Abstract
The landscape of rotavirus (RV) infection has changed substantially in recent years. Autoimmune triggering has been added to clinical spectrum of this pathology, which is now known to be much broader than diarrhea. The impact of RV vaccines in these other conditions is becoming a growing field of research. The importance of host genetic background in RV susceptibility has been revealed, therefore increasing our understanding of vaccine effectiveness and giving some clues about the limited efficacy of RV vaccines in low-income settings. Also, interaction of RV with intestinal microbiota seems to play a key role in the process of infection vaccine effect. This article reviews current findings on the extraintestinal impact of RV infection and their widening clinical picture, and the recently described mechanisms of host susceptibility to infection and vaccine effectiveness. RV infection is a systemic disease with clinical and pathophysiological implications beyond the gut. We propose an “iceberg” model for this pathology with almost hidden clinical implications away from the gastrointestinal tract and eventually triggering the development of autoimmune diseases. Impact of current vaccines is being influenced by host genetics and gut microbiota interactions and these factors must be taken into account in the development of public health programs.
Introduction
For many years, rotavirus (RV) pathology has remained an undervalued condition and limited only to the gastrointestinal tract in the eyes of most clinicians. However, recent evidence from hidden systemic implications of RV infection has renewed interest in this pathology.Citation1 It is now clear that RV goes beyond the gastrointestinal infection. The classic term “acute gastroenteritis (AGE) by RV” is increasingly replaced by “pathology by RV”, reflecting the well-established systemic implications of the infection.Citation2
This “rotavolution” – or change in the traditional clinical perception of RV infection – has been encouraged by the impact of RV vaccines,Citation2 through a series of published unexpected benefits that have scrambled the long-held perception of diarrhea as the main or only clinical effect of RV.Citation3,Citation4 In fact, diarrhea is not even necessary for the diagnosis of RV infection since an important percentage of rotaviremic patients show no clinical intestinal manifestations.
Furthermore, the recently established link between host genetics, gut microbiota and RV susceptibility have focused our interest on the interaction of RV with their host, and on the eventual “natural” resistance of some individuals to the infection or to the systemic spread of the virus.Citation5
The present review aims to expand our understanding of this pathology, providing an updated rationale for the concept “rotavolution”.Citation2,Citation6 We propose an “iceberg” model for the RV pathology () that explains the underestimated or previously ignored clinical implications of this infection beyond the gastrointestinal tract.
Figure 1 The “iceberg” model of RV infection proposal: AGE and diarrhea are just the most obvious and frequent clinical picture of the pathology by RV.
Notes: Systemic viral spreading occurs and might produce several other extraintestinal manifestations such as seizures in the CNS (). Moreover, RV infection may be a trigger for the development of autoimmune pathology in individuals with a specific genetic background through a proposed mechanism of immune tolerance breakdown at early ages.
Abbreviations: AGE, acute gastroenteritis; CNS, central nervous system; RV, rotavirus.
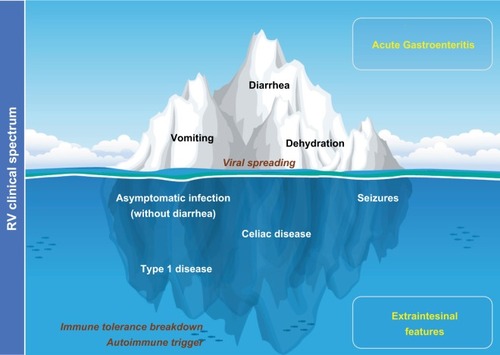
The clinical spectrum of RV
The clinical status of RV infection is updated every year, with growing evidence pointing to a link between RV and the development of a number of autoimmune diseases in susceptible subjects as the most impactful systemic consequence. The RV pathology is systemic; the RV goes beyond the intestinal lumen irrespective of the presence or not of diarrhea.Citation1 Since RV antigenemia detection is not a routine diagnostic tool, the impact of RV as a pathogen in children is underestimated, especially in the absence of diarrhea, where the routine RV diagnostic work-up is not even used. Seizures may constitute the most frequently recognized extraintestinal manifestation of RV infection.Citation7
Acute extraintestinal manifestations
The role of RV infection as the cause of seizure or clinical neurological illness is well established in the scientific literature;Citation8 these are the most common neurological symptoms with an incidence of 4.0% to 7.7% of patients, respectively.Citation7 Furthermore, several reports described diffuse cerebral white matter lesions in neonates with RV-associated seizures.Citation9 The pathophysiological mechanism of RV-induced seizure remains elusive. A hypothesis has been proposed that argues a key role for the viral nonstructural protein 4 (NSP4) through a disruption of Ca2+ homeostasis that may result in neurotox-icity and neurotransmitter dysregulation.Citation10 NSP4 has been demonstrated to act as an enterotoxin-inducing secretion of Cl− ions and water through phospholipase-dependent elevation of cytosolic Ca2+. However, this effect was not limited to the intestinal cells as NSP4 can bind to the surface of various cell types through interaction with glycosaminoglycans.Citation11 Thus, the pathophysiological effects of NSP4 may have a broader cellular tropism and exert a wider range of physiological effects in the host. NSP4 has also been shown to have inherent membrane destabilizing properties.Citation12 In this vein (see also below), it has been proposed that changes in NSP4 susceptibility may affect the impact of RV vaccines in seizures.Citation13
Another possible explanation for the RV-induced seizure is through direct central nervous system (CNS) infection action.Citation14 This hypothesis is supported by several studies demonstrating RV detection on spinal fluid,Citation15 and by experimental animal models.Citation16 However, RV evidence has not been sought in spinal fluid in all cases nor the pathogenic mechanism established.
