Abstract
Background
Ventilator-associated pneumonia (VAP) is a common nosocomial infection associated with high morbidity due to multidrug-resistant (MDR) pathogens. The purpose of this study was to determine the occurrence of extended-spectrum β-lactamase (ESBL) genes, especially blaCTX-M-15, in Klebsiella pneumoniae (K. pneumoniae)-associated VAP and to investigate the antimicrobial resistance patterns and molecular epidemiological characteristics of K. pneumoniae strains.
Materials and methods
From January 2013 to December 2015, we retrospectively collected 89 VAP-causing K. pneumoniae isolates from tertiary-care hospitals in China, among which ESBL-producing strains were assessed for antimicrobial susceptibility. Several antibiotic resistance genes of clinical relevance in K. pneumonia isolates producing ESBL were investigated. Polymerase chain reaction (PCR) and DNA sequencing were employed to characterize the genetic contexts of blaCTX-M-15. Conjugative plasmids carrying blaCTX-M-15 were obtained by mating and further subjected to replicon typing. The genetic relatedness of isolates was assessed by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing.
Results
All of the 30 ESBL-producing isolates identified displayed MDR phenotype, with blaSHV, blaCTX-M, blaOXA, and blaTEM detected in 21, 21, 1, and 20 isolates, respectively. blaCTX-M-15 was the most prevalent ESBL gene (19/30, 63.33%), and ISEcp1 was detected 48 bp upstream of 15 blaCTX-M-15 genes. Based on S1-PFGE analyses, 25 isolates exhibited different plasmid profiles, ranging from ~70 to 320 kb. The blaCTX-M-15 with blaTEM and qnr genes and the ISEcp1 element from eight isolates were co-transferrable to recipients via conjugation, with IncFIB, IncFIC, and IncFII being the most prevalent replicons. Twenty different PFGE patterns and 11 sequence types were identified, with ST304 being dominant.
Conclusion
This work reports the emergence of blaCTX-M-15 in K. pneumoniae-induced VAP in China. We showed that IncFIB, IncFIC, and/or IncFII plasmids carrying blaCTX-M-15 with blaTEM, qnr resistance genes, and the ISEcp1 element mediate the local prevalence in K. pneumoniae-associated VAP.
Introduction
Ventilator-associated pneumonia (VAP) is one of the most frequent hospital-acquired infections occurring in intubated and mechanically ventilated patients. The rate of VAP occurrence is reportedly 9%–27%, with mortality reaching 20%–50%.Citation1,Citation2 Common causative pathogens of VAP include Gram-negative bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli and Gram-positive bacteria such as Staphylococcus aureus.Citation3–Citation6 Extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae is a common organism of nosocomial infections that play an important role in VAP.Citation7,Citation8
Some clinical isolates of pathogens produce ESBLs that are able to hydrolyze expanded-spectrum cephalosporins (eg, ceftriaxone, cefotaxime, and ceftazidime), aztreonam, and related oxyimino-β-lactams, but not carbapenems, and are inhibited by clavulanic acid and tazobactam.Citation9 Genes encoding ESBLs, such as blaSHV, blaCTX-M, blaTEM, blaOXA, blaPER, and blaVEB, are usually located on large plasmids (>30 kb in size) that are highly mobile and often harbor resistance determinants for several unrelated classes of antimicrobials, such as aminoglycosides, trimethoprim/sulfonamides, tetracyclines, and chloramphenicol.Citation10 Therefore, antibiotic therapy for treating such multidrug-resistant (MDR) pathogen infections is limited to a small number of expensive drugs.
CTX-M enzymes are among the most important ESBLs worldwide, with a clearly higher prevalence compared to other ESBLs. Indeed, emergence and outbreaks of the CTX-M enzyme have been described in bacteria from Africa, Europe, South America, and Asia.Citation11 At present, more than 90 CTX-M variants have been designated (http://www.lahey.org/Studies/other.asp), of which CTX-M-15 is the most prevalent variant around the world.Citation12 The global spread of blaCTX-M-15 is largely due to E. coli of sequence type (ST) 131, IncFII plasmids, and the genetic platforms of the blaCTX-M gene, such as ISCR1, ISEcp1, and IS26, which act as promoters for expression of various resistance genes and influence the mobilization of blaCTX-M genes.Citation13,Citation14
Many recent reports have indicated that VAP may be associated with multiresistant pathogens, such as P. aeruginosa and Gram-negative bacilli, characterized by the production of ESBLs.Citation15,Citation16 However, limited data are available regarding the emergence and prevalence of blaCTX-M-15-producing K. pneumoniae isolates from VAP cases in China. To better guide prevention efforts and clinical treatment of infections, this cross-sectional study was performed to investigate the antimicrobial resistance and molecular epidemiology of VAP caused by K. pneumoniae isolates producing ESBLs, especially blaCTX-M-15, over a 3-year period.
