenter hospital-wide studies of all types of HAI. As in other national-level studies,Citation7,Citation10 we attempted to make best use of available data from a national point prevalence study of HAIs that was combined with patient follow-up and linkage to national registry data to make projections at the national level.
Comparison of results between different studies remains difficult mainly because of differences in patient case mix and methodology.Citation12 Compared to ECDC data from 33 countries,Citation1 the overall HAI prevalence and incidence rates reported in this study rank Greece as 4th and 8th highest in Europe, respectively, affirming a significant burden to the Greek hospital system. The 30-day case-fatality rate in patients with HAI in this study appears to be lower than that reported in hospital-wide studies in Finland and Norway (8.2% vs 9.8% and 10.8%, respectively),Citation10,Citation11 but the longer term case-fatality appears similar to that reported in France and previously in Greece and Cyprus (25.1% vs 21.6% and 27.9%, respectively).Citation8,Citation12 As shown in other studies,Citation7,Citation8,Citation11 we also found that patients with BSI or LRTI had increased risk of dying during the follow-up period, even after adjusting for the effects of age, comorbidity, underlying disease severity, and other important risk factors for death. SSIs and UTIs were not associated with increased mortality risk, which has also been seen by others.Citation7,Citation8,Citation11,Citation25
The present study is unique, however, in showing the significant independent impact of AMR in patient mortality, even outside the critical care setting. Indeed, hospital-wide case-fatality rates at 90 days in this study reached 37% in patients infected by third-generation cephalosporin-resistant Enterobacteriaceae, 33% in patients infected by carbapenem-resistant Enterobacteriaceae, and 35% in those infected by carbapenem-resistant A. baumannii. Correcting for the effects of other important risk factors for death, the daily risk of dying within 90 days of admission was shown to increase by 90%–110% in patients infected by these resistant pathogens compared to uninfected patients.
Our overall estimate of the excess LOS due to HAIs (4.3 days) is almost identical to the estimate of 4 days from the seminal 1981 study of Haley et al,Citation26 which used direct attribution by expert reviewers to assess the prolongation of LOS due to HAI. By contrast, our excess LOS estimate is considerably lower than those given in comparative attribution studies in Belgium (7.3 days), Greece and Cyprus (10.1 days), and England (14.1 days).Citation7,Citation12,Citation27 The latter were based on time-invariant methods that cannot fully account for the timing of infection and thereby have most likely overestimated the effect of HAI on excess LOS.Citation13,Citation20 In agreement with the site-specific estimates of excess LOS obtained in this study, a cohort study of hospitalized patients in Australia controlling for a comprehensive set of confounders found that UTIs were not associated with prolongation of LOS, while LRTIs had an excess LOS of 2.6 (95% CI 1.8–3.7) days.Citation6 The latter also illustrated that many factors, other than HAI, are associated with increased LOS, and omitting these confounders from analysis leads to inflated estimates of excess LOS due to HAI.Citation6 The multistate modeling used in the current study provided estimates of excess LOS that were time adjusted but not fully adjusted for other confounders. Time adjustment is probably more important than adjustment for confounders as was illustrated by Beyersmann et al who reported that accounting for a large number of potential confounders did not redeem the overestimation of excess LOS.Citation28
UTI was the single type of HAI that was associated with decreased LOS in this study. This contradicts with the findings of other time-adjusted estimates of excess LOS due to UTI obtained from multicenter studies in Spain (4.6 days) and Australia (4.0 days). Citation7,Citation24 Both of the latter seem high and might be the result of rather complicated cases of UTIs, mainly in elderly patients surviving a prolonged hospitalization.Citation7 Indeed, UTIs in intensive care units in 10 developing countries were seen to prolong LOS by only 1.6 (95% CI 0.6–2.6) extra days.Citation29 It is possible, however, that accurately estimating excess LOS requires further adjusting for patient case mix in addition to accounting for time-dependent bias, which is a gap in available statistical methods.