Other possible acute extraintestinal manifestations are listed in ; these are mainly based on case reports and therefore it is more difficult to estimate their real burden.
Table 1 Extraintestinal manifestations of RV infection.
Clinical significance of antigenemia
RV antigenemia and ribonucleic acid (RNAemia) detection are common findings in RV infectionCitation17,Citation18 even in the absence of diarrhea;Citation16 furthermore, these features have recently been linked to increased severity of fever and vomiting by an unknown mechanism,Citation19 and particularly associated to the RV genotype G1P[8]Citation7 infections. However, no correlation between RV viremia or antigenemia and diarrhea has been found.
Previously, the activation of dendritic cells in the acute phase of infection appeared to correlate with levels of antigenemia, and a high prevalence of NSP4 gene was detected in peripheral blood mononuclear cells, suggesting white blood cells as the source of extraintestinal viral replication.Citation20 Furthermore, a correlation between cytokine levels and RV antigenemia was found in patients with fever, suggesting that the severity of systemic infection contributes to the systemic manifestation of disease.Citation21
Trigger for autoimmune diseases
The role of RV as an environmental trigger of several autoimmune diseases has been the focus of interest in the last few years.Citation22 Special attention has been paid to celiac disease, an autoimmune enteropathy, where a high frequency of RV infections may increase the risk of celiac disease in childhood in genetically predisposed individuals.Citation23 Frequent RV infections during infancy predicted a higher risk of celiac disease in childhood with a relative risk of 3.76 for individuals with two or more infections.Citation23 A study carried out in Italian patients demonstrated that children born in the summer were at higher risk to develop celiac disease than subjects born in other seasons; this study pointed to the coincidence in the timing of the first introduction of gluten and the highest peak of RV infection as possible causes.Citation24
In a recent study Kemppainen et alCitation25 showed that this risk was modified by human leukocyte antigen (HLA) genotype, gluten consumption, breastfeeding and also RV vaccination, indicating complex interactions among infections, genetics, and diet in the development of celiac disease.
The mechanism for this association is unclear. Several authors have proposed a hypothesis of molecular mimicry between RV capsid protein VP7 and the human-tissue transglutaminase, the main autoantigen of the celiac disease.Citation26 It has been shown that VP7 can be recognized by certain anti-transglutaminase antibodies present in the serum of celiac patients; moreover, these antibodies are present before the onset of the celiac disease, preceding the detection of anti-transglutaminase and anti-endomysium antibodies. However, Ziberna et alCitation27 have recently questioned this hypothesis in a study that showed lack of evidence for this RV-dependent molecular mimicry as a trigger for celiac disease.
Lastly, another interesting mechanism has been proposed by Bouziat et alCitation28 supporting a role for infection with reovirus (the double-stranded RNA virus family to which the RV belongs to) in triggering the development of celiac disease. Using a viral infection model, the authors showed that reovirus infection disrupts intestinal immune homeostasis at inductive and effector sites of oral tolerance, by suppressing peripheral regulatory cells conversion and promoting an exacerbated immune response to dietary antigens, in a type one interferon (IFN)-related pathway.Citation28
Similarly, RV infection has been claimed as a triggering factor for type I diabetes mellitus, an autoimmune endocrinopathy leading to selective destruction of insulin-producing pancreatic beta cells.Citation29 Data from experimental animals as well as in vitro studies indicate that RV, like other viruses, is clearly able to modulate the development of diabetes via different mechanisms, including direct-beta cells lysis, bystander activation of autoreactive T cells, suppression of regulatory cells, and molecular mimicry.Citation30,Citation31 However, the exact mechanism is not entirely clear and some authors consider this association unlikely.Citation32
Using a bioinformatics approach, RV VP6 protein has also been identified as a potential threat for myasthenia gravis, a chronic muscular neurodegenerative autoimmune disorder.Citation33 In this in silico study, most conserved structural protein VP6 matches at two regions with ryanodine receptor, the autoimmune target associated with the myasthenia. Furthermore, it was observed that these regions remain conserved in all circulating RV strains and showed significant antigenicity with respect to myasthenia-associated HLA haplotypes.