Materials and methods
Clinical isolates and ESBL phenotype confirmation
A retrospective cross-sectional study was conducted at the intensive care unit (ICU) of the First Affiliated Hospital of Dalian Medical University, a 3,700-bed tertiary-care hospital with five ICU wards, from January 2013 to December 2015. K. pneumoniae strains were collected via endotracheal aspiration from mechanically ventilated patients with suspected pneumonia and stored at −80°C before use. VAP was defined as pneumonia occurring 48 hours or more after endotracheal intubation with at least two of the following criteria: fever greater than 38.3°C, leukocytosis or leucopenia, and purulent tracheal secretions (greater than 25 neutrophils observed per high-power field).Citation17 In addition, one or more of the following criteria had to be met: new or persistent infiltrates on chest radiographs, the same microorganism isolated from pleural fluid and tracheal secretions, radiographic cavitation or histopathological demonstration of pneumonia, and positive cultures obtained from bronchoalveolar lavage (greater than 104 colony forming units per mL).Citation18–Citation20 A MicroScan Walk-Away 96 Plus instrument (Siemens AG, Munich, Germany) was used for bacterial identification. Polymicrobial infections were excluded from analysis. All of the K. pneumoniae isolates were screened and confirmed using a double-disk synergy test for ESBL production.
Antimicrobial susceptibility testing
The antimicrobial susceptibility of ESBL-producing K. pneumoniae (EPKP) isolates and recipient (J53AzR) and trans-conjugant strains was determined using the standard broth microdilution method according to the recommendations of the Clinical and Laboratory Standards Institute.Citation21 The following antimicrobial compounds were assessed: cefuroxime, cefotaxime, ceftazidime, cefepime, imipenem, aztreonam, amikacin, ciprofloxacin, levofloxacin, and tigecycline. E. coli ATCC 25922 was used as a reference strain. MDR K. pneumoniae strains were defined as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories.
Molecular detection of resistance genes
To identify ESBL genes carried by the K. pneumoniae isolates, cell lysates were subjected to polymerase chain reaction (PCR) detection of blaCTX-M, blaSHV, blaTEM, and blaOXA genes. All PCR products amplified from β-lactamase genes were commercially sequenced, and subsequent searches in PubMed using the BLAST program (https://blast.ncbi. nlm.nih.gov/) were performed. Specific PCR assays were conducted as previously described to identify the possible association of blaCTX-M-15 with ISEcp1 or with the IS26 insertion element.Citation13,Citation22 Furthermore, plasmid-mediated quinolone resistance (PMQR) genes (qnrA, qnrB, qnrS, and aac(6¢)-Ib-cr) were confirmed by multiplex PCR using a previously described protocol.Citation23 Additional genes of antibiotic resistance, such as 16S rRNA methylase-encoding genes (armA, rmtB, and rmtC), were assessed to further characterize the identified strains.Citation24
Multidrug efflux pump gene expression
The expression of genes encoding the multidrug efflux pumps AcrB, OqxB, and KpgB and their global transcriptional regulators RarA and RamA in clinical EPKP isolates was assessed by quantitative reverse-transcription PCR using previously described oligonucleotide primers.Citation25 Total bacterial RNA was extracted using an E.Z.N.A.™ bacterial RNA Kit (Omega Bio-Tek, Norcross, GA, USA) and was reverse transcribed to complementary cDNA using a PrimeScript RT Reagent Kit (Takara, Dalian, China) according to the manufacturer’s instructions. The cDNA was amplified using a SYBR® Premix Ex TaqTM II Kit (Takara) and a Stratagene Mx3005P qPCR System (Stratagene Agilent, Santa Clara, CA, USA) with 40 cycles of 5 seconds at 95°C and 34 seconds at 60°C. Each strain was amplified in triplicate. The expression levels of each target gene were normalized to a housekeeping gene (rrsE). Data were analyzed using Agilent MxPro software based on the 2−ΔΔCt method.