Controlling for time-dependent bias, we found that AMR is a major contributing factor in prolonging hospital LOS, with the mean excess LOS ranging from 9.6 days in MRSA or VRE infections to more than 20 days in infections caused by major carbapenem-resistant gram-negative pathogens. Using similar statistical methods, a cohort study at a Swiss university hospital found that excess LOS attributable to MRSA infection was 11.5 (95% CI 7.9–15) days,Citation23 which resembles our findings. To the best of our knowledge, no other study has assessed the excess LOS due to infections caused by carbapenem-resistant gram-negative pathogens in hospital-wide settings to date.
Particular limitations in this study should be acknowledged when interpreting our findings. The first relates to the absence of global national surveillance data in Greece, which compelled us to rely entirely on data from the only existing national point prevalence survey to assess the global burden of HAIs. It is well known that cohorts of patients gathered through sampling prevalent cases tend to have longer survival times than those obtained in an incident cohort study. Our approach to estimating excess mortality assumes that the composition of the two patient groups (with HAI and without HAI at the time of the baseline survey) and their underlying conditions remained constant during follow-up. We cannot exclude the possibility that some subjects without HAI at baseline may have developed a HAI at a later time; depending on the extent of this misclassification, we might have underestimated the effect of HAI on mortality. The incidence-to-prevalence conversion used in this study also requires data from an incidence series of HAIs; we used median values to estimate the average duration of HAI correcting for the skewness towards patients with longer duration of infection in our sample. Simulations based on Europe-wide surveillance data in intensive care units have confirmed that incidence-to-prevalence conversion performs well using this method,Citation1 although the use of antibiotic treatment as a proxy for infection has been reported to improve the method.Citation30 Nevertheless, we must acknowledge that incident sampling, although logistically difficult and more expensive, remains the gold standard for estimating HAI incidence and impact on LOS and mortality.
Another limitation relates to the fact that we relied on antibiotic susceptibility tests available at the day of the baseline survey and thereby were able to assess resistance phenotypes for about half of the HAIs recorded in this study. This led to reduced sample sizes and thereby wide CIs for excess LOS and mortality associated with specific resistance phenotypes. Previous experience has shown that extending the period of recording microbiology data to a week following the detection of an active HAI in a prevalent cohort study may increase culture and antibiogram availability to about 70% of detected infections,Citation12 thereby improving pathogen-specific burden estimation. Moreover, we did not account for treatment factors in our analysis because our objective was to assess the real-life effect of AMR. Whether this effect was due to intrinsic pathogen factors or treatment failure was beyond the scope of this study.
Conclusion
This assessment of the burden of HAIs from a public health-care provider’s perspective showed that the incidence of HAIs, alongside their associated impact on LOS and mortality, presents a significant burden to the Greek hospital system. These findings, together with the increasing AMR in hospital settings, suggest that it is time to consider systematic interventions to reduce HAI incidence, including the potential of developing a global national surveillance system. Burden estimates obtained in this study will be valuable in future evaluations of the cost-effectiveness of infection prevention programs.
Author contributions
Study conception: EIK, AG; study design: EIK, EA, MR, EI, AG; data acquisition: EIK, FK, EA, MR, EI, AG; data management and statistical analysis: EIK; drafting of the manuscript: EIK; data interpretation and critical revision of the manuscript: EIK, FK, EA, MR, EI, AG. All authors approved the final manuscript and are accountable for all aspects of this work.
Acknowledgments
We thank the infection control teams in all of the participating hospitals for their cooperation and support.
Disclosure
The authors report no conflicts of interest in this work. No external funding was received for this work.
