Abstract
Background
The emergence of the plasmid-mediated mcr colistin-resistance gene in bacteria poses a potential threat for treatment of patients, especially when hospitalized. The aims of this study were to search for the presence of mcr-1 and mcr-2 genes among colistin-resistant Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae) isolates from clinical specimens and to determine the fingerprint of strains by enterobacterial repetitive intergenic consensus sequences PCR (ERIC-PCR) method.
Methods
In this study, 712 nonduplicate Enterobacteriaceae isolates from clinical specimens were examined. All of the isolates were subcultured on suitable media, and the isolated colonies were identified by standard biochemical tests. Antimicrobial susceptibility test on 7 antibiotics was performed by disk diffusion method, and minimal inhibitory concentration (MIC) of isolates to colistin was determined by the E-test method. These isolates were typed by ERIC-PCR method, and the presence of mcr-1 and mcr-2 genes was investigated by PCR method.
Results
Out of 712 nonduplicate Enterobacteriaceae, 470 isolates, including 351 (74.7%) E. coli and 119 (25.3%) K. pneumoniae, were detected. The results of antibiogram tests showed that most of the isolates (81.3%) were resistant to ceftazidime; however, the most susceptibility among of E. coli and K. pneumoniae isolates was observed (81.5%) to colistin. The typing results by ERIC-PCR method showed 36 and 23 fingerprint patterns for colistin-resistant E. coli and K. pneumoniae strains, respectively. Among 64 (13.6%) colistin-phenotypically-resistant Enterobacteriaceae, 8 isolates (1.7%) had mcr-1 gene. These 8 isolates were attributed to E. coli and K. pneumoniae with 6 and 2 isolates, respectively. Whereas no isolates carrying the mcr-2 gene was found. These colistin-resistant isolates displayed colistin MIC values >2 μg/ml in the antibiotic concentration by E-test method.
Conclusion
Spreading of Enterobacteriaceae strains harboring plasmid-mediated mcr could fail the colistin-included therapy regimen as the last line of treatment against multidrug-resistant bacterial infections.
Keywords:
Introduction
Gram-negative rods, especially Enterobacteriaceae, are considered as the main organisms that cause nosocomial infections.Citation1,Citation2 E. coli and K. pneumoniae are among the main strains associated with such infections. E. coli causes some major infections, including bacteremia, neonatal meningitis, urinary tract infections, intra-abdominal infections, sepsis and gastroenteritis in developing countries. K. pneumoniae causes early acquired lobar pneumonia, wound, soft tissue and urinary tract infections.Citation3
Recently, the emergence of multidrug-resistant (MDR) phenotype as the consequence of antibiotics overconsumption in the treatment of human and animal diseases caused a global challenge to health systems. MDR was defined as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories.Citation4–Citation6
Carbapenems are the choice drugs to treat such infections. However, with increasing global incidence of carbapenem resistance, colistin is now widely used as the last resort antibiotic for the treatment of carbapenem-resistant Enterobacteriaceae. Among carbapenem-resistant K. pneumoniae isolates, the rate of resistance to colistin has been reported to be 15% which is very worrying.Citation7
Colistin uses lipopolysaccharide (LPS) as the bacterial target and disrupts the negative charge of the outer membrane.Citation8 Several studies proposed two main mechanisms for colistin resistance including LPS modifications and LPS removal. Other possible mechanisms are overexpression of efflux pumps, overproduction of capsule polysaccharide and production of colistinase.Citation8
Resistance to colistin in gram-negative bacteria is attributed to a reduction in binding affinity to the outer membrane of LPS. Two-component systems (TCSs) including PhoPQ and PmrAB, as the regulatory systems, reduce the negative charge of lipid A and subsequently reduce the binding affinity of colistin to LPS.Citation9,Citation10
