Abstract
Tafenoquine is an 8-aminoquinoline with activity against all human life cycle stages of Plasmodium vivax, including dormant liver stages – so called hypnozoites. Its long half-life of ~15 days is allowing for a single exposure regimen. It has been under development since 1980 and received approval by the US Food and Drug Administration in summer 2018 as an anti-relapse drug for P. vivax malaria in patients aged 16 years and older and for prophylaxis of malaria caused by any Plasmodium species in adults. Prior to tafenoquine administration, glucose-6-phosphate dehydrogenase (G6PD) deficiency needs to be excluded by testing. Individuals with a deficient G6PD activity are at risk of tafenoquine-induced hemolysis – as is the case for primaquine, the mainstay drug for P. vivax radical cure. A wealth of clinical studies have been conducted and are still ongoing to assess the safety, tolerability, and efficacy of tafenoquine. This review focuses on data emerging from the latest clinical trials on P. vivax radical cure with tafenoquine, the key studies for regulatory approval of tafenoquine, and elucidates the latest hypothesis on the mode of action.
Introduction
Tafenoquine (TQ) is an 8-aminoquinoline derivative that had just received approval by the US Food and Drug Administration (FDA, July 2018) and the Australian Therapeutic Goods Administration (TGA, September 2018) for a radical cure of Plasmodium vivax malaria (US/Australia brand name Krintafel/Kozenis, developed by GlaxoSmithKline [GSK, London, UK]) together with Medicines for Malaria Venture (MMV, Geneva, Switzerland) and for prophylaxis of malaria (US/Australia brand name Arakoda/Kodatef, developed by 60 Degrees Pharmaceuticals (Washington DC, USA) together with the US Army).Citation1 TQ passed more than four decades of development as an antimalarial drug, and was initially under investigation as a prophylactic antimalarial agent for preventing Plasmodium falciparum malaria but without final submission of the dossier for market authorization. Clinical development slowed down and only gained pace again in the last 10 years when containment and treatment of P. vivax malaria appeared on the WHO agenda.Citation2 Previously a neglected pathogen, P. vivax has received more attention in recent years. Due to its unique biologic features, P. vivax is nowadays considered a huge challenge for the control and elimination of malaria.Citation3 P. vivax is globally the most widespread human malaria parasite species, and indeed causes more significant morbidity and mortality than previously thought.Citation4 Unlike P. falciparum, P. vivax develops undetectable dormant liver stages (hypnozoites) in the infected host. These hypnozoites are spontaneously activated by unknown mechanisms weeks (3–6 weeks) to several months (6–12 months) after the primary infection and cause blood stage infections called relapses, resulting in recurrent disease.Citation5,Citation6 As hypnozoites are not affected by standard antimalarial treatments, eg, chloroquine (CQ) or artemisinin based combination therapies (ACTs), the treatment is a particular challenge to P. vivax malaria control.
Until FDA approval of TQ this summer, the only licensed drug for treatment of hypnozoites was primaquine (PQ), also an 8-aminoquinoline (8-AQ) known to act against dormant liver stages. It is given together with a 3-day treatment of CQ to provide radical cure, ie, curing the acute asexual blood stage infection and additionally clearing hypnozoites, thus preventing P. vivax relapses.Citation7–Citation9 PQ side effects include gastrointestinal disturbances (GID), leukopenia, and methemoglobinemia (MHb).Citation9 Of special concern is hemolysis in people with glucose-6-phosphate-dehydrogenase (G6PD)-deficiency, a genetic disorder frequently found in populations of malaria-endemic regions.Citation7–Citation10 This impedes PQ to be given without prior testing of the G6PD-status. In addition, PQ has to be metabolized by liver enzymes (CYP2D) to exert its action and will not be fully active in low metabolizers, leading to treatment failure.Citation7,Citation8 A major limitation is the short biological half-life (3–6 hours) of PQ, implying daily administration for 14 days, presenting a major issue for compliance.Citation11
GSK supported by MMV were spearheading the recent TQ development program for a radical cure of P. vivax. These efforts resulted into FDA approval of TQ as a single-dose (300 mg) oral treatment for P. vivax hypnozoites in patients older than 16 years given together with appropriate acute P. vivax antimalarial treatment (full course CQ or ACT).
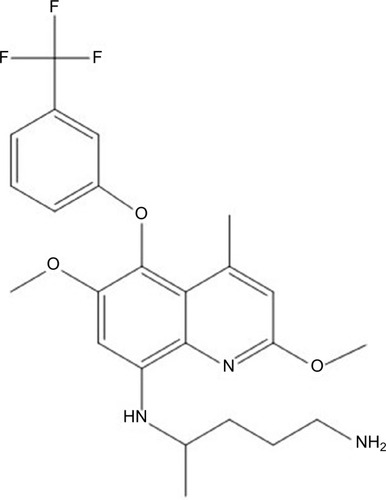
TQ (2,6-dimethoxy-4-methyl-5-[{3trifluoromethyl}-phenoxy]–8-[{4-amino-1-methylbutyl}amino] quinoline) () is a long-acting PQ analog active against both blood and liver stages of P. vivax developed in the 1980s as an alternative to PQ by the Walter Reed Army Institute of Research in the USA, under the identifier WR238605.Citation12,Citation13
In total, 33 clinical studies with TQ as an anti-malarial agent have been conducted until October 2018. Results of the first TQ clinical trial in humans were published in 1998, showing that TQ was safe and well tolerated.Citation14 The molecule’s promising half-life of 15 days initiated a series of clinical studies that led to established and consolidated safety, tolerability, and efficacy data.Citation12,Citation15–Citation23
Three key studies with promising efficacy outcomes encouraged/fueled the current TQ anti-hypnozoite development program: DETECTIVE Part 1Citation15 (phase II study) and Part 2 (NCT1376167, phase III study) and GATHER (NCT2216123, phase III study). Overall, TQ at a 300 mg single dose was highly efficacious in preventing P. vivax recurrences until 6 months after treatment. In DETECTIVE Part 2, recurrence-free efficacy was 63% (95% CI = 55–69) for CQ + TQ, compared to 28% (95% CI = 20–36) for CQ alone. When CQ + TQ was compared to CQ + PQ, recurrence-free efficacy was 73% (95% CI = 65–79), compared to 75% (95% CI = 64–83), respectively.Citation24
The aim of this review is to provide an update on what is known about the mode of action of TQ and to give an overview of the data emerging from the latest clinical trials conducted/being conducted in P. vivax infected patients. Studies leading to approval of TQ as a prophylactic antimalarial drug against all Plasmodium species are beyond the scope of this review, and will only be briefly summarized.