Plasmid analysis
S1-nuclease (Takara) digestion followed by pulsed-field gel electrophoresis (S1-PFGE) analysis was performed for all the EPKP isolates and transconjugants. For plasmid size estimation, comparison with the molecular weight marker Salmonella braenderup H9812 was performed. Plasmid replicons were determined using the PCR-based replicon typing scheme (PBRT) with 18 pairs in PCR for detecting F, FIA, FIB, FIC, HI1, HI2, I1-Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, and FII replicons, as described by Carattoli et al.Citation26
Resistance transfer determination
To determine whether plasmids coding for antibiotic resistance enzymes can be transferred, conjugation experiments were performed with all isolates carrying blaCTX-M-15 using a broth mating protocol. K. pneumoniae isolates were mated with the sodium azide-resistant E. coli strain J53AzR. Transconjugants were selected on LB agar plates containing sodium azide (100 µg/mL) and cefotaxime (10 µg/mL). PCR amplification, antimicrobial susceptibility testing, and plasmid replicon typing were performed for all transconjugants to determine the presence of resistance determinants, antibiotic phenotypes, and incompatibility groups, respectively.
PFGE and multilocus sequence typing (MLST)
The genetic relatedness of the identified K. pneumoniae isolates was examined by PFGE and MLST. DNA was extracted and digested with 45 U XbaI (Takara) for 2 hours at 37°C. PFGE was performed for the EPKP isolates using a CHEF-DRIII apparatus (Bio-Rad Laboratories, Hercules, CA, USA) as previously described.Citation27 MLST analysis was conducted by sequencing fragments of seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB), and STs were assigned using the K. pneumoniae MLST website (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae).
Ethical clearance
The collection of K. pneumoniae in this study was part of routine hospital laboratory procedure. This retrospective study was performed using samples for secondary use, free of the need for informed consent and ethics committee approval.
Results
Antimicrobial susceptibility testing
From January 2013 to December 2015, 89 mechanically ventilated patients were identified with K. pneumoniae-induced VAP in our tertiary-care hospital. The patients, 60 (67.4%) of whom were male and 29 (32.6%) were female, had a mean ± SD age of 56.0±14.0 years. Among the patients, 30 clinical K. pneumoniae isolates were screened and confirmed for ESBL production (30/89, 33.71%). High-level resistance to cephalosporins (cefuroxime [28/30, 93.33%; MIC range: 8 to >512 µg/mL], ceftazidime [29/30, 96.67%; MIC range: 8–512 µg/mL], cefotaxime [28/30, 93.33%; MIC range: 2–512 µg/mL], and cefepime [24/30, 80.00%; MIC range: 4–128 µg/mL]) as well as noteworthy resistance to fluoroquinolones (ciprofloxacin [26/30, 86.67%; MIC range: 1–256 µg/mL], levofloxacin [23/30, 76.67%; MIC range: 2–256 µg/mL]), and aminoglycosides (amikacin [16/30, 53.33%; MIC range: 4 to >512 µg/mL]) were found. All 30 EPKP isolates exhibited a MDR phenotype and were examined in subsequent experiments. Their susceptibility profiles for ten antimicrobial agents are shown in .
Table 1 Resistance features of the ESBL-producing K. pneumoniae isolates, transconjugants, and recipients
Identification of antibiotic resistance genes and the blaCTX-M-15 genetic context
Of the 30 EPKP isolates, blaSHV, blaCTX-M, blaOXA, and blaTEM were identified in 21, 21, 1, and 20 isolates, respectively. Among the ESBL genes detected in this study, the blaCTX-M-15 allele was the most prevalent (19/30, 63.33%), followed by blaSHV-28 (2/30, 6.67%) and blaTEM-53 (2/30, 6.67%). DNA sequence analysis revealed the insertion of ISEcp1 48 bp upstream of the start codon of the 15 CTX-M-15 genes (except EPKP4, EPKP12, EPKP16, and EPKP27). However, PCR amplification with primers specific for IS26 was negative for all K. pneumoniae isolates carrying blaCTX-M-15. Regarding β-lactamases other than ESBLs, blaSHV-11 (16/30, 53.33%) and blaTEM-1 (16/30, 53.33%) alleles predominated; other minor β-lactamases, including blaCTX-M-14, blaCTX-M-22, blaOXA-10, blaTEM-1b, blaSHV-33, and blaSHV-27, were also detected. For PMQR genes, 17 isolates were found to carry qnrB, 21 isolates qnrS, and two other variants such as qnrA or aac-(6¢)-Ib-cr. Resistance genes for other antibiotics included armA in 17 isolates and rmtB in two ().