Recently, a plasmid transmitted resistance has been reported as the mobilized colistin resistance (mcr), and has been designated as mcr-1, which is the most prevalent mcr type.Citation11 The mcr-1 gene is a phosphatidylethanolamine transferase, which adds this residue to lipid A component of the LPS, leading to a lower binding affinity of colistin to its target.Citation12 The mcr-2 gene is a rare variant of mcr. The locations of mcr-1 and mcr-2 genes are on conjugative plasmid which may accelerate the transmission between strains and bacterial species, leading to breakdown of the therapeutic regimen.Citation13–Citation16 The reports on the role of mcr-1 and mcr-2 genes indicated the increase in the resistance of E. coli and K. pneumoniae isolates.Citation7
Several studies have reported the variants such as mcr-3, mcr-4 and mcr-5 in E. coli and Salmonella isolates.Citation17 Furthermore, a new plasmid-mediated colistin-resistance gene called mcr-7.1 has been identified in K. pneumoniae in China.Citation18
There are different methods to evaluate the clonality and genetic diversity of Enterobacteriaceae such as pulsed-field gel electrophoresis, multilocus sequence typing, multiple-locus variable-number tandem repeat analysis and enterobacterial repetitive intergenic consensus sequences PCR (ERIC-PCR).Citation19
In this study, we investigate the prevalence of mcr-1 and mcr-2 genes in colistin-resistant E. coli and K. pneumoniae clinical isolates. Also, the clonality of colistin-resistant E. coli and K. pneumoniae strains was evaluated by ERIC-PCR-based dendrogram. ERIC-PCR is cost-effective, easy to perform and a rapid method, which can be applied for comparison of clusters generated.Citation20
Materials and methods
Bacterial isolates
A total of 470 isolates of E. coli and K. pneumoniae were detected among 712 Enterobacteriaceae isolates. These samples were isolated from different clinical specimens such as blood, urine, wound, etc. except stool. These isolates were collected from the laboratories of teaching hospitals collaborated with Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran) from January to June 2017. This study was approved by the Institutional Review Board (IRB) and Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences. The isolates were transferred to the Medical Microbiology Department and were subcultured on blood agar and eosin methylene blue agar (EMB Agar) media (Merck, Germany) for double checking and taking pure culture. After incubation of plates at 37°C for 18–24 hrs, the isolated colonies were identified by Gram staining and standard biochemical tests such as triple-sugar iron (TSI), Simmonʼs citrate, sulfide indole motility (SIM), urea, ornithine and lysine decarboxylase tests (Merck).Citation21
Antimicrobial susceptibility testing (AST)
AST was performed using disk diffusion method (Kirby–Bauer) on Mueller–Hinton agar (Merck) plates according to the Clinical and Laboratory Standards Institute (CLSI) Guidelines.Citation22 The tested antibiotic disks were colistin (10 μg), ciprofloxacin (5 μg), tetracycline (30 μg), imipenem (10 μg), ceftazidime (30 μg), azithromycin (15 μg) and amikacin (30 μg) (MAST Co., UK). The phenotype of Enterobacteriaceae was defined as MDR according to the International Expert proposal for Interim Standards Guidelines.Citation6
Minimum inhibitory concentration (MIC)
Colistin MICs were measured by E-test strips (Liofilchem, Italy) and interpreted based on European Committee on Antimicrobial Susceptibility Testing (EUCAST, Ver. 6, 2016) guidelines. After measuring the MIC with E-test method, the E. coli and K. pneumoniae isolates with a MIC higher than 2 μg/ml were considered as “resistant”.Citation23,Citation24 E. coli ATCC 25,922 was used as a quality control for antimicrobial susceptibility testing.
DNA extraction and PCR amplification
DNA extraction was directly carried out by using the G-spin™ Total DNA Extraction Kit (iNtRON Biotechnology, South Korea) according to the manufacturer’s instructions. The concentrations of extracted DNA from E. coli and K. pneumoniae isolates were measured by using a nanodrop (Thermo Fisher Scientific, Waltham, MA, USA).