Methods
The literature databases NCBI PubMed and Cochrane Library, as well as the clinical trial registry databases ClinicalTrial.gov, FDA homepage fda.gov, and the WHO International Clinical Trial Registry Platform were searched. Search terms included tafenoquine, mode of action, clinical trials, human, P. vivax malaria, and malaria in combination. The last search was done in October 2018. Any clinical trial on TQ was included, irrespective of study type. Data on two key studies (Detective Part II and Gather) are not yet published in peer-reviewed journals, but results were available through the FDA Application Review files available on the FDA webpage.
In total, 33 clinical studies on TQ were done or are still ongoing (). To date (October 2018) TQ has been evaluated in more than 4,000 individuals enrolled in ten phase I, nine phase II, eleven phase II/III, and three phase III clinical studies.
Table 1 List of studies with a primary focus on safety, tolerability, and pharmacokinetics of TQ
Mechanism of action
As is true for many antimalarial compounds, neither the exact mechanism of antiplasmodial activity of TQ nor of the much longer available/licensed drug PQ is well understood. TQ is active against both liver stages and blood stages of P. vivax and P. falciparum. Current hypotheses claim that different compound metabolites and, hence, different modes of action account for TQ’s parasite stage specific activity. As identified for PQ, CYP/CYP450 metabolism of TQ plays a crucial role for P. vivax liver stage activity, but seems not to be relevant for its blood schizonticidal activity.Citation25 For PQ’s liver stage and anti-relapse activity liver enzyme CYP/CYP450 2D6 (CYP-2D6) metabolism is crucial. This was shown in transgenic mice that had a knockout of the P450 2D isozyme gene cluster. PQ was only active against P. berghei liver stages if the knockout was rescued with human CYP-2D6.Citation26 This finding is completed by a report from a P. vivax human challenge trial, where two study participants experienced P. vivax relapses after CQ and PQ treatment, who were retrospectively identified as poor CYP-2D6 metabolizer genotypes.Citation27
The most likely mechanism of PQ’s antiplasmodial activity is its metabolism via CYP-2D6 generating hydroxyl PQ metabolites. Redox cycling of these metabolites results in formation of reactive oxygen species leading to parasite death.Citation28,Citation29 The CYP-2D6 enzyme is highly polymorphic in human populations, and activity of the enzyme can be categorized in ultrarapid metabolizer, extensive metabolizer, intermediate metabolizer, and poor metabolizer.Citation30,Citation31 Frequencies of poor metabolizers vary between populations, but can be as high as 19%, in addition to poor metabolizers, also intermediate metabolizers might not be able to sufficiently metabolize the drug.Citation25,Citation27,Citation32,Citation33 Studies in the CYP-2D/human CYP-2D6 mice model indicated that CYP-2D metabolism is also crucial for TQ activation, although the extent of CYP-2D6 involvement in TQ liver stage efficacy is not clear.Citation25,Citation32 Indeed, evidence from human studies suggested that the mechanisms of TQ activation might be different. Retrospective analysis of CYP-2D6 genotypes in a phase IIb TQ trial did not reveal an effect on the anti-relapse efficacy of TQ nor on TQ pharmacokinetics, whereas these data further support the liability of CYP-2D6 metabolism of PQ for efficacy against hypnozoites.Citation34 Despite its well described activity against P. falciparum and P. vivax blood stages, forming the rational for the prophylaxis development program, the mechanism of action is not known, but seems to be independent from the CYP-2D6 status.Citation35 Potential mechanisms include interference with heme polymerization or oxidative mechanisms. In contrast to PQ, TQ is highly active against Plasmodium blood stages.Citation34,Citation36 Indeed in the mouse malaria model, TQ was 9-times more active than PQ against blood stages of drug-sensitive P. berghei strains.Citation37 However, TQ is less active than CQ regarding the activity against blood stages of CQ-sensitive parasites.Citation35 Currently the most likely explanation for PQ failures after a full treatment course (14 days) is rather based on polymorphisms of CYP-2D6 and mostly not resistance of the parasite to PQ.Citation32
Safety and tolerability
More than 4,000 participants received at least one dose of TQ, and safety and tolerability profiles were generally good. See for an overview of studies with a particular focus on safety, tolerability, and pharmacokinetics, although safety was also studied and reported within efficacy trials (phase II and phase III). All studies were conducted in adults, except one that included children and young adults aged 12–20 years.Citation17 The adverse event (AE) profile was similar between single dose and weekly or monthly treatments. Most of the AEs reported were mild and transient. Low doses of TQ (200 mg) were associated with fewer AEs compared to high doses (400, 500, and 600 mg).Citation38 The most frequently reported AE was GID with diarrhea and abdominal pain.Citation39–Citation43
Pooled data of the three key studies for registration revealed that pruritus was the most frequent AE in participants exposed to CQ + TQ 300 mg single dose (37/317) followed by dizziness and nausea.Citation44
AEs of special interest (AESIs) include cardiac, ophthalmic, and hematologic side effects, in addition to neurologic and psychiatric side effects, as these are known to be prevalent at various degrees for related drugs.
Cardiac side effects
Cardiac effects of TQ were assessed in two studies with different doses (300, 450, 600, and 1,200 mg) given as monotherapyCitation39,Citation45 or in combination with CQ.Citation45 No major clinical significant effect of TQ up to 1,200 mg on the QTcF was detected, and co-administration of TQ + CQ did not prolong the QT interval more than CQ monotherapy.