References
- European Centre for Disease Prevention and ControlPoint Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care HospitalsStockholmECDC201310.2900/86011
- HuttnerAHarbarthSCarletJAntimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections ForumAntimicrob Resist Infect Control2013213124237856
- FalagasMETansarliGSKarageorgopoulosDEVardakasKZDeaths attributable to carbapenem-resistant Enterobacteriaceae infectionsEmerg Infect Dis20142071170117524959688
- HarbarthSSaxHGastmeierPThe preventable proportion of nosocomial infections: an overview of published reportsJ Hosp Infect200354425826612919755
- UmscheidCAMitchellMDDoshiJAAgarwalRWilliamsKBrennanPJEstimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costsInfect Control Hosp Epidemiol201132210111421460463
- GravesNWeinholdDTongEEffect of healthcare-acquired infection on length of hospital stay and costInfect Control Hosp Epidemiol200728328029217326018
- VrijensFHulstaertFDevrieseSvan de SandeSHospital-acquired infections in Belgian acute-care hospitals: an estimation of their global impact on mortality, length of stay and healthcare costsEpidemiol Infect2012140112613621320376
- Fabbro-PerayPSottoADefezCMortality attributable to nosocomial infection: a cohort of patients with and without nosocomial infection in a French university hospitalInfect Control Hosp Epidemiol200728326527217326016
- García-MartínMLardelli-ClaretPJiménez-MoleónJJBueno-CavanillasALuna-del-CastilloJDGálvez-VargasRProportion of hospital deaths potentially attributable to nosocomial infectionInfect Control Hosp Epidemiol2001221170871411842992
- KanervaMOllgrenJVirtanenMJLyytikäinenOPrevalence Survey Study GroupEstimating the annual burden of health care-associated infections in Finnish adult acute care hospitalsAm J Infect Control200937322723019111367
- KochAMNilsenRMEriksenHMCoxRJHarthugSMortality related to hospital-associated infections in a tertiary hospital; repeated cross-sectional studies between 2004–2011Antimicrob Resist Infect Control2015415726719795
- KritsotakisEIDimitriadisIRoumbelakiMCase-mix adjustment approach to benchmarking prevalence rates of nosocomial infection in hospitals in Cyprus and GreeceInfect Control Hosp Epidemiol200829868569218643744
- NelsonRENelsonSDKhaderKThe Magnitude of Time-Dependent Bias in the Estimation of Excess Length of Stay Attributable to Healthcare-Associated InfectionsInfect Control Hosp Epidemiol20153691089109426041436
- European Centre For Disease Prevention and ControlPoint Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Protocol Version 4.3. Full Scale Survey and CodebookStockholmECDC201210.2900/53482
- CharlsonMEPompeiPAlesKLMacKenzieRA new method of classifying prognostic in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- FreemanJHutchisonGBPrevalence, incidence and durationAm J Epidemiol198011257077236969024
- RhameFSSudderthWDIncidence and prevalence as used in the analysis of the occurrence of nosocomial infectionsAm J Epidemiol198111311117457475
- MagillSSEdwardsJRBambergWEmerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey TeamMultistate point-prevalence survey of health care-associated infectionsN Engl J Med2014370131198120824670166
- AllignolASchumacherMBeyersmannJEmpirical transition matrix of multi-state models: the etm PackageJ Stat Softw2011384115
- BeyersmannJGastmeierPWolkewitzMSchumacherMAn easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimationJ Clin Epidemiol200861121216122118619803
- LambertMLSuetensCSaveyAClinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort studyLancet Infect Dis2011111303821126917
- VincentJRelloJMarshallJInternational study of the prevalence and outcomes of infection in intensive care unitsJAMA2009302212323232919952319
- Macedo-ViñasMDe AngelisGRohnerPBurden of meticillin-resistant Staphylococcus aureus infections at a Swiss University hospital: excess length of stay and costsJ Hosp Infect201384213213723608003
- MitchellBGFergusonJKAndersonMSearJBarnettALength of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state modelJ Hosp Infect2016931929926944900
- RoumbelakiMKritsotakisEITsioutisCTzilepiPGikasASurveillance of surgical site infections at a tertiary care hospital in Greece: incidence, risk factors, microbiology, and impactAm J Infect Control2008361073273818834729
- HaleyRWSchabergDRCrossleyKBVon AllmenSDMcGowanJEJrExtra charges and prolongation of stay attributable to nosocomial infections: a prospective interhospital comparisonAm J Med198170151587457491
- PlowmanRGravesNGriffinMAThe rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposedJ Hosp Infect200147319820911247680
- BeyersmannJKneibTSchumacherMGastmeierPNosocomial infection, length of stay, and time-dependent biasInfect Control Hosp Epidemiol200930327327619193018
- RosenthalVDDwivedyACalderónMEInternational Nosocomial Infection Control Consortium (INICC) MembersTime-dependent analysis of length of stay and mortality due to urinary tract infections in ten developing countries: INICC findingsJ Infect201162213614121168440
- KingCAylinPHolmesAConverting incidence and prevalence data: an update to the ruleInfect Control Hosp Epidemiol201435111432143325333444