Detection of mcr-1 and mcr-2 genes by polymerase chain reaction (PCR)
DNA amplification was performed using a thermocycler (Eppendorf, Hamburg, Germany), and the set of primers as listed in .Citation25,Citation26
Table 1 Sequences of primers used for detection of mcr-1 and mcr-2 genes and ERIC-PCR
The master mix was prepared in a final volume of 25 μL containing 10× PCR buffer, 50 mM MgCl2, 10 mM dNTPs, 10 μM of each primer, 5 U/μL of Taq DNA polymerase and 5 μL of extracted DNA as template. The DNA amplification was performed based on the following program: initial denaturation at 94°C for 5 mins, 25 cycles of denaturation at 94°C for 1 min, annealing at 51°C for 30 s, extension at 72°C for 30 s and a final extension at 72°C for 5 mins. DNAs from E. coli KP81 and E. coli KP37 strains harboring mcr-1 and mcr-2, respectively, were used as the positive controls. Genomic DNA from colistin-susceptible E. coli ATCC 25,922 was used as the negative control. The PCR products were separated by electrophoresis on a 1.5% agarose gel containing 0.5 µg/ml ethidium bromide. The bands were visualized under UV light using a gel documentation system (Protein Simple, Santa Clara, CA, USA).Citation23 PCR products were sequenced, checked with BLAST and annotated to GenBank.
Genbank accession number
The partial nucleotide sequences of mcr-1 gene have been deposited in the GenBank database under accession number of MH627973 (mcr-1 from E. coli strain EC9).
DNA fingerprinting
ERIC-PCR was applied using the ERIC-1 and ERIC-2 primers for colistin-resistant E. coli and K. pneumoniae isolates ().Citation27 The ERIC-PCR conditions were regulated based on the published report by Smith et alCitation28. Analyses of the DNA fingerprints were performed by using GelCompar II; version 6.5 (Applied Maths, NV, Keistraat, Belgium). A cutoff value of 80% similarity was applied to define the clusters. The similarity between the profiles was evaluated with the band matching Dice coefficient, and dendrograms for each species were produced by the unweighted pair group method with arithmetic averages (UPGMA). Based on the UPGMA dendrograms, identical strains were defined as isolates with >97% similarity, closely related isolates with ≥95% similarity and isolates with <95% similarity as unrelated strains.
Statistical analysis
The results were analyzed by using SPSS version 22 software (IBM Armonk, North Castle, NY, USA). Statistical analyses were carried out by applying the Mann–Whitney, Chi-square and Kolmogorov–Smirnov tests, with a statistical significant P-value <0.05.
Results
In the present study, among 712 examined Enterobacteriaceae isolates, 470 isolates were identified as E. coli (n=351, 74.7%) and K. pneumoniae (n=119, 25.3%). The remaining 242 isolates belonged to other Enterobacteriaceae species which were not in the scope of this study. These 470 isolates were collected from different clinical specimens and were screened for antibiotic resistance and presence of mcr-1and mcr-2 genes. The distributions of E. coli and K. pneumoniae isolates in the specimens were as follows: urine, 87.4% (n=411); tracheal, 4.9% (n=23); blood, 3.4% (n=16); wound, 2.1% (n=10); discharge, 1.3% (n=6); and cord spinal fluid, 0.8% (n=4). It is important to mention that most isolates were collected from urine specimens.
Also, 55.95% (n=263) of isolates were from female, while 44.04% (n=207) of isolates were from male patients. The prevalence of E. coli and K. pneumoniae in different hospital wards is listed in . Outpatient clinic with 258 isolates (54.9%) and infectious ward with 2 isolates (0.4%) showed the highest and lowest rates, respectively.