Ophthalmic side effects
Eye disorders (mild vortex keratopathy, photophobia) were reported from one study in 69/74 (93%) Australian soldiers who received weekly TQ 200 mg for 6 months as a prophylactic regimen.Citation40 The ophthalmic changes were not associated with visual disturbances or impaired acuity/color vision. The keratopathy resolved within 1 year in all cases (incidence at 3 month 39% and at 6 month 10%). Fundoscopic assessments were made 3 months after the end of prophylactic treatment, and abnormalities were found in 27/69 individuals who took TQ and 4/17 individuals who took mefloquine. An independent group of experts investigated these findings and concluded that there is no sign of TQ-induced retinal toxicity. Induced ophthalmic changes are also known from other drugs, including the 4-aminoquinoline antimalarial CQ.Citation46,Citation47 Results of three following studies aimed to provide further evidence of the ophthalmic effects of TQ and did not find a clinically significant effect of TQ on ophthalmic function,Citation19,Citation41,Citation45 even so a mild keratopathy was also observed with TQ when given for a short course (3 days).Citation41 One unpublished study (NCT2658435) submitted with the dossier to FDA was a phase I study designed to investigate ophthalmic safety of the 300 mg TQ dose for radical cure. The study did not find a signal for retinal toxicity of this TQ dose.Citation24 Ophthalmic safety will remain of special interest in future studies.
Hematologic side effects
The main safety concern of TQ, like with other 8-AQ, is hemolysis in patients with G6PD-deficiency. G6PD-deficiency is frequently found in malaria endemic areas and is a hereditary, X-linked genetic defect so that males have either deficient G6PD activity (<30% of normal) or normal (>70% activity), whereas females can have intermediate (30%–60% activity) in addition to deficient and normal activity.Citation48
One study assessed the hemolytic risk of different doses of TQ (100, 200, and 300 mg) vs the standard 14-day regimen of PQ in healthy G6PD-deficient females with a G6PD enzyme activity of 40%–60% of the site median normal value in comparison to G6PD “normal” control individuals.Citation49 Dose-limiting toxicity (≥2.5 g/dL decline in hemoglobin and ≥7.5% decrease in hematocrit vs pre-treatment) was reached in 3/3 individuals receiving TQ 300 mg and 3/5 individuals receiving PQ. Following these results, further female individuals with a higher enzyme activity (61%–80%) were recruited to receive a 200 mg dose of TQ. Only two individuals could be identified (six planned), both had lower hemolysis compared to the individuals with lower enzyme activity. Overall, hemolysis induced by TQ was higher in individuals with low G6PD-activity levels and increased with TQ dose. A TQ 300 mg single dose had similar hemolytic potential as PQ 15 mg for 14 days. All other clinical studies with TQ excluded individuals with a G6PD-activity <70%. Despite of this, eight incorrectly typed G6PD-deficient individuals were included in five clinical studies, all recovered without sequelae, but one individual experienced a serious adverse event (SAE), she was hospitalized and needed blood transfusion.Citation24 These results further supported the contraindication of giving TQ to individuals with G6PD-activity <70%.
Neurologic/psychiatric side effects
Other clinical AEs that occurred with TQ administration were nervous and psychiatric disorders: insomnia, anxiety, depression, and mainly headache and dizziness. AEs were mild-to-moderate and transitionalCitation14,Citation15,Citation17,Citation40,Citation50 DETECTIVE-Part 2 and GATHER. A limited number of serious psychiatric adverse reactions, such as depression, suicidal behaviour, and psychosis, have been reported, mainly in relation with multiples doses and in individuals with a prior history of psychiatric disorders.Citation24 Many participants of these trials were military personal that were in a “psychologically hostile environment”, however, prescription is contraindicated in people with a history of psychotic disorders.Citation24
Serious adverse events
In the three key studies, a total of 29 SAEs occurred in 483 individuals receiving CQ + TQ. Of those, hemoglobin (Hb) decrease was the most frequent SAE. An Hb decrease ≥30% or >39 g/L from baseline or a drop in Hb below 60 g/L was a predefined SAE described in the study protocol.
The safety profile led to the addition of the following points to the prescribing information of TQ. G6PD testing has to be done in all individuals before prescription of the drug. Contraindications include unknown G6PD status, G6PD deficiency (<70% activity), pregnancy, breastfeeding in cases where the G6PD deficiency of the child is unknown/child is G6PD deficient, and individuals with a history of psychotic disorders. Mothers should not breastfeed infants of unknown/deficient G6PD status for 3 months after the last dose of TQ.Citation51
Pharmacokinetics
The pharmacokinetic profile of TQ was evaluated in several studies.Citation14,Citation20,Citation42,Citation43,Citation52–Citation54 TQ is slowly absorbed, with maximum plasma concentrations reached 12–16 hour post-administration. TQ has a large volume of distribution (~1,600 L), a long elimination half-life of 13–19 days, and is enriched in erythrocytes vs plasma. Administration of TQ with food increases adsorption and minimizes gastrointestinal side-effects.Citation42 A population modeling approach combined data from different studies and showed that body weight, age, gender, and ethnicity did not have a large impact on phar-macokinetics.Citation52 TQ pharmacokinetics were not affected by co-administration of commonly prescribed antimalarial drugs such as CQ or ACTs (dihydroartemisinin-piperaquine [DHA-PQP] or artemether-lumefantrine) so that TQ as well as the co-administered drug can be given without dose adjustment.Citation55 The area under the curve (AUC) of TQ after a single dose of 300 mg TQ in adults is ~100 µgh/mL and a Cmax of ~200 ng/mL.Citation55,Citation56 Modeling revealed that this dose (300 mg) would provide in 90% of individuals an AUC greater than 56.4 µg h/mL – a concentration that was a significant breakpoint as a predictor of relapse to infection, so that individuals with a higher AUC have a high probability of being relapse-free at 6 months.Citation56
Efficacy of TQ against P. vivax hypnozoites
In July/September 2018, FDA/TGA approved TQ (Krintafel/Kodatef) for radical cure of P. vivax malaria administered as a 300 mg single dose together with a standard P. vivax antimalarial to patients ≥16 years. Three keys studies (DETECTIVE Parts 1 and 2, and GATHER) (NCT1376167, NCT2216123)Citation24 provided evidence for efficacy and safety of this dose and led to dossier submission applying for registration.Citation1
In total, eight clinical studies investigated the anti-relapse efficacy of TQ in more than 2,700 adults ≥16 years with P. vivax malaria, of which more than 1,800 received TQ. Of these, 483 patients received a CQ (standard regimen)+TQ 300 mg single dose in three pivotal studies summarized below. All studies but one (7/8)Citation57 randomized study participants, and 5/8 were multicentric studies (NCT2216123, NCT1376167).Citation15,Citation19,Citation57 The anti-relapse efficacy of TQ was determined by assessing the proportion of overall recurrent P. vivax parasitemia in the Kaplan–Meier analysis at 6 months follow-up. All studies were conducted in malaria-endemic areas where P. vivax reinfections can occur. Unfortunately, for P. vivax no method is established to reliably distinguish relapses caused by hypnozoites from new infections. See for an overview of clinical studies focusing on TQ efficacy for radical cure as well as for malaria prophylaxis.
