Abstract
Background
Of all blood stream infections (BSI), candidaemia poses the greatest threat with a high fatality rate among children. There has been an increase in the number of reports of non-C. albicans species and antifungal resistance has progressively emerge.
Aim
The present study aimed to demonstrate the prevalence of candidaemia among children and to characterize the involved species and their susceptibility to antifungal agents.
Methodology
Microbes were isolated from blood samples and identified via standard microbiological procedures. Chromogenic media was used to characterize the Candida species. The susceptibility of the isolates to the antifungal agents; caspofungin, amphotericin, itraconazole, and fluconazole was determined with the E-test.
Statistical methods
The data were analysed with Statistical Package for the Social Science SPSS; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows. Comparisons between the study groups were performed using the Chi square (χ2) test. p-values less than 0.05 were considered significant.
Results
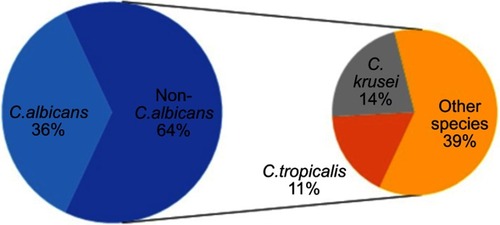
Candidaemia accounted for 17.3% of all BSIs. C. albicans and non-C. albicans species accounted for 36% and 64% of the cases of candidaemia, respectively. Caspofungin, amphotericin, itraconazole, and fluconazole antifungals had activities of 99%, 97%, 73% and 64%, respectively. In total, 64% of patients with candiaemia died.
Conclusion
The prevalence of candidaemia was high, the fatality rate was alarming and non-C. albicans species were predominant. Fluconazole was the least effective of the tested antifungal agents owing to the high level of resistance.
Keywords:
Introduction
Invasive candidiasis is a life-threatening condition that endangers critically ill patients and is associated with a high fatality rate. The mortality rate is unacceptably high, ranging from 29% to 76%.Citation1 Neonates, elderly patients and those admitted to intensive care units (ICUs) are at greater risk of death than other categories of patients.Citation2 Candidaemia is defined as the presence of Candida species in the blood determined by at least one positive blood culture in patients with fever and signs of a blood stream infection (BSI).Citation3
Reports of Candida BSIs have significantly increased worldwide over the past 20 years. Candida BSIs are the fourth most common type of nosocomial BSIs in the USA and they are the sixth most common in Europe.Citation1,Citation4
The World Health Organization (WHO) and the Joint United Nations Program on HIV/AIDS (UNAIDS) reported that in 2011, there were 4,127 cases of candidaemia in the total population of 82,500,000.Citation5 Immunosuppression, prolonged antibiotic intake, inserted devices, extreme age and ICU admission are all predictive risk factors for invasive candidiasis.Citation6
Several studies have reported a change in the type of Candida isolated from blood, with an increasing prevalence of non-C. albicans species.Citation7 C. tropicalis and C. krusei are commonly observed in leukaemic and neutropenic patients. C. parapsilosis causes 30% of candidaemia cases among new-borns and 10–15% of cases among adults.Citation8
The emergence of antifungal resistance among Candida species is considered a leading cause of therapeutic failure and the high mortality rate. The present work aimed to calculate the prevalence of candidaemia among pediatric patients, identify the risk factors, characterize the involved species and determine the susceptibility of the isolated strains to antifungal agents, specifically caspofungin, amphotericin B, itraconazole and fluconazole.
Methodology
This study was conducted with positive blood cultures from children suspected of having BSIs in tertiary pediatric hospitals of Cairo University -2017.
Clinical data
The clinical characteristics of the patients with blood cultures positive for Candida were collected including; the hospital site, history of previous administration of antibiotics, associated indwelling devices, factors affecting immunosuppression and 30-day mortality after the diagnosis of Candida BSI. The clinical data were retrieved from the electronic medical records and the associated request form of routine patients’ samples delivered to the laboratory. A written informed consent was obtained by the parents or legal guardians of the patients, and the study was conducted in accordance with the Declaration of Helsinki. The study was reviewed by the Research Ethics Committee (REC) of the Clinical Pathology department at the Faculty of Medicine, Cairo University for all the required ethical statements and was approved with an ID code: I-201008.
Growth detection and species identification
The blood samples were delivered to the laboratory in pediatric blood culture bottles and microbial growth was detected by measuring the signal production with an automated Bact/Alert system (Biomerieux, Craponne, France).
Subculturing of the positive blood cultures was performed on blood, chocolate and MacConkey agar (Oxoid, England) to isolate bacteria and on Sabouraud dextrose medium (BioMérieux, France, REF 43 651) to isolate fungi.Citation9,Citation10 The primary differentiation of the isolated Candida species into C. albicans and non-C. albicans species was determined by germ tube test.Citation11 Further characterization of the Candida species was performed by subculturing on chromogenic Candida agar, which discriminates among different Candida species by color (Oxoid, England, REF: CM1002B).Citation10 All Candida isolates were stored in glycerol broth at −80 °C.