Table 2 Prevalence of E. coli and K. pneumoniae in different hospital wards
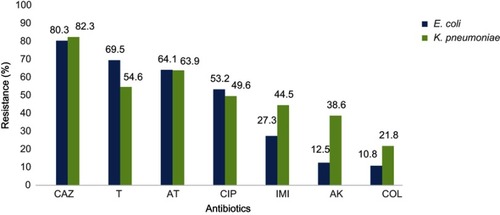
On the basis of the results of AST, most isolates (81.3%) showed high resistance to ceftazidime. However, the most susceptibility among E. coli and K. pneumoniae isolates was attributed to colistin (81.5%). Antibiotics resistance patterns of E. coli and K. pneumoniae isolates which have been presented in show that 68.5% of the isolates are MDR phenotype.
Figure 1 Antimicrobial resistance pattern of E. coli and K. pneumoniae isolates.
Abbreviations: CAZ, ceftazidime; T, tetracycline; AT, azithromycin; CIP, ciprofloxacin; IMI, imipenem; AK, amikacin; COL, colistin.
Among 470 isolates, 13.6% (n=64) were identified as colistin resistant by disk diffusion method. Among colistin-resistant isolates, 59.4% (n=38) were E. coli and 40.6% (n=26) were K. pneumoniae. These isolates were then examined by E-test to evaluate the MICs. By using the E-test, only 30 isolates were confirmed as colistin resistant. In this study, a significant difference was between MIC and disk diffusion methods (P=0.001).
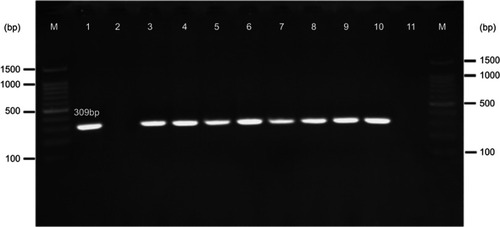
Based on the PCR results, 1.7% (n=8) of E. coli and K. pneumoniae strains carried mcr-1 gene (); however, none of the isolates harbored mcr-2 gene. All mcr-1 positive isolates were collected from urine specimens. There was not any statistically significant relationship between the presence of mcr genes with the aforementioned hospital wards (P=0.13) as well as with the gender of the patients (P=0.92).
Figure 2 Gel electrophoresis of mcr-1 gene (309 bp) encoding for colistin resistance. M: 100 bp DNA marker, line 1: positive control (E. coli KP81), line 2: negative control (E. coli 25,922), line 3: EC9, line 4: EC10, line 5: KP10, line 6: EC16, line 7: EC21, line 8: EC25, line 9: EC26, line 10: KP21, line 11: negative patient sample (E. coli isolates lack mcr-1 gene).
The majority of mcr-1-positive E. coli and K. pneumoniae isolates were resistant to colistin, with MIC values in the rates of >2 µg/ml. Two mcr-1-positive isolates were reported to be colistin susceptible by the E-test method but were resistant to colistin by the disk diffusion method.
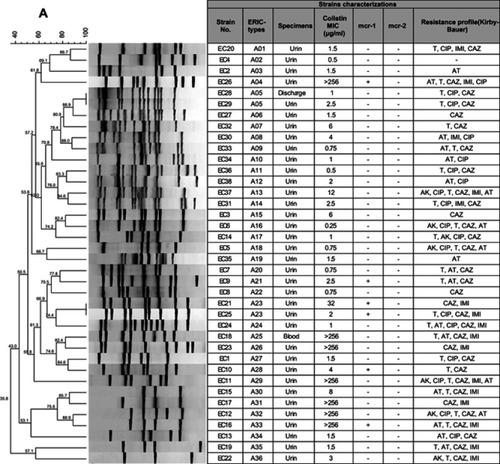
The determination of genomic diversity of 38 strains of phenotypic colistin-resistance E. coli and 26 strains of K. pneumoniae was carried out using the ERIC-PCR fingerprinting method. By analyzing the ERIC-PCR profiles, the 38 E. coli isolates were categorized into 36 ERIC-types, including 14 main clusters and 34 singletons. These 36 distinct types were then called A01 to A36. Moreover, 26 K. pneumoniae isolates were categorized into 23 ERIC types (called B01 to B23), including 11 main clusters and 20 singletons (). The results of ERIC-PCR showed that the colistin-resistant strains in this study were categorized into different genotypes. However, 2 ERIC-types named ERIC-type A05 and ERIC-type A23 had the same genetic background. Complex fingerprint patterns were obtained for all the strains. Generally, the electrophoretic analysis of the PCR reaction products has revealed that the number of bands in particular electrophoretic paths ranged from 3 to 15. The sizes of the PCR products ranged from 100 bp to about 1,500 bp.