Table 2 List of clinical studies focusing on TQ efficacy
Key studies
The following three key studies established 300 mg of TQ as an effective single dose treatment for radical cure of P. vivax malaria combined to CQ standard treatment.
DETECTIVE, Part 1 (NCT1376167), was a phase IIb/III dose finding study conducted in four different countries (Brazil, Peru, India, and Thailand).Citation15 Single doses of 50, 100, 300, or 600 mg TQ (combined to a standard regimen of 3 days CQ) were compared to 14 days PQ (15 mg/day) + CQ and CQ only. Of 329 participants enrolled, 225 received CQ + TQ. Recurrence-free efficacy 6 months after treatment with CQ + TQ 300 mg was 89% (95% CI = 77–95) compared to 38% (95% CI = 23–52) receiving CQ only and 77% (95% CI = 63–87) in the CQ + PQ group, corresponding to 48/57, 21/54, and 34/50 participants without a recurrence at 6 months, respectively. Recurrence-free efficacy at 6 months for the other doses was for CQ + TQ 50 mg 58% (95% CI = 43–70), and for CQ + TQ 100 mg 54% (95% CI = 40–66), and for CQ + TQ 600 mg 92% (95% CI = 80–97). Based on the safety and efficacy data from this study, TQ 300 mg was selected as the lowest efficacious dose for further evaluations in part 2 of DETECTIVE study.Citation15
DETECTIVE, Part 2 (NCT1376167, was a phase III study which confirmed efficacy of the selected TQ 300 mg single dose, and the study was conducted in Brazil, Peru, Colombia, Thailand, Philippines, and Ethiopia.Citation24 Among the 522 included P. vivax malaria patients, recurrence-free efficacy at 6 months was for CQ + TQ 62% (95% CI = 55–69; n=155/260), for CQ + PQ 70% (95% CI = 60–77; n=83/129 and 28% (95% CI = 20–36; n=35/133) for CQ.Citation24
GATHER (NCT2216123) was a phase III study conducted in Brazil, Colombia, Ethiopia, Peru, Thailand, and Vietnam to assess the effect of the single dose of 300 mg CQ + TQ and 14-day course of CQ + PQ on clinically relevant hemolysis defined as an Hb decrease of ≥30% or >30 g/L from baseline or an overall drop in Hb below 60 g/L. In total, 251 participants were enrolled and, of those, 166 received a single dose of TQ 300 mg compared to PQ. Occurrence of clinically relevant hemolysis was similar between both groups: 2.4% for CQ + TQ vs 1.28% for CQ + PQ. The 6 months recurrence-free efficacy was similar between both regimens: 73% (95% CI = 65–79; n=112/166) for CQ + TQ and 75% for CQ + PQ (95% CI = 64–83; n=60/85).Citation24
These three studies showed an acceptable safety profile of the TQ 300 mg dose, when given together with CQ during a 6 month follow-up period and similar efficacy against recurrence for CQ + TQ compared to CQ + PQ.
Supporting studies
Several additional trials have been conducted evaluating TQ efficacy against hypnozoites at different TQ doses and regimens (1-, 3-, and 7-day treatment). The findings of studies published until 2015 were summarized by a Cochrane review.Citation58 A trial conducted in Thailand compared a high single dose (600 mg) of TQ vs a 3-day regimen of daily 600 mg TQ or 7 days 300 mg TQ.Citation20 Another study in Thailand tested a slightly lower single dose (500 mg) of TQ vs 3 days 500 mg TQ followed by the same dose (3 times 500 mg) 1 week laterCitation21 or vs 300 mg TQ given daily for 7 days. In both studies, TQ was compared to CQ alone and in the first study additionally to CQ + PQ. Data from these studies suggested that a single day treatment is sufficient to prevent P. vivax relapses. The rate of protection in the first study of the different TQ doses combined was 93% (95% CI = 7–99.9) and 91% in the second study (95% CI = 39–99). A third study performed in Thailand compared TQ 400 mg monotherapy (without CQ) for 3 days to CQ + PQ.Citation22 This study revealed that monotherapy of TQ is efficacious, but parasite and fever clearance is slower compared to treatment with CQ, so that combination therapy should be given. Apart from one study where doxycycline was given with TQ,Citation19 all participants were treated with a standard full course of CQ regimen (1,500 mg for 3 days) to treat the P. vivax infections. Pregnant/lactating women, children, individuals with G6PD-deficiency, or a mixed infection with malaria parasites other than P. vivax were not included in these studies.
In all these studies, activity against recurrences was evaluated without differentiating between relapse and new infection. Only samples of Detective Part 1 (NCT1376167) were additionally analyzed by a genotyping approach using three discriminatory markers.Citation59 Recurrent infections that differed by at least one marker from pre-treatment infections were defined as heterologous. The authors suggested that analysis of the frequency of homologous and heterologous recurrences could be used for estimation of TQ’s anti-hypnozoite efficacy against P. vivax, as they saw a dose response effect, ie, a reduction in homologous recurrences (probably relapses) at high doses of TQ, but not in heterologous recurrences (probably new infections). A separate analysis investigated, by mathematic modelling, the exposure response effect in time-to-recurrence of the infection. This revealed that a 300 mg dose led to a systemic exposure of TQ greater than AUC 56 µg/h/mL in 90% of individuals, an exposure that would lead to a significant lower 6 months recurrence rate.Citation56
Two studies assessing anti-recurrence efficacy are still ongoing: NCT2802501, a phase III study in Indonesia, testing the superiority of DHA + PPQ + TQ against DHA + PPQ alone and DHA + PPQ + PQ for the prevention of P. vivax malaria recurrence at 6 months in 150 healthy adult volunteers; and NCT2563496, a phase II, prospective, open-label, multicenter, non-comparative, single arm study of children with P. vivax malaria in Colombia, Thailand, and Vietnam to the assess safety and efficacy of CQ + TQ single dose in a total of 60 pediatric individuals aged 6 months to <16 years.