The lessons learnt from RV vaccination
Implementation of RV vaccines has substantially decreased hospitalizations from RV and all-cause AGE among children <5 years of age.Citation34 Vaccination has also had an indirect effect among unvaccinated older children and young adults.Citation35 More surprisingly, the introduction of RV vaccine has also impacted on extraintestinal RV manifestations in a way that we are just beginning to understand.
Impact of RV vaccine on seizures
Payne et alCitation4 were the first to demonstrate that a full course of RV vaccination significantly reduced the risk of childhood seizures during the year following vaccination, with an 18–21% decrease in relative risk of seizures requiring hospitalization as compared with children not receiving the vaccine. Several other teams have found similar protective effects for RV vaccine against seizures and convulsions,Citation13,Citation36 even in a moderate vaccine coverage scenario (). The unexpected benefit of RV vaccination in these studies seems more marked in the youngest infants (<5 years). Yeom et alCitation13 described changes in the clinical characteristics of RV-associated seizures after the introduction of RV vaccines, with more common afebrile seizures and a longer interval between gastroenteritis symptoms and the onset of seizures. Action on NSP4 is identified as the cause of this altered clinical course, related to changes in NSP4 immunity and the generation of anti-NSP4 IgG antibodies after vaccination.Citation37
Figure 2 Data from different studies indicating the relation existing between RV vaccination coverage and seizures/convulsions expressed as RRs and their 95% CI.
Notes: RR values below one suggest a heterologous effect or an unforeseen direct effect of the RV vaccine that would favor a lower incidence of seizures/convulsions in the child population. aPayne et alCitation4 first-ever seizures; bPayne et alCitation4 all seizures; cPardo-Seco et alCitation3 (AKS) in year 2007; dPardo-Seco et alCitation3 AKS in year 2010; ePardo-Seco et alCitation3 convulsions in year 2007; fPardo-Seco et alCitation3 convulsions in year 2012; gSheridan et alCitation72 any emergency department presentation; hSheridan et alCitation72 any hospital admission; iSheridan et alCitation72 first emergency department presentation; jSheridan et alCitation72 first hospital admission; kPringle et alCitation73 seizure rates; lOrrico-Sánchez et alCitation38 vaccine coverage 1%–19%; mOrrico-Sánchez et alCitation38 vaccine coverage 20%–39%; nOrrico-Sánchez et alCitation38 vaccine coverage >39; °Burke et alCitation74 full vaccination; pBurke et alCitation74 partially vaccinated; Unpublished data AKS and RV vaccination coverage 1%–14%; Unpublished data AKS and RV vaccination coverage 15%–29%; Unpublished data AKS and RV vaccination coverage >29%; Unpublished data convulsions and RV vaccination coverage 1%–14%; Unpublished data convulsions and RV vaccination coverage 15%–29%; Unpublished data convulsions and RV vaccination coverage >29%.
Abbreviations: AKS, all kinds of seizures; RV, rotavirus.
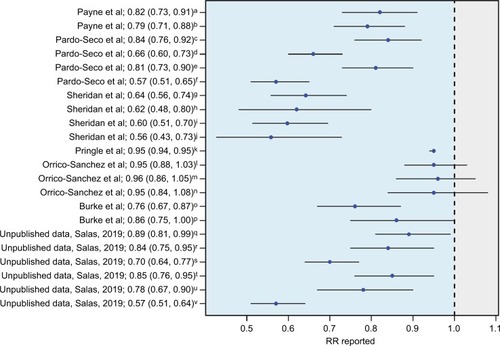
In contrast, a recent ecological study carried out in Spanish (Valencia; southeast Spain) children <5 years old by Orrico-Sánchez et alCitation38 has reported a lack of impact of RV vaccine on seizure hospitalization rates. There are some issues in the study by Orrico-Sánchez et alCitation38 that might help to explain the differences with all the other studies (). For instance, the authors included primary care patients and used absolute figures instead of rates. In addition, these authors used a mixed Poisson regression model involving multiple variables (including vaccination coverage) to avoid confounder effects. However, this model includes variables such as time since vaccine introduction, which could be highly correlated with vaccination coverage (as time goes by, the vaccination coverage should increase), which can result in overfitting and statistical noise. Despite this the study states no impact of RV vaccine on seizures hospitalization, it is remarkable that the trend shown by their data indicates a relative risk in the same direction as the studies with positive findings.Citation38 More recently, Biggart et alCitation39 have also published a lack of effect of the monovalent RV vaccine on childhood seizure hospitalizations in the UK using an interrupted time series analysis.
If there is a beneficial impact on seizures and whether there is a potential benefit exerted by RV vaccines mainly due to the prevention of RV infections in infants otherwise susceptible to the neurological tropism of RV, and/or a true heterologous effect of the vaccine, remains unknown.Citation2 Indeed, now more than ever, more studies are needed to clarify the effect of RV vaccines on seizures.