Discussion
The spread of antibiotics resistance to a wide variety of antibiotics such as beta-lactams, aminoglycosides and carbapenems is a global challenge to the health systems. Using colistin is regarded as the last resort for treating infections caused by MDR-gram negative rods, especially Enterobacteriaceae.Citation23,Citation29 However, its nephrotoxicity and neurotoxicity impacts have reduced its application as a routine prescribed drug.Citation30
In this study, ERIC-PCR typing method showed 36 patterns for 38 clinical E. coli isolates. 89.4% (n=34) of these isolates displayed a separate ERIC profile. The uniqueness of ERIC patterns suggests that they had a nonclonal transmission. However, 10.5% (n=4) of the isolates had identical patterns, indicating the similar origin of dissemination. In addition, ERIC-PCR typing method showed 23 patterns for 26 clinical K. pneumoniae isolates. Which 76.9% (n=20) of these isolates displayed a single ERIC profile, whereas, 23% (n=6) showed shared patterns. The isolates of the ERIC types, namely A05 and B08, had similar antibiotic resistance patterns. The ERIC type A23 dissemination with similar mcr-1 gene could be causes anxiety in society. Genetic analysis using ERIC-PCR showed that majority of colistin-resistant isolates were clonally unrelated, suggesting that dissemination of these isolates was not due to a clonal outbreak.Citation31
The results of this study showed a low rate of resistance against colistin for E. coli and K. pneumoniae (10.8% and 21.8%, respectively). The isolates were generally susceptible to amikacin; however, most of them were resistant to ceftazidime and tetracycline. In addition to colistin, most of the mcr-1-positive isolates were resistant to ceftazidime (87.5%) and were variably resistant to tetracycline (62.5%), azithromycin (62.5%), imipenem (50%) and ciprofloxacin (25%). Consistently, high resistance to ceftazidime and tetracycline was reported in previous studies.Citation32 The susceptibility rates of these isolates to amikacin were 100%.Citation33
In our study, 53.1% (n=34) of the colistin-resistant isolates tested by disk diffusion were susceptible when examined by E-test. This observation suggested that the specificity and sensitivity of the E-test were higher than the disk diffusion method. In the E-test method, 10% of the K. pneumoniae strains were resistant to colistin, which was consistent with the study conducted by Singh et al (10.5%).Citation34
The recent reports have shown plasmid harboring novel colistin-resistance genes, mcr-1 and mcr-2, in some isolates such as E. coli and K. pneumoniae.Citation25,Citation26 An E. coli isolate containing mcr-1 gene which was sensitive to colistin has been before reported by Liassine et al.Citation35 Before Liassine et al, Pham et al reported the first mcr-1-positive but colistin-susceptible isolate, a Shigella sonnei.Citation36
Our PCR results showed that among 470 E. coli and K. pneumoniae isolates, 6 E. coli isolates (1.2%) and 2 K. pneumoniae isolates (0.4%) had mcr-1 gene. Consistently, Quan et alCitation29. reported that among 1,494 E. coli and 571 K. pneumoniae isolates, only 20 (1%) isolates and 1 (<1%) isolate were mcr-1 positive, respectively. The mcr-2 gene was not detected in our study, consistent with other studies.Citation37–Citation39
The results of previous studies have shown the presence of mcr-1 gene in 7 countries in Southeast Asia (China, Thailand, Laos, Japan, Vietnam, Cambodia and Malaysia).Citation40 In the studies conducted in Asia, the isolation rate of mcr-1 gene is about 1%, which is consistent with our results (1.7%) ().Citation25,Citation29,Citation41
Table 3 Comparison of the previous studies with the current study
In this study, among 8 isolates of positive mcr-1 gene, 2 isolates were colistin sensitive (25%), which was in agreement with the Newton-Foot et al study (26%).Citation23 In both the studies, PCR was used as the molecular method which does not examine the gene expression. The identification of a colistin-susceptible/mcr-1-positive isolate in this study indicates that the silent spread of this gene might happen. The gene expression as well as the expression of the gene by real-time PCR, in samples with higher MIC, could demonstrate the inactivation of mcr-1 gene in some strains.Citation23 These studies show the importance of approving phenotypic methods by molecular methods.