Differential expression of efflux pump genes
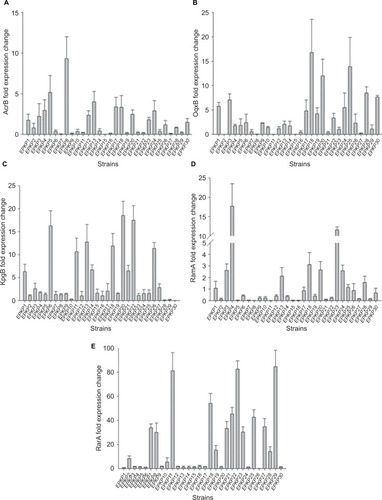
The levels of differential gene expression of the assayed multidrug efflux pumps and their global regulators among the 30 EPKP isolates are presented in . Real-time PCR analysis showed uniformly high expression levels of acrB (1.80- to 4.00-fold) and ramA (2.12- to 17.68-fold) of the RamA/AcrB pathway in seven isolates (EPKP3, EPKP4, EPKP13, EPKP18, EPKP20, EPKP23, and EPKP24). Twelve isolates (EPKP3, EPKP4, EPKP6, EPKP9, EPKP10, EPKP17, EPKP18, EPKP20, EPKP22, EPKP25, EPKP28, and EPKP29) exhibited simultaneously high expression levels of oqxB (1.08- to 16.77-fold) and rarA (1.18- to 84.50-fold) genes. In addition, 13 isolates (EPKP1, EPKP3, EPKP6, EPKP11, EPKP13, EPKP14, EPKP17, EPKP18, EPKP20, EPKP21, EPKP22, EPKP26, and EPKP27) showed upregulation of the kpgB gene (2.07- to 18.53-fold).
Figure 1 Differential gene expression of multidrug efflux pumps and global regulators among the 30 EPKP isolates.
Notes: Fold change in the expression of genes encoding (A) the multidrug efflux pump AcrB; (B) the multidrug efflux pump OqxB; (C) the multidrug efflux pump KpgB; (D) the global regulator RamA; and (E) the global regulator RarA. Fold changes in gene expression were determined after normalizing to that of the 16S rRNA gene (rrsE) in each strain and then comparing the expression of each gene with corresponding genes in the tigecycline-susceptible Klebsiella pneumoniae strain ATCC 13883.
Abbreviations: EPKP, ESBL-producing K. pneumoniae; ESBL, extended-spectrum β-lactamase.
Plasmid analysis
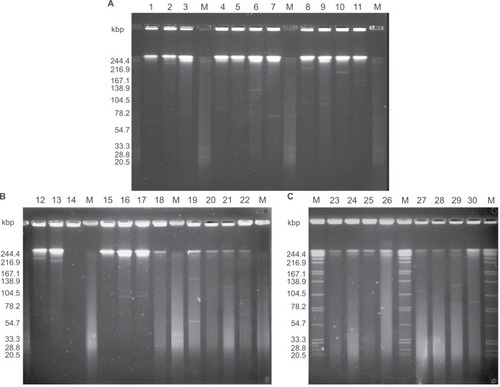
We examined the plasmid profiles of 30 EPKP isolates by S1-PFGE analysis. As shown in , 25 different plasmid profiles were observed among the 30 EPKP isolates (ranging from ~70 to 320 kb). Fourteen strains (EPKP2, EPKP4, EPKP6, EPKP7, EPKP10, EPKP12, EPKP13, EPKP16, EPKP17, EPKP21, EPKP24, EPKP27, EPKP28, and EPKP30) harbored single plasmids of different sizes, and eleven strains (EPKP1, EPKP3, EPKP5, EPKP8, EPKP9, EPKP11, EPKP19, EPKP20, EPKP22, EPKP26, and EPKP29) harbored two to four plasmids. Conversely, five isolates (EPKP14, EPKP15, EPKP18, EPKP23, and EPKP25) contained no detectable plasmid elements.