Antifungal susceptibility testing
Candida isolates were tested for their susceptibility to 4 antifungals; namely, itraconazole, fluconazole, amphotericin B and caspofungin; through the detection of the minimal inhibitory concentrations (MICs) with E-test strips (bioMérieux) and GM-MH agar (Mueller-Hinton agar containing 2% glucose and methylene blue 5 microg/mL).Citation9 As reported by Lee et al, 2009, the results of the E-test with the GM-MH agar plate are strongly correlated with the results of the antifungal macrodilution susceptibility test.Citation12 The MICs for the antifungals were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI M27-S4) CLSI, 2012.Citation13 According to CLSI M27-S4, the sensitive and resistant MICs for C.albicans and C.tropicalis are (≤2 and ≥8) μg/ml for fluconazole, (≤0.125 and ≥0.5) μg/ml for itraconazole and (≤0.25 and ≥1) μg/ml for caspofungin. For C.krusei, sensitive and resistant MICs are (≤0.125 and ≥0.5) μg/ml for itraconazole and (≤0.25 and ≥1) μg/ml for caspofungin. The European Committee on Antimicrobial susceptibility testing (EUCAST) guidelines were followed for amphotericin B; the MICs for which have not been defined by the CLSI.Citation14
Quality control with reference strains
The reference strains C. albicans ATCC 90028, C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used to ensure the quality of antifungal susceptibility tests.Citation14
Statistical methods
Data were statistically analysed with Statistical Package for the Social Science (SPSS; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows. Comparisons between the study groups were made with the Chi square (χ2) test. p-values less than 0.05 were considered significant.
Results
Among the 1934 pediatric blood cultures sent to the microbiology laboratory in 2017, 578 (29.8%) were positive for different microorganisms; with 100/578 (17.3%) positive for Candida.
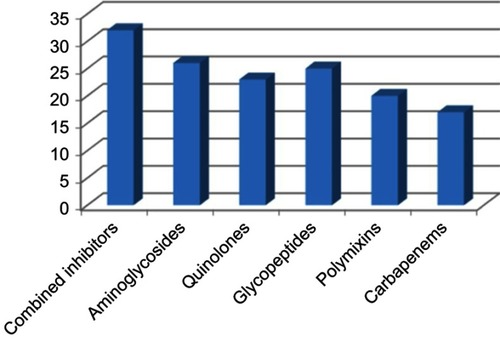
A history of previous antibiotic intake for >2 weeks was observed in 75% (75/100) of candidaemia cases. The antibiotics are listed in . Immunosuppression was detected in 35 cases in the form of neutropenia (18), steroid intake (10) and both neutropenia and steroid intake (7).The isolated Candida species are shown in ; these isolates were from ICUs and wards in 59% and 41% of the cases, respectively, as shown in .
Table 1 Distribution of Candida species in different wards and ICUs
Figure 1 History of previous antibiotic intake prior to candidaemia. Combined inhibitors: β lactam- β lactamase inhibitors (Ampicillin –Sulbactam, Piperacillin- Tazobactam).
The ages of the enrolled patients with candidaemia ranged from birth to 12 years, and they were categorized into the following age groups: 0–28 days (35), >28 days-2 years (41), >2–6 years (22) and >6–12 years (2). Indwelling medical devices were observed in 83% of candidaemia cases, with multiple devices sometimes found to a single patient, especially in the ICU. The Candida species isolated from patients with devices are shown in .
Table 2 Associated indwelling devices with candidaemia
The susceptibility of C. albicans to the 4 tested antifungals is shown in ; caspofungin and amphotericin were the most efficacious, itraconazole and fluconazole were the least efficacious. The MICs for fluconazole, itraconazole and amphotericin B for C. parapsilosis ATCC 22019 ranged from 1 to 4 μg/mL, from 0.12 to 0.5 μg/mL, and from 0.5 to 1 μg/mL, respectively; for C. albicans ATCC 90028 the MICs ranged from 0.125 to 0.5 μg/mL, from 0.064 to 0.25 μg/mL, and from 0.125 to 0.5 μg/mL, respectively and for C. krusei ATCC 6258 the MICs ranged from 16 to 128 μg/mL, from 0.25 to 1 μg/mL, and from1 to 4 μg/mL, respectively; which were all meeting the expected ranges according to the required guidelines by the Clinical and Laboratory Standards Institute.