Prophylactic efficacy of TQ
In August 2018, the FDA also approved TQ, under the name ARAKODA, and shortly later Australia TGA, under the name KODATEF, for prophylaxis against all species of Plasmodium in patients aged 18 years and older for up to 6 months continuous dosing.Citation1 For this indication, TQ was co-developed by the US Army and 60 Degrees Pharmaceuticals. The proposed prophylactic regimen is a loading dose of 200 mg once daily for 3 days before travel to a malaria endemic region, followed by a weekly 200 mg maintenance dose during the stay and one final 200 mg dose in the week following exit from the malaria area.Citation38
Several studies have been undertaken since the first trial done in 1998 showed a protection in three out of four individuals (75%) exposed to experimental infection with P. falciparum by mosquito bite after intake of a 600 mg single dose TQ.Citation12 Since then, different doses and regimens have been evaluated that are nicely summarized in previous reviews.Citation60–Citation62 Five pivotal/key studies provided evidence for safety and prophylactic efficacy that led to drug approval: three phase II conducted in Africa (two in Kenya, one in Ghana), endemic for P. falciparum malariaCitation42,Citation50 (NCT2488980), and one phase III prophylactic efficacy study in non-immune Australian soldiers deployed in East Timor.Citation40
More than 1,250 adults, semi-immune and naïve, aged 18–55 years were included in the TQ prophylactic efficacy studies. The first time the now licensed prophylactic regimen (3 days 200 mg/day followed by weekly 200 mg) was given was in a study in Kenya comparing this regimen to 1) 3 days 400 mg/day followed by weekly placebo, 2) 3 days 400 mg/day followed by 400 mg weekly, and 3) placebo.Citation42 After 13 weeks of prophylaxis in the placebo group, 54/94 had malaria, in group 1, only receiving a loading dose, 16/54 had malaria (68% protective efficacy), and similar efficacy was found between the 200 mg and 400 mg prophylactic regimen, with 7/53 and 6/57 acquiring malaria, and a protective efficacy of 86% and 89%, respectively. As the 200 mg regimen was better tolerated, it was taken forward to the following studies, and the 200 mg regimen (n=492 individuals) was compared in a phase III trial to a standard regimen of mefloquine (162 individuals) in Australian soldiers going to East-Timor.Citation40 No case of malaria occurred in both groups during the 6 months deployment. Another study in semi-immune adults from Ghana evaluated a 3-day loading dose with a following maintenance dose of TQ: 1) 25 mg, 2) 50 mg, 3) 100 mg, 4) 200 mg, vs 5) mefloquine 250 mg, or 6) placebo for 13 weeks. Results showed that 86/94 individuals were infected with malaria in the placebo group, for the regimen: 1) protective efficacy was 32% and 58/93 individuals got infected, 2) 84%, 13/91, 3) 87%, 11/94, 4) 86%, 12/91, and 5): 86, 6/46. These results showed that all weekly TQ doses starting from 50 mg were similarly effective compared to mefloquine for prophylaxis.
Investigations revealed that TQ plasma concentrations of 40 ng/mL were not able to suppress asexual parasites where both P. falciparum and P. vivax are endemic. TQ plasma concentrations of 80 ng/mL should be achieved to prevent malaria, a concentration that is usually reached with the now prescribed 200 mg regimen in 95% of individuals.Citation38
Schizonticidal activity of TQ chemoprophylaxis against P. falciparum has been confirmed by a phase Ib double-blinded, placebo-controlled study in Australia. Two cohorts of eight healthy non-immune participants were assigned to either TQ 200 mg loading doses from day 1 to day 3 followed by another 200 mg on day 10 or placebo. Both groups were inoculated with ~2,800 viable P. falciparum parasites on day 13. None of the TQ recipients, but all placebo recipients, developed parasitemia, confirming TQ’s schizonticidal activity.Citation63
Discussion
Since 1950, PQ has been the drug of choice for treatment of the hypnozoites of P. vivax and P. ovale. Major shortcomings are PQ’s short half-life, implying a 14 day administration leading to poor compliance and a much lower effectiveness if applied in real life conditions. PQ is not safe in G6PD-defi-cient individuals, and has to be metabolized by cytochrome 2D6 to be active, a feature that is less pronounced in TQ.
TQ is a long-awaited alternative drug to prevent P. vivax relapse that has been licensed after a long clinical development. The activity of TQ was superior to PQ against blood-and liver-stage parasites in vitro. The major advantage of TQ compared to PQ is its long half-life (2–3 weeks), allowing for a single dose regimen to clear hypnozoites. It is generally safe and well tolerated and clinical evidence shows that it is similarly efficacious as PQ against relapses of P. vivax. No effectiveness study has been conducted evaluating TQ and PQ in a real-life setting head to head. However, it is obvious that a single dose regimen will be superior, closing the efficacy–effectiveness gap.