Figure 2 S1 endonuclease pulsed-field gel electrophoresis analysis of plasmids from the 30 VAP-inducing EPKP isolates in China.
Notes: (A) “M” indicates the molecular weight marker; Lanes 1–11 show the plasmid profiles of isolates EPKP1–EPKP11, respectively; (B) Lanes 12–22 show the plasmid profiles of isolates EPKP12–EPKP22, respectively; and (C) Lanes 23–30 show the plasmid profiles of isolates EPKP23–EPKP30, respectively.
Abbreviations: VAP, ventilator-associated pneumonia; EPKP, ESBL-producing Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamase.
Resistance transfer and PBRT
The resistance profiles of the eight transconjugants were similar to those of the blaCTX-15-producing K. pneumoniae donor strains, demonstrating the transfer of antimicrobial resistance, including the ESBL phenotype. In addition, resistance to several non-β-lactam-based antimicrobial compounds, such as fluoroquinolones, was also cotransferred along with β-lactam resistance; in contrast, resistance to tigecycline was not transferred. For the transconjugants, the most commonly detected resistance genes included blaCTX-M-15 (n=6), blaTEM-1 (n=3), qnrS (n=4), and qnrB (n=4), while the ISEcp1 element was detected in six isolates ().
Plasmid replicon typing showed that in the K. pneumoniae isolates carrying blaCTX-M-15, the plasmids had different replicons, including IncFIC (n=11), IncFIB (n=8), IncFIA (n=2), IncF (n=1), IncFII (n=1), IncK (n=7), and IncL/M (n=1) (). However, PCR replicon typing of the transconjugants identified only three replicons, IncFIB, IncFIC, and IncFII, which were present in both donors and transconjugants and were associated with the transfer of the ESBL phenotype ().
Table 2 Antibiotic resistance genes and plasmid replicon types in transferable blaCTX-15-producing Klebsiella pneumoniae donors and their transconjugants
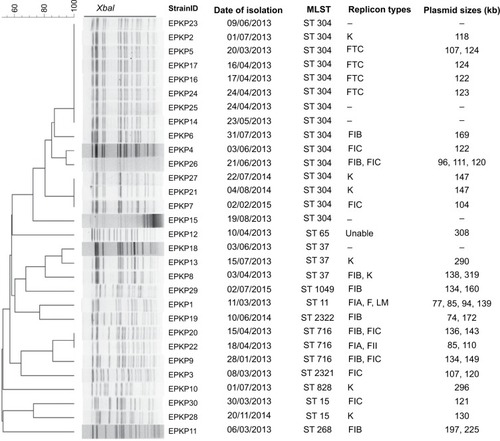
Figure 3 Genetic relatedness, plasmid size, and replicon type of the 30 VAP-inducing EPKP isolates in China.
Note: Dendrogram of patterns generated by PFGE.
Abbreviations: MLST, multilocus sequence typing; ST, sequence type; –, not detected; VAP, ventilator-associated pneumonia; EPKP, ESBL-producing K. pneumoniae; ESBL, extended-spectrum β-lactamase; PFGE, pulsed-field gel electrophoresis.
PFGE and MLST of isolates
The 30 EPKP isolates were assigned to 20 distinct PFGE clusters sharing ≥80% band similarity as well as eleven ST types (ST11, ST15, ST37, ST65, ST268, ST304, ST716, ST828, and ST1049), including two new STs (ST2321 and ST2322) (EPKP3 [02-01-02-01-03-01-25] and EPKP19 [03-20-01-01-01-01-04]). The most prevalent ST was ST304 (n=15, 50%), followed by ST716 (n=3, 10%) and ST37 (n=3, 10%). No clear relationship between replicon and sequence type was observed among the isolates identified in this study ().
