Abstract
Objectives
To assess the performance of the (1,3)-β-D-glucan (BDG) detection assay in a large cohort of patients with suspected candidemia who were admitted to non-intensive care unit hospital wards.
Methods
This observational, retrospective cohort study was conducted in a 1,100-bed university hospital in Rome, where an infectious disease consultation team has been operational. Two groups of patients were included in the analysis: Group 1, patients with Candida bloodstream infection (BSI) who had at least one BDG test performed ±48 hours from the first positive blood culture (Candida BSI Group) and Group 2, patients with risk factors for candidemia who had at least one BDG test but had negative blood cultures (Control Group). Both Group 1 and Group 2 did not receive prior antifungal therapy. Different BDG cutoff values were considered: 80, 200, 300, 400, and ≥500 pg/mL. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic curve were calculated.
Results
A total of 1,296 patients were studied. Of them, 100 patients (candidemic) were in Group 1 and the remaining 1,196 patients (controls) were in Group 2. There were no differences in demographic characteristics between patients of the two groups. According to the above cutoff values, sensitivity (%) and specificity (%) of the BDG assay ranged from 91 to 60.7 and 87.7 to 97.8, respectively, whereas the PPV (%) and NPV (%) ranged from 38.2 to 68.3 and 99.1 to 97.0, respectively.
Conclusion
Serum BDG has a very high NPV in a population witĥ10% prevalence of candidemia. This NPV may support decisions to discontinue antifungal therapy in those patients who were empirically treated because of the suspect of candidemia.
Background
Bloodstream infections (BSIs) due to Candida spp. are a frequent clinical condition in hospitalized patients, which significantly contributes to their morbidity and mortality. Candida spp. account for 9% of all BSIs and up to 25% of BSIs associated with central venous catheter (CVC).Citation1 The mortality rate of candidemia has been found to be high in the past two decades, being estimated as 5%–71%.Citation2–Citation4
The gold standard method for the diagnosis of Candida BSIs are blood cultures, which usually take about 2 days to yield positive results, but their sensitivity is as low as 50%.Citation5,Citation6 Many studies have investigated the risk factors for candidemia; however, the list of risk factors is largeCitation7 and most of the hospitalized patients shared at least one of these risk factors. Several scores have been evaluated to help in identifying patients at the highest risk of Candida BSIs, but they are often difficult to calculate in nonintensive care unit (non-ICU) settings.Citation8,Citation9 Therefore, when suspecting candidemia, criteria that allow physicians to start antifungal therapy remain controversial. Anyway, appropriate antifungal therapy, especially when early administered, was seen to correlate with a better survival.Citation10
To reduce unnecessary antifungal empirical therapy, many efforts have been devoted to improve the diagnostic sensitivity of culture-independent tests.Citation11 Among them, the T2Candida nanodiagnostic panel (T2 Biosystems, Lexington, MA, USA) and a (1,3)-β-D-glucan (BDG) assay (Fungitell; Associates of Cape Cod, East Falmouth, MA, USA) were cleared by the US Food and Drug Administration for the diagnosis of candidemia and invasive fungal infections, respectively.Citation12 As Bayesian biomarkers, these tests assign a probability of infection; thence, management decisions based on test results will be left to the judgment of physicians .Citation12 However, both molecular and nonmolecular diagnostic tests could be a strong support for more rapidly identifying patients who should start empirical antifungal treatment.Citation13
With regard to BDG, studies conducted in the ICU setting showed that BDG assay can be used to specifically improve the early discrimination between patients with culture-documented candidemia and patients with suspected candidemia.Citation14–Citation16 Particularly, high negative predictive values (NPVs) of BDG (≥97%) in patients at risk for candidemiaCitation12 could be useful for an early discontinuation of antifungal therapy. To the best of our knowledge, very few studies were conducted in non-ICU wards until now. In the present study, we aimed to assess the performance of the BDG assay in a large cohort of patients with suspected candidemia admitted to non-ICU hospital wards.
Methods
This observational, retrospective, case–control study was conducted at a 1,100-bed university hospital (Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy), where an inpatient infectious disease consultation team (IDCT) has operated since November 2012. The team is made up of four infectious disease specialists. Any hospital physician operating in medical and surgical units can request an infectious disease consultation via the hospital’s computerized information system. The IDCT takes charge of patients at the bedside within 24 hours of the request. The service is not active in Hematology unit and ICU, so patients from these wards were not included in the study. Follow-up consultations are provided only after a request by the ward physician. An alert system for blood cultures is active from Monday to Saturday: a microbiologist informs the in-charge infectious disease specialist of any positive blood culture at the time of identification. Data from every consultation is daily added into a standardized database.