Hemolysis in G6PD-deficient individuals and formation of MHb remain the biggest restriction to the future usefulness of TQ. Especially in Asia, where P. vivax is most prevalent, a gene variant conferring severe G6PD-deficiency is frequent.Citation64 TQ should be only given to individuals with a G6PD enzyme activity >70%, whereas for PQ the threshold is >30%, therefore more individuals (usually females) will not be eligible to receive TQ treatment. A model was created by Watson et alCitation48 estimating the number of individuals that will not be covered by PQ or TQ treatment due to their underlying genetic deficiency. Most available methods to test for the G6PD-deficiency status need trained staff and significant laboratory infrastructure, lacking at many field sites. The most frequently used method includes the fluorescent spot test, a semi-quantitate method based on UV spectrophotometry that can discriminate between normal (>70%) and the deficient status (<30%), but does not reliably determine the intermediate enzyme activities of heterozygous females.Citation65,Citation66 Reference testing for this includes flow cytometry to determine the fraction of G6PD-deficient erythrocytes in heterozygous females.Citation67 The need of easy to use quantitative point-of-care-tests has been realized, and several organizations (eg, PATH) support the development.Citation68,Citation69 A number of new point-of-care tests are in development and will likely facilitate accurate testing in the future.Citation70
Overall, the safety data of TQ are good, and higher doses than for PQ can be administered to most individuals, as TQ has a greater therapeutic window. However, here the longer half-life shows its disadvantage: G6PD-deficient individuals are at a potential greater risk of hemolysis, as active concentrations of TQ will remain in the body for several weeks, whereas the PQ course can be stopped if hemolysis aggravates. In addition, TQ shares the shortcoming of PQ that it cannot be given to pregnant or lactating women. Even though clinical infection with P. vivax is associated with maternal anemia and may be deleterious for the infant’s health,Citation71 application is contraindicated in this group because of the danger of causing acute hemolytic anemia in the fetus. PQ can be given to children in a weight-adjusted dose. So far, the safety of TQ in children has not been established, but a pediatric trial is ongoing (NCT2563496), so that the indication will hopefully be expanded in future. Combination of TQ with other antimalarial drugs showed no major interactions, an important feature as combination therapy will be the choice for any future drug regimen, for acceleration of parasite clearance, but also for preventing the development of resistances.Citation55
Currently no other drug for hypnozoite treatment is in the drug development pipeline. The activity of antimalarial drugs against hypnozoite stages is only poorly understood and candidate drugs are scarce. This might be because, on the one hand, this stage is difficult to target as it is less metabolically active, and on the other hand because none of the Plasmodium parasite species that form hypnozoites can be kept in long-term continuous in vitro culture. In addition, in vivo and in vitro hypnozoite assays have only become available recently and are not yet implemented in a large scale in the malaria research community.Citation72–Citation76 Availability, simplification, and standardization of these assays might lead to new drug candidates for this indication that do not belong to the 8-aminochinolines.
In addition to the application as a drug for radical cure against P. vivax hypnozoites, TQ also gained approval for chemical prophylaxis against infections of all Plasmodium species. Its long half-life allows for a once weekly regimen for this indication, whereas most other currently recommended prophylactic drugs need daily administration (doxycycline, atovaquone-proguanil) leading to poor compliance. Mefloquine can be given with a weekly dosing for malaria prophylaxis, but neurologic and psychiatric side effects have been associated with its use for prophylaxis, so that TQ might be a good alternative. Currently, TQ is licensed to be given for the duration of 6 months for prophylaxis. However, a current study is ongoing to evaluate the long-term safety of TQ when it is given as prophylactic regimen for 1 year (NCT3320174), specially focusing on the ophthalmic safety.
Conclusion
WHO recognized in its recent agenda that more attention has to be paid to P. vivax infections to move forward in the elimination efforts. Even though TQ does not overcome all shortcomings of PQ, the TQ single dose anti-relapse treatment for hypnozoites of P. vivax as well as of P. ovale advances the treatment regimens for these malaria species profoundly. The changes in the WHO agenda might have given the final impulse to keep TQ in the development pipeline and finally bring it to the market.Citation19
Currently no other drugs are in the pipeline with potential for anti-relapse therapy.Citation72,Citation77 Considering that development from the early clinical phase to market approval takes ~10 years, shows that no alternative to 8-AQ for anti-relapse therapy will be available in the near future. The present data suggest that TQ will replace current treatment regimens with PQ and play a major role in the treatment agenda for control and elimination of P. vivax malaria.
Disclosure
The authors report no conflicts of interest in this work.
References
- FramptonJETafenoquine: first global approvalDrugs201878141517152330229442
- World Health OrganizationWorld Malaria Report2010 Available from: https://www.who.int/malaria/publications/atoz/9789241564106/en/Accessed October 25, 2018
- BassatQVelardeMMuellerIKey knowledge gaps for Plasmodium vivax control and eliminationAm J Trop Med Hyg2016956 Suppl627127430544
- World Health OrganizationControl and elimination of Plasmodium vivax malaria: a technical brief2017WHO Available from: http://www.who.int/malaria/publications/world-malaria-report-2017/report/en/Accessed June 8, 2018
- MuellerIGalinskiMRBairdJKKey gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasiteLancet Infect Dis20099955556619695492
- PriceRNTjitraEGuerraCAYeungSWhiteNJAnsteyNMVivax malaria: neglected and not benignAm J Trop Med Hyg2007776 Suppl798718165478
- BairdJKHoffmanSLPrimaquine therapy for malariaClin Infect Dis20043991336134515494911