Discussion
This is one of only a few studies performed to date investigating the antimicrobial resistance and molecular epidemiology of K. pneumoniae carrying blaCTX-M-15-caused VAP in China. The prevalence of ESBL reported herein is a matter of concern because MDR pathogens causing infectious diseases are common in this area, limiting therapeutic options for treating severe infections often associated with a poor outcome. Indeed, the incidence of EPKP is increasing among patients receiving mechanical ventilation in the ICU of our tertiary-care hospital. The prevalence rate of ESBL among K. pneumonia isolates causing VAP was 33.71%. Despite the high prevalence of ESBLs reported in this study, it is lower compared with the prevalence of those causing device-associated infections among children in a pediatric ICU of other medical centers.Citation28
MIC determinations showed all EPKP isolates to be highly resistant to cephalosporins, with noteworthy resistance to fluoroquinolones and aminoglycosides also observed. As shown in , although the MDR phenotype reported in our studied isolates is frequently associated with ESBL producers, the concurrent combination of different mechanisms, such as PMQR gene expression, 16S rRNA methylase production, and differential expression of multidrug efflux pump genes may lead to this phenotype. The co-presence of ESBL genes and other resistance determinants on the same plasmid is reported regularly.Citation29 In agreement with our findings, PMQR has been associated with blaCTX-M genes, with genes conferring resistance to aminoglycosides and tetracycline and other bla genes being found on the same plasmids as carrying blaCTX-M.Citation30
Over the past decade, predominant CTX-M-type ESBLs have been described globally, including China, South Korea, and many other countries.Citation11,Citation31,Citation32 In fact, together with blaCTX-M-14, blaCTX-M-15 is currently the most common variant detected worldwide in clinically important Gram-negative bacteria.Citation33,Citation34 In this study, we identified the CTX-M-15 enzyme as the most prevalent ESBL in VAP patients. The insertion sequence ISEcp1 has previously been shown to play an important role in the mobilization and expression of genes encoding blaCTX-M,Citation13,Citation35 therefore, linkage of blaCTX-M-15 with ISEcp1 was assessed and shown to be present in all but four of the K. pneumoniae isolates carrying the blaCTX-M-15 gene. The presence of internal sequences such as IS26, which is related to the transmission of β-lactamase genes, such as DHA-1, CFE-1, ACC-1, and SHV-2a, is typically found for the IncFII plasmid.Citation33,Citation36 Regardless, PCR amplification of the IS26 gene was negative for all 19 CTX-M-15 genes.
In our study, PBRT and conjugation experiments showed that among the clinical K. pneumoniae isolates from mechanically ventilated patients, IncFIB, IncFIC, and IncFII replicons were present in the transconjugants and the blaCTX-M-15 gene was co-transferred to the recipient strain with blaTEM and qnr genes and the ISEcp1 element. These results indicate that IncF-related plasmids carrying blaCTX-M-15 are a major vehicle mediating the local prevalence of resistance determinants in K. pneumoniae isolates. Nonetheless, it was previously reported that the blaCTX-M-15 gene can also be found in IncN, IncR, IncFIIk, or IncL/M types.Citation11,Citation37–Citation39
Regarding our S1-PFGE analysis, the existence of five EPKP isolates (EPKP14, EPKP15, EPKP18, EPKP23, and EPKP25) containing no plasmid suggests that these “plasmid-encoded” resistance genes have been integrated into the chromosome. In addition, smaller plasmids may not have been detected by S1-PFGE analysis.Citation40 Further studies are needed to investigate other resistance genes possibly carried on smaller plasmids.
A previous retrospective study of 49 mechanically ventilated patients in tertiary hospitals in China showed that ST23 was dominant among hypervirulent K. pneumoniae strains,Citation41 yet ST304 was the most prevalent in our collection of “classic” K. pneumoniae isolates (n=15, 50.00%). To the best of our knowledge, this is the first report of ST304 in K. pneumoniae, and no clear relationship between replicon and sequence type was observed among the current isolates. This result suggests that this is not a result of the dissemination of particular clones but rather is due to the spread of multiple specific clones and/or mobile genetic elements.
The emergence of MDR pathogens as causative agents of VAP has resulted in a greater administration of inappropriate initial antimicrobial therapies, defined as an antimicrobial regimen that lacks in vitro activity against the isolated organism(s) responsible for the infection.Citation42 Our data highlights the importance of continuous surveillance of both resistant isolates and genetic elements of resistance to monitor the emergence and trends of ESBL-producing isolates to promote adequate therapeutic strategies for managing MDR bacterial infections.
The present study has several limitations. First, because this study was a retrospective analysis and only limited VAP patient information was available, the study focused on the molecular characterization of the prevalence of genes among clinical EPKP. Another limitation of the study included a lack of analysis of other resistance-related determinants, such as the outer-membrane permeability of EPKP isolates. Further studies are needed to address these limitations.