To calculate the sensitivity, specificity, positive predictive value (PPV), and NPV of BDG assay, we identified two groups of people. Group 1 consisted of all patients with culture-documented candidemia who had at least one BDG test performed ±48 hours from the first positive blood culture and had never been treated before with antifungal therapy (ie, Candida BSI Group). Group 2 consisted of all patients with at least one risk factor for candidemia (ie, surgery in the previous 30 days, presence of CVC, antibiotic therapy in the last 90 days, dialysis, or an immunosuppression status) who had at least one BDG test performed but had negative blood cultures and had never been treated with antifungal therapy (ie, Control Group). All consecutive patients from January 2013 to November 2016 who met one of the two study group criteria were enrolled in Candida BSI Group or Control Group, respectively. Candida BSI was defined as the isolation of Candida spp. in at least one blood culture drawn from a patient. Candida catheter-related (CR)-BSI was defined when the time of positivity of blood culture from a peripheral vein was at least 2 hours more than time of positivity of blood culture from a CVC.Citation17 Septic shock at the time of blood culture was also assessed. During the study period, the rate of Candida BSIs was 16% of all BSIs identified at our Institution.
The Fungitell assay (Associates of Cape Cod) was used for BDG measurement. BDG results were evaluated using different cutoff values: 80, 200, 300, 400, and ≥500 pg/mL. Candida spp. were isolated from blood using the Bactec (Thermo Fisher Scientific, Waltham, MA, USA) or BacT/Alert (bioMérieux, Marcy l’Etoile, France) system and were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.Citation18 For clinical use, the BDG test was considered positive if BDG level was above the manufacturer’s recommended cutoff (80 pg/mL).
No informed consent was required because the activity of the IDCT constitutes routine clinical practice and only anonymized data were analyzed. The need for informed consent was waived by the local Institutional Review Board of the Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, that approved the study (protocol number 49/18).
Statistical analysis
Demographic, epidemiological, and clinical characteristics of patients in Candida BSI Group were compared to those of Control Group. Normally distributed values were expressed as mean (±SD), and nonnormally distributed values as median (interquartile range). Chi-squared or Fisher’s exact test was used to compare the distribution of categorical variables, and Student’s t-test or Mann–Whitney U test was used to compare quantitative variables. A two-sided P-value <0.05 was considered statistically significant. To calculate the diagnostic performance of BDG assay, the sensitivity, specificity, PPV, and NPV of the test and their 95% CIs were calculated.
The 30-day crude mortality rate (death from any cause within 30 days from the first positive blood cultures in Candida BSI Group and from the BDG assay in Control Group) was also calculated for both the study groups stratifying for the BDG result. The discriminatory power of the BDG assay was assessed by the area under the receiver operating characteristic (ROC) curve (AUROC) calculation. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
We identified 1,296 patients with at least one BDG result, of which 100 patients were in Candida BSI Group and 1,196 patients were in Control Group. The mean age was 65 (±20) years, and 774 patients (59.7%) were males. Approximately two-thirds of the study population was admitted to Medicine wards and a third to General Surgery (and other surgical specialties) wards. The clinical characteristics of the study population are shown in . Patients in Candida BSI Group were more likely to have septic shock (9.1% vs 0.7%; chi-squared test, P<0.001). Risk factors for Candida infection, such as the presence of CVC or a previous hospital stay, were significantly more common in Candida BSI Group. In 38 cases (38%) of candidemia, a CR-BSI was identified. Sixty-two percent of Candida BSIs were caused by Candida albicans, 24.2% by Candida parapsilosis, and 5% by Candida glabrata. The 30-day mortality rate was significantly higher in Candida BSI Group than in Control Group (30.3% vs 8.6%; chi-squared test, P<0.0001).
Table 1 Characteristics of the study population
The mean of risk factors for candidemia was 0.86 (SD 0.80). It was higher in people with positive BDG result (1.13 [SD 0.84]) than in people with negative BDG result (0.80 [SD 0.78]) (t-test, P<0.001). Only few patients (33, 2.5%) had three or more risk factors.