- FernandoDRodrigoCRajapakseSPrimaquine in vivax malaria: an update and review on management issuesMalar J201110135122152065
- HillDRBairdJKPariseMELewisLSRyanETMagillAJPrimaquine: report from CDC expert meeting on malaria chemoprophylaxis IAm J Trop Med Hyg200675340241516968913
- ChuCSBanconeGMooreKAHaemolysis in G6PD heterozygous females treated with primaquine for Plasmodium vivax malaria: a nested cohort in a trial of radical curative regimensPLoS Med2017142e100222428170391
- LeslieTRabMAAhmadzaiHCompliance with 14-day primaquine therapy for radical cure of vivax malaria – a randomized placebo-controlled trial comparing unsupervised with supervised treatmentTrans R Soc Trop Med Hyg200498316817315024927
- BruecknerRPCosterTWescheDLShmuklarskyMSchusterBGProphylaxis of Plasmodium falciparum infection in a human challenge model with WR 238605, a new 8-aminoquinoline antimalarialAntimicrob Agents Chemother1998425129312949593172
- CooperRDMilhousWKRieckmannKHThe efficacy of WR238605 against the blood stages of a chloroquine resistant strain of Plasmodium vivaxTrans R Soc Trop Med Hyg19948866916927886774
- BruecknerRPLasseterKCLinETSchusterBGFirst-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarialAm J Trop Med Hyg19985856456499598455
- Llanos-CuentasALacerdaMVRueangweerayutRTafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (detective): a multicentre, double-blind, randomised, phase 2B dose-selection studyLancet201438399221049105824360369
- NasveldPKitchenerSEdsteinMRieckmannKComparison of tafenoquine (WR238605) and primaquine in the post-exposure (terminal) prophylaxis of vivax malaria in Australian Defence Force PersonnelTrans R Soc Trop Med Hyg200296668368412625150
- LellBFaucherJ-FMissinouMAMalaria chemoprophylaxis with tafenoquine: a randomised studyLancet200035592202041204510885356
- LearyKJRielMARoyMJA randomized, double-blind, safety and tolerability study to assess the ophthalmic and renal effects of tafenoquine 200 mg weekly versus placebo for 6 months in healthy volunteersAm J Trop Med Hyg200981235636219635898
- ElmesNJNasveldPEKitchenerSJKociskoDAEdsteinMDThe efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the southwest PacificTrans R Soc Trop Med Hyg2008102111095110118541280
- WalshDSWilairatanaPTangDBRandomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapseClin Infect Dis20043981095110315486831
- WalshDSLooareesuwanSWilairatanaPRandomized dose-ranging study of the safety and efficacy of WR 238605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in ThailandJ Infect Dis199918041282128710479159
- FukudaMMKrudsoodSMohamedKA randomized, double-blind, active-control trial to evaluate the efficacy and safety of a three day course of tafenoquine monotherapy for the treatment of Plasmodium vivax malariaPLoS One20171211e018737629121061
- WalshDSEamsilaCSasipraphaTEfficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant P. falciparum malariaJ Infect Dis200419081456146315378438
- Drug approval package: KRINTAFEL (tafenoquine) Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210795Orig1s000TOC.cfmAccessed December 3, 2018
- VuongCXieLHPotterBMDifferential cytochrome P450 2D metabolism alters tafenoquine pharmacokineticsAntimicrob Agents Chemother20155973864386925870069
- PybusBSMarcsisinSRJinXThe metabolism of primaquine to its active metabolite is dependent on CYP 2D6Malar J201312121223782898
- BennettJWPybusBSYadavaAPrimaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malariaN Engl J Med2013369141381138224088113
- IdowuORPegginsJOBrewerTGKelleyCMetabolism of a candidate 8-aminoquinoline antimalarial agent, WR 238605, by rat liver microsomesDrug Metab Dispos19952311177720510
- Vásquez-VivarJAugustoOHydroxylated metabolites of the antimalarial drug primaquine. Oxidation and redox cyclingJ Biol Chem199226710684868541313024
- ZhouSFPolymorphism of human cytochrome P450 2D6 and its clinical significance: part IClin Pharmacokinet2009481168972319817501
- Ingelman-SundbergMGenetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversityPharmacogenomics J20055161315492763
- MarcsisinSRSousaJCReichardGATafenoquine and NPC-1161B require CYP 2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compoundsMalar J2014131224386891
- BernardSNevilleKANguyenATFlockhartDAInterethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implicationsOncologist200611212613516476833
- St JeanPLXueZCarterNTafenoquine treatment of Plasmodium vivax malaria: suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the phase 2B detective trialMalar J20161519726888075
- VennerstromJLNuzumEOMillerRE8-Aminoquinolines active against blood stage Plasmodium falciparum in vitro inhibit hematin polymerizationAntimicrob Agents Chemother199943359860210049273
- RobertABenoit-VicalFDechy-CabaretOMeunierBFrom classical antimalarial drugs to new compounds based on the mechanism of action of artemisininPure Appl Chem200173711731188
- PetersWRobinsonBLMilhousWKThe chemotherapy of rodent malaria. LI. Studies on a new 8-aminoquinoline, WR 238,605Ann Trop Med Parasitol19938765475528122915
- 8242018FDA approves Arakoda for malaria prophylaxis in adults Available from: https://www.healio.com/infectious-disease/emerging-diseases/news/online/%7bb8c1d6f6-0622-4f6d-b592-677f47a75c0b%7d/fda-approves-arakoda-for-malaria-prophylaxis-in-adultsAccessed October 2, 2018
- GreenJAPatelAKPatelBRTafenoquine at therapeutic concentrations does not prolong Fridericia-corrected QT interval in healthy subjectsJ Clin Pharmacol2014549995100524700490
- NasveldPEEdsteinMDReidMRandomized, double-blind study of the safety, tolerability, and efficacy of tafenoquine versus mefloquine for malaria prophylaxis in nonimmune subjectsAntimicrob Agents Chemother201054279279819995933
- WarrasakSEuswasAFukudaMMComparative ophthalmic assessment of patients receiving tafenoquine or chloroquine/primaquine in a randomized clinical trial for Plasmodium vivax malaria radical cureInt Ophthalmol Epub 2018 Sep 29
- ShanksGDOlooAJAlemanGMA new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malariaClin Infect Dis200133121968197411700577
- EdsteinMDNasveldPEKociskoDAKitchenerSJGattonMLRieckmannKHGender differences in gastrointestinal disturbances and plasma concentrations of tafenoquine in healthy volunteers after tafenoquine administration for post-exposure vivax malaria prophylaxisTrans R Soc Trop Med Hyg2007101322623016814823
- US FDA approves Krintafel (tafenoquine) for the radical cure of P. vivax malaria | medicines for malaria venture Available from: https://www.mmv.org/newsroom/press-releases/us-fda-approves-krintafel-tafenoquine-radical-cure-p-vivax-malariaAccessed August 24, 2018