Conclusion
Although ESBL-producing members of Enterobacteriaceae have been reported in China, very limited data are available regarding the susceptibility and molecular characterization of K. pneumoniae isolates from mechanically ventilated patients. This study highlights the emergence of ESBLs, particularly the CTX-M-15 type, in K. pneumoniae-induced VAP in Chinese hospitals. We showed that the blaCTX-M-15 gene was cotransferred with the blaTEM and qnr genes and the ISEcp1 element, conferring a high level of resistance to most antibiotics tested. All transconjugants were associated with IncFIB, IncFIC, and IncFII plasmids.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81401697), the Doctoral Start-up Foundation of Liaoning Province (grant number 201501159), the Postdoctoral Research Foundation of China (grant number 2015M571314), and the Program for High-Level Entrepreneurial and Innovative Talents of Dalian, China (2017RQ120). This work was also supported by the Liaoning Provincial Program for Top Discipline of Basic Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
- KirschenbaumLAzziESfeirTTietjenPAstizMEffect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory careCrit Care Med20023091983198612352030
- CunnionKMWeberDJBroadheadWEHansonLCPieperCFRutalaWARisk factors for nosocomial pneumonia: comparing adult critical-care populationsAm J Respir Crit Care Med199615311581628542110
- ChastreJFagonJYVentilator-associated pneumoniaAm J Respir Crit Care Med2002165786790311934711
- JonesRNMicrobial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumoniaClin Infect Dis201051Suppl 1S81S8720597676
- AlcónAFàbregasNTorresAHospital-acquired pneumonia: etiologic considerationsInfect Dis Clin North Am200317467969515008591
- KollefMHShorrATabakYPGuptaVLiuLZJohannesRSEpidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumoniaChest200512863854386216354854
- KrishnamurthyVGSVMSKHVPRPERNPhenotypic and genotypic methods for detection of extended Spectrum β lactamase producing Escherichia coli and Klebsiella pneumoniae isolated from ventilator associated pneumoniaJ Clin Diagn Res2013791975197824179913
- BassettiMRighiEFasceREfficacy of ertapenem in the treatment of early ventilator-associated pneumonia caused by extended-spectrum beta-lactamase-producing organisms in an intensive care unitJ Antimicrob Chemother200760243343517540673
- PatersonDLBonomoRAExtended-spectrum beta-lactamases: a clinical updateClin Microbiol Rev200518465768616223952
- WangJStephanRZurfluhKHächlerHFanningSCharacterization of the genetic environment of bla ESBL genes, integrons and toxin-antitoxin systems identified on large transferrable plasmids in multi-drug resistant Escherichia coliFront Microbiol2014571625610429
- YounesAHamoudaADaveJAmyesSGPrevalence of transferable blaCTX-M-15 from hospital- and community-acquired Klebsiella pneumoniae isolates in ScotlandJ Antimicrob Chemother201166231331821131694
- PitoutJDLauplandKBExtended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concernLancet Infect Dis20088315916618291338
- PoirelLDecousserJWNordmannPInsertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase geneAntimicrob Agents Chemother20034792938294512936998
- BonnetRGrowing group of extended-spectrum beta-lactamases: the CTX-M enzymesAntimicrob Agents Chemother200448111414693512
- DeyABairyIIncidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: a nine months’ prospective studyAnn Thorac Med200722525719727346
- BaileyKLKalilACVentilator-Associated Pneumonia (VAP) with Multidrug-Resistant (MDR) Pathogens: Optimal Treatment?Curr Infect Dis Rep201517849426092246
- American Thoracic Society; Infectious Diseases Society of AmericaGuidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumoniaAm J Respir Crit Care Med2005171438841615699079
- KoenigSMTruwitJDVentilator-associated pneumonia: diagnosis, treatment, and preventionClin Microbiol Rev200619463765717041138
- PorzecanskiIBowtonDLDiagnosis and treatment of ventilator-associated pneumoniaChest2006130259760416899866
- WuCLYangDieWangNYKuoHTChenPZQuantitative culture of endotracheal aspirates in the diagnosis of ventilator-associated pneumonia in patients with treatment failureChest2002122266266812171848