Impact of RV vaccines on autoimmune manifestations
Vaarala et alCitation40 reported that RV vaccination did not alter the risk of celiac disease and type I diabetes. In contrast, the recent study by Kemppainen et alCitation25 proposed a protective association between RV vaccine and the development of celiac disease, considering RV as an important environmental factor for triggering autoimmunity. This study showed a reduced risk of celiac disease autoimmunity in children vaccinated against RV who had been introduced to gluten before 6 months of age, with a HR of 0.57. More studies are needed to clarify the effects of RV vaccines on autoimmune manifestations.
Molecular mechanisms involved in the systemic interaction of RV
The underlying molecular mechanism of RV attachment and entry into host is now well established (). Trypsin-like proteases from the host intestinal lumen cleave the VP4 capsid protein to produce an N-terminal VP8* and the C-terminal VP5* peptides. This proteolytic processing of the outer viral spike VP4 seems essential for infectivity,Citation41 leading to a more stable, rigid spike structure and displaying the distal lectin domain of VP8* molecule for receptor binding and attachment to the mucosal epithelia.Citation42
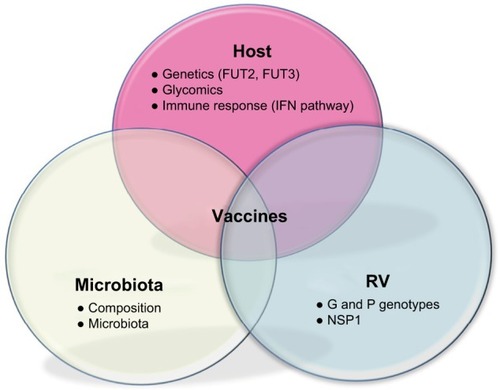
Figure 3 Interactions in RV pathology: host, microbiota and RV.
Notes: Host background includes genetics through expression of histo-blood groups in epithelia, glycomics with the importance of sugars for RV interaction with host cells, and the IFN pathway, key in immune host defense. Intestinal microbiota composition and metagenome influence the course of RV infection through interactions between commensal microbial flora and pathogen. RV proteins (VP4 and VP7) determined by G and P genotypes and enterotoxin NSP1 play an important role in RV attachment, cell internalization and pathogeny. Overall, effectiveness of RV vaccines may be influenced by all these elements.
Abbreviations: IFN, interferon; RV, rotavirus; NSP1, nonstructural protein1.
The ligands for VP8* in host epithelia have been identified as oligosaccharides of the histo-blood group antigens (HBGAs) family, present in mucosal secretion, epithelial intestinal, and red blood cells.Citation43 All of these HBGAs have in common an α-1,2-fucose linked residue, added by the α-1,2-fucosyltransferase enzyme, encoded by the FUT2 gene. FUT2-null homozygotes determine the non-secretor phenotype; the evidence suggesting that homozygote carriers develop a natural resistance to RV infection. Thus, FUT2 expression seems to be relevant for viral infectivity and serves as a marker for host susceptibility.
However, the mechanism for viral dissemination from the gut is not clear. A neonatal mouse model of RV infection suggested that extraintestinal spread occurs via a lymphatic pathway, is primarily determined by non-structural protein NSP3, and can be modified by the VP6 capsid protein.Citation44 Cells from the lymphocytic or myeloid lineage were proposed as viral replication sites during the extraintestinal spread.Citation20
RV tropism toward neuronal cells could in some way explain why the CNS is the main focus of extraintestinal affectation, and the viral dissemination mechanism could involve the attachment to HBGAs or other specific cell receptors. A mechanism of retrograde axon transport has also been proposed, whereby synaptic vesicles returned to the CNS from axon terminals, as described for other pathogens (herpesvirus, rabies, polio virus).Citation45
Overall, it seems that viral spread depends on viral factors (NSP3, VP6), but other components of the process might also be related to genetic host. Recently, a blood-whole transcriptome analysis has revealed that the host downregulates glycophorin expression in a suggested mechanism of viral spread inhibition.Citation46 Glycophorins are cell-membrane glycoproteins rich in sialic acid, a monosaccharide often associated in the literature with RV interaction.Citation47 The role of this mechanism in avoiding systemic spread remains to be clarified.
The role of the host in RV infection
Host genetic component in RV infection
It has been proposed that the HBGAs of the host play an important role in RV cell attachment.Citation48 These HBGAs contain a carbohydrate structure, namely H type I antigen, whose synthesis is dependent on the FUT2 gene expression, which determines the secretor status. There is abundant evidence in the literature suggesting a strong association between the FUT2 gene and risk of infection with RV and other enteropathogens such as norovirus.Citation5,Citation49,Citation50 Individuals with secretor phenotype have an increased susceptibility to RV, especially to P[8] genotype; conversely, severe RV gastroenteritis is virtually absent among children without FUT2 expression in the intestinal epithelium.Citation51 In addition, FUT3 – also related to HBGA expression and determinant of the Lewis antigen – has been proposed as a potential determinant of host susceptibility to RV.Citation49 The recent meta-analysis by Bustamante et alCitation50 points to the single-nucleotide polymorphism rs601338 (W154X) in the FUT2 gene as the causal variant in diarrhea at 1 year of age. The A-allele at this position results in a truncated protein and a lower risk of diarrhea caused by RV and norovirus.Citation50
The host susceptibility to RV infection mediated by FUT2 and FUT3 is RV genotype dependent.Citation49 RV P[8] infects exclusively Lewis- and RV secretor-positive children, in contrast to RV P[6] strains that infect mainly Lewis-negative children, regardless of their secretor FUT2 status.