Using the 80 pg/mL cutoff value, a negative BDG result was found in nine (9%) of the 100 patients in Candida BSI Group, and in 1,049 (87.7%) of the 1,196 patients in Control Group. Only one of the nine patients with candidemia and negative BDG result had a BSI due to C. parapsilosis. The number of patients who had candidemia despite a negative BDG result was nine out of 1,058 (0.85%). In Control Group, 34 (2.8%) patients had a BDG cutoff of ≥500 pg/mL. The sensitivity, specificity, PPV, and NPV of BDG test at different cutoff values are shown in . Time to BDG test did not correlate with the BDG value (linear regression, P=0.34).
Table 2 Diagnostic performance of the BDG detection assay at different cutoff values
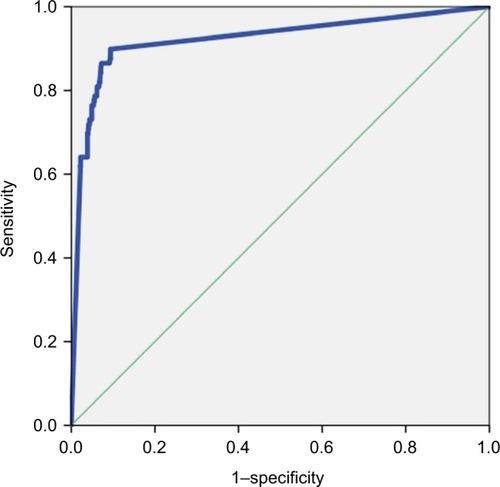
With the increase of the cutoff value (80 to ≥500 pg/mL), sensitivity of the biomarker decreased (from 89.8% to 60.7%), whereas specificity remained >90% (up to 97.8%). The AUROC was ≥0.79 for all the cutoff values of BDG. shows the AUROC calculated using the cutoff of 80 pg/mL as a diagnostic value.
Figure 1 Area under the ROC curve showing the diagnostic sensitivity and specificity of the BDG detection assay.
Abbreviations: BDG, (1,3)-β-D-glucan; ROC, receiver operating characteristic.
Overall, the 30-day mortality of patients with a positive BDG result was higher than that of patients with a negative BDG result (19.8% vs 8.0%; chi-squared test, P<0.001). Only in Control Group, patients with a positive BDG result trended toward higher mortality compared to patients with a negative BDG result (12.2% vs 8.0%; chi-squared test, P=0.08). In particular, 18 of 34 patients with a positive BDG result died within 30 days without starting antifungal therapy (mean of survival, 7.9 days [SD 7.3]), and six of these patients had a BDG value of ≥500 pg/mL (mean of survival, 8 days [SD 8.6]).
Discussion
In the present study that involves non-ICU patients, we show that the BDG detection assay (cutoff, 80 pg/mL) had a high NPV (>99%), thus confirming the good accuracy of the assay for identifying patients without Candida BSI. Our findings may support strategies aimed at discontinuing empirical antifungal therapy when BDG results are negative.
Several studies have evaluated the performance of BDG assay in different clinical contexts. Studying a small population of ICU patients in 2011, Posteraro et al showed that the NPV of BDG was higher (98.7%) than that of Candida score (≥3, 97.2%) or colonization index (≥0.5, 91.7%).Citation14 In a study on 89 surgical ICU patients with abdominal candidiasis, Tis-sot et al found an NPV of 78%,Citation15 whereas in a study on 100 patients, most of them admitted to ICU, the NPV of BDG was 86%–90%.Citation16 Giacobbe et al recently found a high PPV (96%) and a high NPV (93%–95%) when using both BDG and procalcitonin for the diagnosis of Candida BSI in ICU patients.Citation19 In two meta-analyses,Citation20,Citation21 the AUC for BDG was 0.88 and 0.84. In a study on application of mass spectrometry in diagnosis of fungal infections, Mery et al showed that mass spectrometry disaccharide index potentially comple ments BDG detection.Citation22 Although it is difficult to compare our results with those of previous reports because of differences in the study design, invasive candidiasis definition, patient populations, and negative controls, the NPV of BDG was always excellent. Unlike previous studies, we included a very large population and, to maximize the stringency of the study, we used as controls those patients with risk factors for candidemia but who did never receive antifungal therapy. Some authors highlighted that BDG test result could be more frequently negative in patients with C. parapsilosis BSI.Citation23 However, in our population only one of nine patients with a negative BDG result had a C. parapsilosis BSI.