- CLSIPerformance Standars for Antimicrobial Susceptibility Testing, CLSI Supplement M10027th edWayne, PAClinical and Laboratory Standards Institute2017
- WoodfordNWardMEKaufmannMECommunity and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UKJ Antimicrob Chemother200454473574315347638
- KimHBParkCHKimCJKimECJacobyGAHooperDCPrevalence of plasmid-mediated quinolone resistance determinants over a 9-year periodAntimicrob Agents Chemother200953263964519064896
- LuoYYangJZhangYYeLWangLGuoLPrevalence of β-lactamases and 16S rRNA methylase genes amongst clinical Klebsiella pneumoniae isolates carrying plasmid-mediated quinolone resistance determinantsInt J Antimicrob Agents201137435235521376540
- XuHZhouYZhaiXEmergence and characterization of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae isolates from blood samples of patients in intensive care units in northern ChinaJ Med Microbiol201665875175927324378
- CarattoliABertiniAVillaLFalboVHopkinsKLThrelfallEJIdentification of plasmids by PCR-based replicon typingJ Microbiol Methods200563321922815935499
- OuWCuiLLiYZhengBLvYEpidemiological characteristics of blaNDM-1 in Enterobacteriaceae and the Acinetobacter calcoaceticus-Acinetobacter baumannii complex in China from 2011 to 2012PLoS One2014912e11385225469701
- IsmailAEl-Hage-SleimanAKMajdalaniMHanna-WakimRKanjSSharara-ChamiRDevice-associated infections in the pediatric intensive care unit at the American University of Beirut Medical CenterJ Infect Dev Ctries201610655456227367002
- GoudarziMAzadMSeyedjavadiSSPrevalence of Plasmid-Mediated Quinolone Resistance Determinants and OqxAB Efflux Pumps among Extended-Spectrum β-Lactamase Producing Klebsiella pneumonia Isolated from Patients with Nosocomial Urinary Tract Infection in Tehran, IranScientifica (Cairo)2015201551816726301114
- BoydDATylerSChristiansonSComplete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, CanadaAntimicrob Agents Chemother200448103758376415388431
- HuangSYPanKYLiuXQAnalysis of the drug-resistant characteristics of Klebsiella pneumoniae isolated from the respiratory tract and CTX-M ESBL genesGenet Mol Res2015144120431204826505351
- KimJSKimJKimSJCharacterization of CTX-M-type extended-spectrum beta-lactamase-producing diarrheagenic Escherichia coli isolates in the Republic of Korea during 2008–2011J Microbiol Biotechnol201424342142624509253
- CantónRGonzález-AlbaJMGalánJCCTX-M Enzymes: Origin and DiffusionFront Microbiol2012311022485109
- D’AndreaMMArenaFPallecchiLRossoliniGMCTX-M-type β-lactamases: a successful story of antibiotic resistanceInt J Med Microbiol20133036–730531723490927
- EckertCGautierVArletGDNA sequence analysis of the genetic environment of various blaCTX-M genesJ Antimicrob Chemother2006571142316291869
- PartridgeSRZongZIredellJRRecombination in IS26 and Tn2 in the evolution of multiresistance regions carrying blaCTX-M-15 on conjugative IncF plasmids from Escherichia coliAntimicrob Agents Chemother201155114971497821859935
- LavollayMMamloukKFrankTClonal dissemination of a CTX-M-15 beta-lactamase-producing Escherichia coli strain in the Paris area, Tunis, and BanguiAntimicrob Agents Chemother20065072433243816801423
- CoelhoAGonzález-LópezJJMiróECharacterisation of the CTX-M-15-encoding gene in Klebsiella pneumoniae strains from the Barcelona metropolitan area: plasmid diversity and chromosomal integrationInt J Antimicrob Agents2010361737820392607
- NovaisACantónRMoreiraRPeixeLBaqueroFCoqueTMEmergence and dissemination of Enterobacteriaceae isolates producing CTX-M-1-like enzymes in Spain are associated with IncFII (CTX-M-15) and broad-host-range (CTX-M-1, -3, and -32) plasmidsAntimicrob Agents Chemother200751279679917145793
- LiuBTYangQELiLDissemination and characterization of plasmids carrying oqxAB-bla CTX-M genes in Escherichia coli isolates from food-producing animalsPLoS One201389e7394724040123
- YanQZhouMZouMLiuWEHypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in ChinaEur J Clin Microbiol Infect Dis201635338739626753990
- KollefMHBroad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up frontClin Infect Dis200847Suppl 1S3S1318713047