In view of these findings, differences in host genetic susceptibility could have implications in vaccine efficacy and management. The P[8] genotype is the main component of the two licensed RV vaccines, therefore the proportion of Lewis-negative individuals must be taken into account in order to assess the vaccine efficacy. Thus, for instance, it is reasonable to speculate about a lower vaccine efficacy in Lewis-negative individuals;Citation49 evidence in this direction has been recently provided by Bucardo et al by analyzing Nicaraguan children.Citation52 In particular, the predominance of Lewis-negative phenotype among African populations is worth noting,Citation53 as these population differences in HBGA expression may be responsible for discrepancies in the vaccine protection detected for the current RV vaccines in low-income vs high-income settings.Citation54
Additionally, it was proposed that neonatal resistance to the P[8] and P[4] genotypes could be explained by the absence of Lewis antigen on the cell surface, as young children are usually Lewis-negative until 1–2 months of age.Citation55 Thus, neonatal children might be susceptible to RV P[6] genotypes only, and not to P[8] and P[4] genotypes.
The host microbiota perspective
New evidence points to the host gut microbiota as a key player necessary for a viral pathogen to cause infection.Citation56 Data from experimental studies demonstrated that the use of germ-free animals or antibiotic treatments results in a reduced rate of RV infection.Citation57 These findings highlight the importance of the presence of certain bacterial types in the gut microbiota for RV attachment and infection. This microbiome composition is also related to HBGA and host genetics.Citation5 The intestinal microbiota itself affects host intestinal glycosylation patterns and mucin production, including fucosylation of HBGA.Citation58 The relationship between gastrointestinal viruses and commensal bacteria remains to be elucidated, although there is growing evidence indicating that RV susceptibility and infectivity must be understood within an integrated framework, whereby host genetic and gut microbiota factors cannot be separated.
Alternatively, HBGA, host genetic and microbiota interactions may be also modulating vaccine strain replication. The composition of the bacterial microbiota may shape the response to RV vaccines,Citation59 and this may contribute to their low efficacy in low-income settings. In a study conducted in Pakistani population, Harris et alCitation6 have recently argued that RV vaccine response correlates with the infant gut microbiota composition. The response to the monovalent RV vaccine (RV1) correlated with a higher relative abundance of Clostridium and Proteobacteria, including Serratia and Escherichia coli.Citation6 Therefore, identification of key bacteria that correlate with RV vaccine efficacy could be important for designing future interventions in low-efficacy vaccine settings.
Conversely, RV vaccination appears to be inconsequential for the process of individual microbiome establishment, as recently demonstrated.Citation60 Accordingly, it seems that microbial colonization of the intestine occurs during the first months of life, and oral RV vaccination does not show any major effect upon the infant gut microbiota.
The immunological perspective
The mechanisms responsible for immunity to RV in humans are not completely understood but it seems clear that immunological factors are crucial in susceptibility to RV infection and systemic spread. RV infects primarily enterocytes, and the virus is detected by cytoplasmic pattern recognition receptors (retinoic acid-induced gene-1, also known as RIG-I, and IFN-induced helicase C domain-containing protein 1, also known as MDA5). These immune receptors recognize viral RNACitation61 and induce type I and type III IFN responsesCitation62 in host. At this point, a real battle for controlling the IFN machinery occurs between RV and the host.Citation63 The nonstructural RV protein (NSP1) downregulates IFN expression, inducing degradation of multiple members of the family of the IFN regulatory factors,Citation64 which might underlie the poor innate immune response to RV in the natural infection.Citation65 In addition, toll-like receptor 3, another pattern recognition receptor, has been recently associated to the age-dependent resistance to RV disease in experimental animal models, as both proteins are expressed at higher levels in adult animals.Citation66
A combination of two innate cytokines, IL-18 and IL-22, has been proposed as a key mediator in the clearance of RV by the innate immune system in mice.Citation67 However, RV infection evades the innate immune system efficiently, indicating that the mediators of RV clearance might be cells of the adaptive immune system, as CD8+ cytotoxic T cells that can be detected in the blood of children with RV disease.Citation68 However, some authors consider that circulating RV-specific CD8+ T cells have a poor functional profile and are B cells and antibodies primary determinant in clearance of primary infection and absolutely necessary for development of immunity against reinfection.Citation69
Data from mouse experimental models indicate that immunological effectors responsible for clearance of RV from blood and from intestine are similar, but it is unknown if these effectors are induced solely in blood or intestine or at both sites. Furthermore, recent studies have shown that polymorphisms in genes encoding factors of the immune system can influence the host response to infection and the course of disease in RV and other viral infections.Citation70 Data from animal models showed that IFN-λ genetic polymorphisms affected host control of RV infection;Citation71 thus, genetic variation of key immune mediators could potentially influence the course of the disease and determine the degree of viral spread.