The pretest probability in the present cohort was 8%, slightly lower than in other published cohorts. This is probably due to the non-ICU setting of the study. It should be considered that in extremely high-risk patients (such as those in ICU with >30% of pretest likelihood), NPV could be lower than that in our study.
Confirming previously published results, the role of BDG in the diagnosis of Candida BSI is probably less relevant than excluding it. In fact, sensitivity of BDG is suboptimal, ranging from 77% to 91%.Citation24,Citation25 In our study, even at the highest cutoff (≥500 pg/mL), sensitivity was 61%. BDG can be found in many biological compounds, and several factors, such as antimicrobial therapy, nutritional intake, albumin, surgical sponges and/or gauze, and hemodialysis products, are known to be possible causes of false positivity.Citation26 According to some studies, even systemic bacterial infections may result in false-positive BDG results.Citation27 However, since 18 patients in Control Group (1.5%) died within 30 days, we cannot definitively rule out the presence of Candida infection considering that the blood culture sensitivity is suboptimal.Citation28
Empirical antifungal therapy accounts for about 40% of antifungal use.Citation16,Citation29–Citation31 Hence, efforts should be focused on improving the diagnostic sensitivity and specificity of available tests. Considering the well-recognized limitations of blood cultures in diagnosing invasive candidiasis,Citation32,Citation33 an accurate and rapid biomarker could be very useful in treatment decision for patients with suspected candidemia. BDG results can be available in 12–24 hours. Considering the NPV of our study, clinicians could stop treatment based on negative result rapidly but not before the results of negative blood cultures are known (if possible, never before the first 48–72 hours). Determination of BDG is costly, and there are no cost-benefit studies of BDG determination in non-ICU patients. For these reasons, in our opinion, its use should be limited to professionals with a great expertise in the infectious diseases field and the management of invasive fungal infections.
People with positive BDG result had a higher mortality in our study. This result confirms previous findings.Citation34–Citation36 It should be noted that the mean of survival in people with positive BDG result within the Control Group was very short. We do not know whether an earlier initiation of empirical antifungal therapy in these patients could have modified the survival.
The study has several limitations. First, since it is a monocentric study, the generalizability of the results must be demonstrated. Second, this is not an intervention study. The real efficacy of a BDG-based strategy to control overtreatment with antifungal regimens is not widely demonstrated. Unfortunately, only retrospective studies were published on the use of BDG in daily practice and almost all of them were done in ICU patients. Third, the usefulness of BDG in uncertain Candida infections is unknown. Fourth, no data on invasive candidiasis in the absence of candidemia (ie, abdominal candidiasis) are reported and the performance of BDG in such a situation was not evaluated.
Conclusion
The NPV value of BDG result was optimal even in a real-world setting, possibly supporting prospective studies on discontinuation of empirical antifungal therapy when BDG results are negative. The control of overtreatment is a cornerstone of antimicrobial stewardship: cost-effectiveness studies on the use of BDG in the context of a suspected invasive candidiasis are strongly warranted as well as studies on a BDG-based therapeutic approach.
Disclosure
The authors report no conflicts of interest in this work.
References
- WisplinghoffHBischoffTTallentSMSeifertHWenzelRPEdmondMBNosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance studyClin Infect Dis200439330931715306996
- FalagasMEApostolouKEPappasVDAttributable mortality of candidemia: a systematic review of matched cohort and case-control studiesEur J Clin Microbiol Infect Dis200625741942516773391
- TumbarelloMFioriBTrecarichiEMRisk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospitalPLoS One201273e3370522479431
- BassettiMMerelliMRighiEEpidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and SpainJ Clin Microbiol201351124167417224108614
- ClancyCJNguyenMHFinding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient careClin Infect Dis20135691284129223315320
- AvniTLeiboviciLPaulMPCR diagnosis of invasive candidiasis: systematic review and meta-analysisJ Clin Microbiol201149266567021106797
- ScudellerLViscoliCMenichettiFAn Italian consensus for invasive candidiasis management (ITALIC)Infection201442226327924272916