- MillerAKHarrellEYeLPharmacokinetic interactions and safety evaluations of coadministered tafenoquine and chloroquine in healthy subjectsBr J Clin Pharmacol201376685886723701202
- HollanderDAAldaveAJDrug-induced corneal complicationsCurr Opin Ophthalmol200415654154815523201
- MarmorMFKellnerULaiTYMellesRBMielerWFAmerican Academy of OphthalmologyRecommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision)Ophthalmology201612361386139426992838
- WatsonJTaylorWRJBanconeGChuCSJittamalaPWhiteNJImplications of current therapeutic restrictions for primaquine and tafenoquine in the radical cure of vivax malariaPLoS Negl Trop Dis2018124e000644029677199
- RueangweerayutRBanconeGHarrellEJGreenJAHemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PD Mahidol variant) versus G6PD-normal volunteersAm J Trop Med Hyg201797370271128749773
- HaleBROwusu-AgyeiSFryauffDJA randomized, double-blind, placebo-controlled, dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparumClin Infect Dis200336554154912594633
- Research C for DE and. Drug Approvals and Databases – Drug Trials Snapshots: KRINTAFEL Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ucm615729.htmAccessed October 10, 2018
- ThakkarNGreenJAKohGDuparcSTeneroDGoyalNPopulation pharmacokinetics of tafenoquine, a novel antimalarialAntimicrob Agents Chemother20186211 24 10 2018
- EdsteinMDKociskoDABrewerTGWalshDSEamsilaCCharlesBGPopulation pharmacokinetics of the new antimalarial agent tafeno-quine in Thai soldiersBr J Clin Pharmacol200152666367011736877
- CharlesBGMillerAKNasveldPEReidMGHarrisIEEdsteinMDPopulation pharmacokinetics of tafenoquine during malaria prophylaxis in healthy subjectsAntimicrob Agents Chemother20075182709271517517850
- GreenJAMohamedKGoyalNPharmacokinetic interactions between tafenoquine and dihydroartemisinin-piperaquine or artemetherlumefantrine in healthy adult subjectsAntimicrob Agents Chemother20166012 AAC.01588-16-7332
- TeneroDGreenJAGoyalNExposure-response analyses for Tafenoquine after administration to patients with Plasmodium vivax malariaAntimicrob Agents Chemother201559106188619426248362
- KitchenerSNasveldPEdsteinMDTafenoquine for the treatment of recurrent Plasmodium vivax malariaAm J Trop Med Hyg200776349449617360873
- RajapakseSRodrigoCFernandoSDTafenoquine for preventing relapse in people with Plasmodium vivax malariaCochrane Database Syst Rev20154CD010458
- BeckHPWampflerRCarterNEstimation of the antirelapse efficacy of tafenoquine, using Plasmodium vivax genotypingJ Infect Dis2016213579479926500351
- DowGSLiuJLinGSummary of anti-malarial prophylactic efficacy of tafenoquine from three placebo-controlled studies of residents of malaria-endemic countriesMalar J201514147326610844
- Novitt-MorenoARansomJDowGSmithBReadLTTooveySTafenoquine for malaria prophylaxis in adults: an integrated safety analysisTravel Med Infect Dis201717192728495354
- CrockettMKainKCTafenoquine: a promising new antimalarial agentExpert Opin Investig Drugs2007165705715
- McCarthyJSSmithBLReadLTDowLTA randomized, double-blinded, placebo-controlled study in healthy, non-immune adults to determine the schizonticidal activity of tafenoquine after challenge with Plasmodium falciparum blood stage parasites Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372009. Published June 22, 2018Accessed June 22, 2018
- RechtJAshleyEAWhiteNJUse of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countriesPLoS Negl Trop Dis2018124e000623029672516
- AinoonOAlawiyahAYuYHSemiquantitative screening test for G6PD deficiency detects severe deficiency but misses a substantial proportion of partially-deficient femalesSoutheast Asian J Trop Med Public Health200334240541412971572
- EspinoFEBibitJASornilloJBTanAvon SeidleinLLeyBComparison of three screening test kits for G6PD enzyme deficiency: implications for its use in the radical cure of vivax malaria in remote and resource-poor areas in the PhilippinesPLoS One2016112e014817226849445
- BanconeGKalnokyMChuCSThe G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjectsSci Rep201771982228852037
- PATHThis blood test is the key to curing the most stubborn form of malaria Available from: https://path.org/articles/blood-test-key-curing-most-stubborn-form-malaria/Accessed December 5, 2018
- PalSBansilPBanconeGEvaluation of a novel quantitative test for glucose-6-phosphate dehydrogenase deficiency: bringing quantitative testing for glucose-6-phosphate dehydrogenase deficiency closer to the patientAm J Trop Med Hyg2019100121322130350771
- LeyBBanconeGvon SeidleinLMethods for the field evaluation of quantitative G6PD diagnostics: a reviewMalar J2017161
- BardajíAMartínez-EspinosaFEArévalo-HerreraMBurden and impact of Plasmodium vivax in pregnancy: a multi-centre prospective observational studyPLoS Negl Trop Dis2017116e000560628604825
- HeldJJeyarajSKreidenweissAAntimalarial compounds in phase II clinical developmentExpert Opin Investig Drugs2015243363382
- GuralNMancio-SilvaLMillerABIn vitro culture, drug sensitivity, and transcriptome of Plasmodium vivax hypnozoitesCell Host Microbe201823339540629478773
- The malERA Refresh Consultative Panel on Basic Science and Enabling TechnologiesmalERA: an updated research agenda for basic science and enabling technologies in malaria elimination and eradicationPLoS Med20171411e100245129190277
- MarchSNgSVelmuruganSA microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivaxCell Host Microbe201314110411523870318
- MikolajczakSAVaughanAMKangwanrangsanNPlasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric miceCell Host Microbe201517452653525800544
- Medicines for Malaria VentureMMV Annual Report 2016Medicines for Malaria Venture Available from: https://www.mmv.org/newsroom/publications/mmv-annual-report-2016. Published June 14, 2018Accessed June 14, 2018
- GoyalNMohamedKRolfeKApplication of the stable isotope label approach in clinical development-supporting dissolution specifications for a commercial tablet product with Tafenoquine, a long half-life compoundAAPS J20182047429869298
- EdsteinMDKociskoDAWalshDSEamsilaCCharlesBGRieckmannKHPlasma concentrations of tafenoquine, a new long-acting antimalarial agent, in Thai soldiers receiving monthly prophylaxisClin Infect Dis200337121654165814689348
- DowGSMcCarthyWFReidMSmithBTangDShanksGDA retrospective analysis of the protective efficacy of tafenoquine and mefloquine as prophylactic anti-malarials in non-immune individuals during deployment to a malaria-endemic areaMalar J20141314924502679