Accordingly, deeper knowledge of the immune mechanisms elicited in RV infection, especially at mucosa level, is necessary in order to predict the potential influence of immune mediator genetic variations on the course and evolution of infection. Similarly, to understand the “protective” effect of RV vaccines, we might hypothesize that the factor responsible for this protective association might be the prevention of the exacerbated inflammatory response elicited by the natural infection with the subsequent break of immune tolerance, as opposed to a more controlled immune response of vaccination. Overall, more research is warranted to elucidate the eventual impact of RV vaccines on autoimmune response.
Conclusion
We are witnessing an authentic “rotavolution” in the understanding of RV pathology. RV infection consequences might be described as an “iceberg” model whereby diarrhea is the most visible tip (). Autoimmune triggering through RV infection constitutes an interesting mechanism for certain diseases; if confirmed, this finding points to new ways of intervention in these diseases. The role of current RV vaccines on these widened clinical spectra remains to be elucidated, and it may constitute a possible heterologous effect, an unforeseen direct effect, or a combination of both. In addition, host genetic background and gut microbiota are being revealed as key influential factors for RV infection and for vaccine effectiveness. The design of new vaccines and public health programs would benefit from taking all these interactions into account. It is now known that the causes of infectious diseases are genetically complex and multifactorial, involving complex interactions between the host and pathogen factors. High-throughput “-omic” strategies (genomics, transcriptomics, metabolomics, glycomics, vaccinomics, etc) are now beginning to revolutionize the way we understand mechanisms of viral infection. Application of these strategies to the rotavolution era will shed new and necessary light on the RV iceberg model.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work
Disclosure
This work was supported by grants from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud): project GePEM ISCIII/PI16/01478/Cofinanciado FEDER (AS) and project ReSVinext ISCIII/PI16/01569/Cofinanciado FEDER (FMT); Consellería de Sanidade, Xunta de Galicia (RHI07/2-intensificación actividad investigadora, PS09749 and 10PXIB918184PR), Instituto de Salud Carlos III (Intensificación de la actividad investigadora 2007-2012, PI16/01569), Fondo de Investigación Sanitaria (FIS; PI070069/PI1000540) del plan nacional de I + D + I (FMT), and 2016-PG071 Consolidación e Estructuración REDES 2016GI-1344 G3VIP (Grupo Gallego de Genética Vacunas Infecciones y Pediatría, ED341D R2016/021) (AS and FMT). FMT has received honoraria from GSK, Pfizer, Sanofi Pasteur, MSD, and Janssen for taking part in advisory boards and expert meetings, and for acting as speaker in congresses outside the scope of the submitted work. FMT has also acted as principal investigator in RCTs of the above-mentioned companies as well as Seqirus, Ablynx, Regeneron, Abbot, Novavax and Medimmune, with honorarium paid to his institution. The authors report no other conflicts of interest in this work.
References
- BluttSEKirkwoodCDParreñoVRotavirus antigenaemia and viraemia: a common event?Lancet200336293941445144914602437
- Rivero-CalleIGómez-RialJMartinón-TorresFSystemic features of rotavirus infectionJ Infect201672SupplS98S10527181101
- Pardo-SecoJCebey-LópezMMartinón-TorresNImpact of rotavirus vaccination on childhood hospitalization for seizuresPediatr Infect Dis J201534776977325923425
- PayneDCBaggsJZerrDMProtective association between rotavirus vaccination and childhood seizures in the year following vaccination in US childrenClin Infect Dis201458217317724265355
- PayneDCCurrierRLStaatMAEpidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United StatesJAMA Pediatr2015169111040104526389824
- HarrisVAliAFuentesSRotavirus vaccine response correlates with the infant gut microbiota composition in PakistanGut Microbes2018929310128891751
- LloydMBLloydJCGestelandPHBaleJFRotavirus gastroenteritis and seizures in young childrenPediatr Neurol201042640440820472191
- DifazioMPBraunLFreedmanSHickeyPRotavirus-induced seizures in childhoodJ Child Neurol200722121367137018174553
- LeeKYWeonYCChoiSHOhKWParkHNeurodevelopmental outcomes in newborns with neonatal seizures caused by rotavirus-associated leukoencephalopathySeizure201856141929427833
- YeomJSKimYSParkJSRole of Ca2+ homeostasis disruption in rotavirus-associated seizuresJ Child Neurol201429333133523271755
- DidsburyAWangCVerdonDSewellMAMcIntoshJDTaylorJARotavirus NSP4 is secreted from infected cells as an oligomeric lipoprotein and binds to glycosaminoglycans on the surface of non-infected cellsVirol J2011855122185400