- LeónCRuiz-SantanaSSaavedraPA bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonizationCrit Care Med200634373073716505659
- PittetDMonodMSuterPMFrenkEAuckenthalerRCandida colonization and subsequent infections in critically ill surgical patientsAnn Surg199422067517587986142
- KollefMMicekSHamptonNDohertyJAKumarASeptic shock attributed to Candida infection: importance of empiric therapy and source controlClin Infect Dis201254121739174622423135
- ArvanitisMAnagnostouTFuchsBBCaliendoAMMylonakisEMolecular and nonmolecular diagnostic methods for invasive fungal infectionsClin Microbiol Rev201427349052624982319
- ClancyCJNguyenMHNon-culture diagnostics for invasive candidiasis: promise and unintended consequencesJ Fungi20184127
- ClancyCJNguyenMHDiagnosing Invasive CandidiasisJ Clin Microbiol2018565e019091729444828
- PosteraroBde PascaleGTumbarelloMEarly diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-glucan assay, Candida score, and colonization indexCrit Care2011155R24922018278
- TissotFLamothFHauserPMβ-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasisAm J Respir Crit Care Med201318891100110923782027
- Martínez-JiménezMCMuñozPValerioMVenaAGuineaJBouzaECombination of Candida biomarkers in patients receiving empirical anti-fungal therapy in a Spanish tertiary hospital: a potential role in reducing the duration of treatmentJ Antimicrob Chemother201570113107311526311840
- ParkKHLeeMSLeeSODiagnostic usefulness of differential time to positivity for catheter-related candidemiaJ Clin Microbiol20145272566257224829236
- de CarolisEVellaAVaccaroLDevelopment and validation of an in-house database for matrix-assisted laser desorption ionization-time of flight mass spectrometry-based yeast identification using a fast protein extraction procedureJ Clin Microbiol20145251453145824554755
- GiacobbeDRMikulskaMTumbarelloMCombined use of serum (1,3)-β-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care unitsCrit Care201721117628693606
- HouTYWangSHLiangSXJiangWXLuoDDHuangDHThe screening performance of serum 1,3-beta-D-glucan in patients with invasive fungal diseases: a meta-analysis of prospective cohort studiesPLoS One2015107e013160226146829
- LamothFCrucianiMMengoliCβ-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hemato-logical malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3)Clin Infect Dis201254563364322198786
- MeryASendidBFrançoisNApplication of mass spectrometry technology to early diagnosis of invasive fungal infectionsJ Clin Microbiol201654112786279727605710
- MikulskaMGiacobbeDRFurfaroELower sensitivity of serum (1,3)-β- d -glucan for the diagnosis of candidaemia due to Candida parapsilosisClin Microbiol Infec2016227646.e5646.e8
- Ostrosky-ZeichnerLAlexanderBDKettDHMulticenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humansClin Infect Dis200541565465916080087
- PersatFRanqueSDerouinFMichel-NguyenAPicotSSulahianAContribution of the (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infectionsJ Clin Microbiol20084631009101318160456
- MartyFMKooSRole of (1→3)-beta-D-glucan in the diagnosis of invasive aspergillosisMed Mycol200947Suppl 1S233S24018720216
- AlbertOToubasDStradyCReactivity of (1→3)-β-d-glucan assay in bacterial bloodstream infectionsEur J Clin Microbiol Infect Dis201130111453146021479838
- FriedrichRRappoldEBogdanCHeldJComparative Analysis of the Wako β-Glucan test and the Fungitell® assay for the diagnosis of Candidemia and Pneumocystis jirovecii pneumoniaJ Clin Microbiol2018e004641829899003
- LeónCOstrosky-ZeichnerLSchusterMWhat’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patientsIntensive Care Med201440680881924718642
- ValerioMRodriguez-GonzalezCGMuñozPEvaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently neededJ Antimicrob Chemother20146971993199924659750
- RuhnkeMAntifungal stewardship in invasive Candida infectionsClin Microbiol Infect201420Suppl 6111824661820
- BerenguerJBuckMWitebskyFStockFPizzoPAWalshTJLysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infectionDiagn Microbiol Infect Dis19931721031098243032
- GrollAHShahPMMentzelCSchneiderMJust-NueblingGHuebnerKTrends in the postmortem epidemiology of invasive fungal infections at a university hospitalJ Infect199633123328842991
- BassettiMMerelliMAnsaldiFClinical and therapeutic aspects of candidemia: a five year single centre studyPLoS One2015105e012753426010361
- SbranaFSozioEBassettiMIndependent risk factors for mortality in critically ill patients with candidemia on Italian Internal Medicine WardsIntern Emerg Med201813219920429322386
- PosteraroBTumbarelloMde PascaleG(1,3)-β-d-Glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: an observational studyJ Antimicrob Chemother20167182262226927125554