Among 30 colistin-resistant isolates tested by E-test, only 26.6% (n=8) were harboring the plasmid-mediated colistin-resistance mcr-1 gene. Therefore, other possible resistance mechanisms should be considered. These mechanisms are as follows: 1) inactivation of genes encoding proteins involved in the LPS biosynthesis (lpxA, lpxC, lpxD), 2) LPS modification by the regulatory role of the PhoPQ and PmrAB TCSs and 3) mgrB gene alterations.Citation42
Continuous monitoring is very important for determining the exact frequency of mcr-1 gene among gram-negative bacteria in both human and veterinary medicine. Reevaluation of polymyxins application in animals and implementation of large regular screening of animal isolates for mcr genes would be an important step in preventing the spread of these genes to the human isolates.Citation25
Conclusion
Spreading of Enterobacteriaceae strains harboring mcr containing plasmids could fail the colistin-included therapy regimen which is used as a last line of treatment against MDR gram-negative bacterial infections. Increased surveillance of colistin-resistance mechanisms for monitoring their acquisition and spread is vital. Persistent efforts to ensure the judicious use of colistin (and indeed all antibiotics) both in agriculture and in health-care systems are welcome.
Ethics approval and informed consent
The study was approved by the research committee of the Ahvaz Jundishapur University of Medical Sciences (No: IR.AJUMS.REC.1396.331), Ahvaz, Iran. As the bacterial isolates were collected as a part of routine patient care investigation in the hospital, ethical approval was not required.
Consent for publication
All authors have consent for the manuscript publication.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Prof. Surbhi Malhotra (Department of Medical Microbiology, University of Antwerp, Belgium), and Prof. Ágnes Sonnevend (College of Medicine, Department of Medical Microbiology and Immunology, United Arab Emirates University) for kindly providing us the mcr-1 and mcr-2 as the positive control strains. This study is a part of MSc thesis (by Nasrin Emam) which was approved as a research plan (Grant No: 96109) and was financially supported by the Deputy Vice-Chancellor for Research Affair and Infectious & Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran. The authors gratefully acknowledge all of them.
Disclosure
The authors report no conflicts of interest in this work.
References
- Gu DX, Huang YL, Ma JH, et al. Detection of colistin resistance gene mcr-1 in hypervirulent Klebsiella pneumoniae and Escherichia coli isolates from an infant with diarrhea in China. ACS Infect Dis. 2016;60(8):5099–5100.
- Yao X, Doi Y, Zeng L, Lv L, Liu JH. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis. 2016;16(3):288–289. doi:10.1016/S1473-3099(16)00057-826842777
- Al-Agha AG. Extraction and purification of lipopolysaccharide of Klebsiella pneumoniae isolates. Int J Curr Microbiol Appl Sci. 2017;6(8):90–100. doi:10.20546/ijcmas.2017.608.012
- Cannatelli A, Giani T, Antonelli A, Principe L, Luzzaro F, Rossolini GM. First detection of the mcr-1 colistin resistance gene in Escherichia coli in Italy. ACS Infect Dis. 2016;60(5):3257–3258.