- TianPBallJMZengCQEstesMKThe rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activityJ Virol19967010697369818794341
- YeomJSKimYSKimRBImpact of rotavirus vaccine introduction on rotavirus-associated seizures and a related possible mechanismJ Child Neurol201530672973425117417
- GoldwaterPNRowlandKThesingerMRotavirus encephalopathy: pathogenesis reviewedJ Paediatr Child Health200137220620911328483
- NishimuraSUshijimaHNishimuraSDetection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reactionBrain Dev19931564574597511877
- CrawfordSEPatelDGChengERotavirus viremia and extraintestinal viral infection in the neonatal rat modelJ Virol200680104820483216641274
- BluttSEMatsonDOCrawfordSERotavirus antigenemia in children is associated with viremiaPLoS Med200744e12117439294
- RamaniSPaulASaravanabavanARotavirus antigenemia in Indian children with rotavirus gastroenteritis and asymptomatic infectionsClin Infect Dis201051111284128921039217
- HemmingMHuhtiLRäsänenSSalminenMVesikariTRotavirus antigenemia in children is associated with more severe clinical manifestations of acute gastroenteritisPediatr Infect Dis J201433436637124136370
- MoonSWangYDennehyPSimonsenKAZhangJJiangBAntigenemia, RNAemia, and innate immunity in children with acute rotavirus diarrheaFEMS Immunol Med Microbiol201264338239122176605
- SugataKTaniguchiKYuiAAnalysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritisPediatrics2008122239239718676558
- VojdaniAA potential link between environmental triggers and autoimmunityAutoimmune Dis2014201443723124688790
- SteneLCHoneymanMCHoffenbergEJRotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal studyAm J Gastroenterol2006101102333234017032199
- CapriatiTFrancavillaRCastellanetaSFerrettiFDiamantiAImpact of the birth’s season on the development of celiac disease in ItalyEur J Pediatr2015174121657166326141172
- KemppainenKMLynchKFLiuETEDDY Study GroupFactors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early lifeClin Gastroenterol Hepatol2017157025694702.e5
- DolcinoMZanoniGBasonCA subset of anti-rotavirus antibodies directed against the viral protein VP7 predicts the onset of celiac disease and induces typical features of the disease in the intestinal epithelial cell line T84Immunol Res2013562–346547623572432
- ZibernaFDe LorenzoGSchiavonVLack of evidence of rotavirus-dependent molecular mimicry as a trigger of coeliac diseaseClin Exp Immunol2016186335636327548641
- BouziatRHinterleitnerRBrownJJReovirus infection triggers inflammatory responses to dietary antigens and development of celiac diseaseScience20173566333445028386004
- HoneymanMCCoulsonBSStoneNLAssociation between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetesDiabetes20004981319132410923632
- van der WerfNKroeseFGRozingJHillebrandsJLViral infections as potential triggers of type 1 diabetesDiabetes Metab Res Rev200723316918317103489
- PaneJAFlemingFEGrahamKLThomasHEKayTWCoulsonBSRotavirus acceleration of type 1 diabetes in non-obese diabetic mice depends on type I interferon signallingSci Rep201662969727405244
- BlomqvistMJuhelaSErkkilaSRotavirus infections and development of diabetes-associated autoantibodies during the first 2 years of lifeClin Exp Immunol2002128351151512067306
- SarkarTDasSNandyPBhowmickRNandyAIn silico study of potential autoimmune threats from rotavirus infectionComput Biol Chem201451515624929545
- JonestellerCLBurnettEYenCTateJEParasharUDEffectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006-2016Clin Infect Dis201765584085028444323
- LopmanBACurnsATYenCParasharUDInfant rotavirus vaccination may provide indirect protection to older children and adults in the United StatesJ Infect Dis2011204798098621878425
- ParkSHKimYOKimHKIncidence of benign convulsions with mild gastroenteritis after introduction of rotavirus vaccineBrain Dev201537662563025266417
- YeomJSKimYSJunJSNSP4 antibody levels in rotavirus gastroenteritis patients with seizuresEur J Paediatr Neurol201721236737327847298
- Orrico-SánchezALópez-LacortMMuñoz-QuilesCDíez-DomingoJLack of impact of rotavirus vaccines on seizure-related hospitalizations in children under 5 years old in SpainHum Vaccin Immunother20181461534153829393748
- BiggartRFinnAMarlowRLack of impact of rotavirus vaccination on childhood seizure hospitalizations in England – an interrupted time series analysisVaccine201836314589459229937243
- VaaralaOJokinenJLahdenkariMLeinoTRotavirus vaccination and the risk of celiac disease or type 1 diabetes in Finnish children at early lifePediatr Infect Dis J201736767467528399059
- AriasCFRomeroPÁlvarezVLópezSTrypsin activation pathway of rotavirus infectivityJ Virol1996709583258398709201
- RodríguezJMChichónFJMartín-ForeroENew insights into rotavirus entry machinery: stabilization of rotavirus spike conformation is independent of trypsin cleavagePLoS Pathog2014105e100415724873828
- HuLCrawfordSECzakoRCell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigenNature2012485739725625922504179
- MosselECRamigRFA lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouseJ Virol20037722123521235614581572
- WeclewiczKSvenssonLKristenssonKTargeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neuronsBrain Res Bull19984643533609671265
- SalasAMarco-PucheGTriviñoJCStrong down-regulation of glycophorin genes: a host defense mechanism against rotavirus infectionInfect Genet Evol20164440341127491455
- IsaPAriasCFLópezSRole of sialic acids in rotavirus infectionGlycoconj J2006231–2273716575520
- JiangXLiuYTanMHisto-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategyEmerg Microbes Infect201764e2228400594
- NordgrenJSharmaSBucardoFBoth Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent mannerClin Infect Dis201459111567157325097083
- BustamanteMStandlMBassatQA genome-wide association meta-analysis of diarrhoeal disease in young children identifies FUT2 locus and provides plausible biological pathwaysHum Mol Genet201625184127414227559109
- Imbert-MarcilleBMBarbéLDupéMA FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotypeJ Infect Dis201420981227123024277741
- BucardoFNordgrenJReyesYGonzálezFSharmaSSvenssonLThe Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan childrenSci Rep201881150229367698
- Ferrer-AdmetllaASikoraMLaayouniHA natural history of FUT2 polymorphism in humansMol Biol Evol20092691993200319487333
- KaziAMCorteseMMYuYSecretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infantsJ Infect Dis2017215578678928329092
- Iturriza-GómaraMDallmanTBányaiKRotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance networkEpidemiol Infect2011139689590920707941
- GalePHillAKellyLApplications of omics approaches to the development of microbiological risk assessment using RNA virus dose-response models as a case studyJ Appl Microbiol201411761537154825269811
- UchiyamaRChassaingBZhangBGewirtzATAntibiotic treatment suppresses rotavirus infection and enhances specific humoral immunityJ Infect Dis2014210217118224436449
- FreitasMAxelssonLGCayuelaCMidtvedtTTrugnanGIndigenous microbes and their soluble factors differentially modulate intestinal glycosylation steps in vivo. Use of a “lectin assay” to survey in vivo glycosylation changesHistochem Cell Biol2005124542343316160839
- ParkerEPKPraharajIZekavatiAInfluence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south IndiaVaccine201836226427229217369
- García-LópezRPérez-BrocalVDiez-DomingoJMoyaAGut micro-biota in children vaccinated with rotavirus vaccinePediatr Infect Dis J201231121300130222828641
- BroquetAHHirataYMcAllisterCSKagnoffMFRIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epitheliumJ Immunol201118631618162621187438
- LinJDFengNSenADistinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infectionsPLoS Pathog2016124e100560027128797
- LópezSSánchez-TacubaLMorenoJAriasCFRotavirus strategies against the innate antiviral systemAnnu Rev Virol20163159160927482897
- BarroMPattonJTRotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3Proc Natl Acad Sci U S A2005102114114411915741273
- HollowayGDangVTJansDACoulsonBSRotavirus inhibits IFN-induced STAT nuclear translocation by a mechanism that acts after STAT binding to importin-αJ Gen Virol201495Pt 81723173324814927
- ZhuSDingSWangPNlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cellsNature2017546766066767028636595
- ZhangBChassaingBShiZViral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18Science2014346621186186525395539
- MesaMCGutiérrezLDuarte-ReyCAngelJFrancoMAA TGF-beta mediated regulatory mechanism modulates the T cell immune response to rotavirus in adults but not in childrenVirology20103991778620096911
- FrancoMAGreenbergHBRole of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in miceJ Virol19956912780078067494291
- EgliASanterDMO’SheaDTyrrellDLHoughtonMThe impact of the interferon-lambda family on the innate and adaptive immune response to viral infectionsEmerg Microbes Infect201437e5126038748
- SyedbashaMEgliAInterferon lambda: modulating immunity in infectious diseasesFront Immunol2017811928293236
- SheridanSLWareRSGrimwoodKLambertSBFebrile Seizures in the Era of Rotavirus VaccineJ Pediatric Infect Dis Soc201652206209
- PringleKDBurkeRMSteinerCAParasharUDTateJETrends in Rate of Seizure-Associated Hospitalizations Among Children <5 Years Old Before and After Rotavirus Vaccine Introduction in the United States, 2000-2013J Infect Dis2018217458158829325147
- BurkeRMTateJEDahlRMAliabadiNParasharUDRotavirus Vaccination Is Associated with Reduced Seizure Hospitalization Risk Among Commerciallly Insured U.S. ChildrenClin Infect Dis201867101614161629788180