- Poirel L, Kieffer N, Liassine N, Thanh D, Nordmann P. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis. 2016;16(3):281. doi:10.1016/S1473-3099(16)30197-9
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x21793988
- Di Pilato V, Arena F, Tascini C, et al. Mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016;60(9):5612–5615. doi:10.1128/AAC.01075-1627401575
- Kim Y, Bae IK, Lee H, Jeong SH, Yong D, Lee K. In vivo emergence of colistin resistance in Acinetobacter baumannii clinical isolates of sequence type 357 during colistin treatment. Diagn Microbiol Infect Dis. 2014;79(3):362–366. doi:10.1016/j.diagmicrobio.2014.03.02724809861
- Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria. Proc Natl Acad Sci U S A. 2012;109(22):8722–8727. doi:10.1073/pnas.120131310922589301
- Petrou VI, Herrera CM, Schultz KM, et al. Structures of aminoarabinose transferase ArnT suggest a molecular basis for lipid A glycosylation. Science. 2016;351(6273):608–612. doi:10.1126/science.aad117226912703
- Kline KE. Investigation of first identified mcr-1 Gene in an isolate from a US patient—pennsylvania, 2016. MMWR Surveill Summ. 2016;65(36):977–978.
- Hinchliffe P, Yang QE, Portal E, et al. Insights into the mechanistic basis of plasmid-mediated colistin resistance from crystal structures of the catalytic domain of MCR-1. Sci Rep. 2017;7:39392. doi:10.1038/srep3939228059088
- Falgenhauer L, Waezsada SE, Yao Y, et al. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing gram-negative bacteria in Germany. Lancet Infect Dis. 2016;16(3):282–283. doi:10.1016/S1473-3099(16)00009-826774242
- Bontron S, Poirel L, Nordmann P. Real-time PCR for detection of plasmid-mediated polymyxin resistance (mcr-1) from cultured bacteria and stools. J Antimicrob Chemother. 2016;71(8):2318–2320. doi:10.1093/jac/dkw13927121402
- Du H, Chen L, Tang YW, Kreiswirth BN. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis. 2016;16(3):287–288. doi:10.1016/S1473-3099(16)00056-626842776
- Mediavilla JR, Patrawalla A, Chen L, et al. Colistin-and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. MBio. 2016;7(4):e01191–16. doi:10.1128/mBio.01191-1627578755
- Sun J, Zhang H, Liu YH, Feng Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808. doi:10.1016/j.tim.2018.02.00629525421
- Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73:1791–1795. doi:10.1093/jac/dky11129912417
- Ross IL, Heuzenroeder MW. A comparison of three molecular typing methods for the discrimination of Salmonella enterica serovar Infantis. FEMS Immunol Med Microbiol. 2008;53(3):375–384. doi:10.1111/j.1574-695X.2008.00435.x18625012
- Adzitey F, Huda N, Ali GR. Molecular techniques for detecting and typing of bacteria, advantages and application to foodborne pathogens isolated from ducks. 3 Biotech. 2013;3(2):97–107. doi:10.1007/s13205-012-0074-4
- Till PM. Bailey & Scott’s Diagnostic Microbiology. 14th ed. Missouri: Elsevier; 2016.
- Wayne P. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25; 2015.
- Newton-Foot M, Snyman Y, Maloba MR, Whitelaw AC. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. clinical isolates from the Western Cape region of South Africa. Antimicrob Resist Infect Control. 2017;6(1):78. doi:10.1186/s13756-017-0234-828785405
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0, 2016 Available from: http://www.eucast.org. Accessed March 30, 2016.
- Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR 1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/S1473-3099(15)00424-726603172
- Xavier BB, Lammens C, Ruhal R, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016;21(27):30280. doi:10.2807/1560-7917.ES.2016.21.27.30280
- Ranjbar R, Ghazi FM. Antibiotic sensitivity patterns and molecular typing of Shigella sonnei strains using ERIC-PCR. Iran J Public Health. 2013;42(10):1151.26060624
- Smith JL, Drum DJ, Dai Y, et al. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 2007;73(5):1404–1414. doi:10.1128/AEM.01193-0617194843
- Quan J, Li X, Chen Y, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017;17(4):400–410. doi:10.1016/S1473-3099(16)30528-X28139430
- Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat. 2010;13(4–5):132–138. doi:10.1016/j.drup.2010.05.00220843473
- Peymani A, Farivar TN, Sanikhani R, Javadi A, Najafipour R. Emergence of TEM, SHV, and CTX-M-extended spectrum β-lactamases and class 1 integron among Enterobacter cloacae isolates collected from hospitals of Tehran and Qazvin, Iran. Microb Drug Resist. 2014;20(5):424–430. doi:10.1089/mdr.2013.019124684320
- Sonnevend Á, Ghazawi A, Alqahtani M, et al. Plasmid-mediated colistin resistance in Escherichia coli from the Arabian Peninsula. Int J Infect Dis. 2016;50:85–90. doi:10.1016/j.ijid.2016.07.00727566913
- Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm RK. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY antimicrobial surveillance program in 2014 and 2015. ACS Infect Dis. 2016;60(9):5623–5624.
- Singh S, Pathak A, Kumar A, et al. Emergence of chromosome-borne colistin resistance gene mcr-1 in clinical isolates of Klebsiella pneumoniae from India. Antimicrob Agents Chemother. 2018;62(2):e01885–17. doi:10.1128/AAC.01885-1729133565
- Liassine N, Assouvie L, Descombes MC, et al. Very low prevalence of MCR-1/MCR-2 plasmid-mediated colistin resistance in urinary tract Enterobacteriaceae in Switzerland. Int J Infect Dis. 2016;51:4–5. doi:10.1016/j.ijid.2016.08.00827544715
- Pham TD, Thanh TH, Nguyen TNT, et al. Inducible colistin resistance via a disrupted plasmid-borne mcr-1 gene in a 2008 Vietnamese Shigella sonnei isolate. J Antimicrob Chemother. 2016;71(8): 2314–2317.
- Zurfluh K, Stephan R, Widmer A, et al. Screening for fecal carriage of MCR-producing Enterobacteriaceae in healthy humans and primary care patients. Antimicrob Resist Infect Control. 2017;6(1):28. doi:10.1186/s13756-017-0186-z28316780
- Terveer EM, Nijhuis RH, Crobach MJ, et al. Prevalence of colistin resistance gene (mcr-1) containing Enterobacteriaceae in feces of patients attending a tertiary care hospital and detection of a mcr-1 containing, colistin susceptible E. coli. PLoS One. 2017;12(6):e0178598.28575076
- Schrauwen EJ, Huizinga P, van Spreuwel N, Verhulst C, Kluytmans-van den Bergh MF, Kluytmans JA. High prevalence of the mcr-1 gene in retail chicken meat in the Netherlands in 2015. Antimicrob Resist Infect Control. 2017;6(1):83. doi:10.1186/s13756-017-0242-828828173
- Ye H, Li Y, Li Z, et al. Diversified mcr-1-harbouring plasmid reservoirs confer resistance to colistin in human gut microbiota. MBio. 2016;7(2):e00177–16. doi:10.1128/mBio.00177-1627048797
- He QW, Xu XH, Lan FJ, et al. Molecular characteristic of mcr-1 producing Escherichia coli in a Chinese university hospital. Ann Clin Microbiol Antimicrob. 2017;16(1):32. doi:10.1186/s12941-017-0207-z28420384
- Pragasam AK, Shankar C, Veeraraghavan B, et al. Molecular mechanisms of colistin resistance in Klebsiella pneumoniae causing bacteremia from India—a first report. Front Microbiol. 2017;7:2135. doi:10.3389/fmicb.2016.0214028119670
- Shen Z, Wang Y, Shen Y, Shen J, Wu C. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis. 2016;16(3):293. doi:10.1016/S1473-3099(16)30197-926973308