Table 3 Results of antifungal susceptibility testing among blood stream isolated Candida
The descriptive analysis of the MICs is shown in , with the lowest MIC values were recorded for itraconazole with C. krusei with a susceptible range of (0.0–0.09); for C. albicans with amphotericin, with a susceptible range of (0.0–0.019); and C. tropicalis and other non-C. albicans species with caspofungin, with susceptible ranges of (0.01–0.05) and (0.01–0.8), respectively.
Table 4 The descriptive analysis of susceptible MIC values to antifungals
After excluding the 14 intrinsically resistant C. krusei isolates, fluconazole resistance was recorded in 41.8% (36/86) of the Candida isolates; with resistance found in 52.8% and 47.2% of isolates from patients in ICUs and in wards, respectively. Fluconazole resistant C. albicans and non-C. albicans species accounted for 38.9% and 44% of the total, respectively. As shown in , devices were found in 88.9% of patients with fluconazole-resistant isolates. Previous fluconazole intake was present in 19.4% (7/36) of fluconazole-resistant cases. In total, 64% (64/100) of patients diagnosed with Candida BSI died within 30 days; the descriptive analysis is shown in . Fluconazole resistance was detected in 34.4% of the deceased patients with Candida BSI.
Table 5 The association of Fluconazole resistance with indwelling devices
Table 6 Descriptive analysis of developed mortality among candidaemia cases
Discussion
Candida BSIs pose a serious threat, especially to vulnerable patients. In the present study, candidaemia accounted for 100 of the 578 confirmed BSIs with a prevalence of 17.3%. Other studies in Egypt reported candidaemia prevalences of 16% and 19% among pediatric ICU patients with BSIs.Citation15,Citation16 A higher prevalence of candidaemia (38%) was reported in another study conducted with a different study populations of adult patients in internal medicine wards.Citation17
The results of the present study agreed with those of another Egyptian study, which reported a predominance of non-C. albicans species (60% non-C. albicans versus 40% C. albicans).Citation15 This finding was consistent with the results of a study that reported C. albicans and non-C. albicans BSIs in the neonatal ICU of Child Healthcare, with prevalences of 43.5% vs 56.5%, respectively.Citation18 Several studies also reported this pattern in contrast to the results of older published.Citation6,Citation16,Citation19,Citation20
Another study conducted in Egypt also found that C. tropicalis was the second common non-C. albicans species isolated with a prevalence of 17%; the prevalence rates of C. tropicalis in other studies were 14.8% and 9.6%.Citation16,Citation19,Citation21 However, those studies; reported C. parapsilosis as the most common non-C. albicans species isolated, while C. krusei was the most common species in the present study.
In the present study, candidaemia was most commonly identified in patients in the ICU (59%) and in neonates (35%), which was consistent with the findings of another study.Citation22 Other studies found fewer patients with candidaemia in ICUs which might be due to more strict adherence to infection control measures.Citation21,Citation23
The findings of the present study were compatible with those of other studies with regard to the risk factors for candidaemia; the most important risk factor was previous exposure to antibiotics for >14 days, followed by the presence of a central venous line, and C. albicans was more commonly isolated than non- C. albicans species.Citation20,Citation23
With regards to antifungal resistance, 3% of the isolates were resistant to amphotericin; which was consistent with the results of another study (6.9%). This resistance was due to prior extensive use of amphotericin B in immunocompromised patients, unlike in other studies that reported no amphotericin B resistance.Citation23–Citation25 The present study showed that 27% of the isolates, mostly C. albicans and not C. tropicalis were resistant to itraconazole resistance; which agreed ith some of the previous studies.Citation24,Citation25 Caspofungin resistance rates remained low; the present study found that 1% of the isolates were resistant to caspofungin.Citation26
The rate of fluconazole resistance was 41.8%, and more non- C. albicans species were resistant than C. albicans species (44% versus 38.9%). A higher rate of fluconazole resistance was found in C. tropicalis than in C. albicans, which was similar to the results of one study (7.2% versus 1.3%), while the opposite trend was observed in another study.Citation7,Citation22
Generally, ICUs are considered epicenters of antimicrobial resistance.Citation18,Citation20 In the present study, the majority of fluconazole-resistant isolates (52.8%) were recovered from patients in ICUs, which agreed with the findings of another study, which recovered 94% of the isolates from ICUs.Citation27
The majority of enrolled cases (64%) died within experienced 30 days of diagnosis, of whom 34.4% were resistant to fluconazole; these findings agreed with those of another study that found resistance in 41.1% of deceased cases.Citation28 Several studies reported mortality rates of 51.2% and 42.4% among patients with candidaemia.Citation16,Citation21 As in other studies, we observed higher mortality rates in patients infected with non- C. albicans species than in patients infected with C. albicans (65.6% versus 34.4%, respectively).Citation20,Citation23
Conclusion
In the present study, candidaemia accounted for 17.3% of all BSIs among pediatric patients, with a predominance of non- C. albicans species. Caspofungin had the highest antifungal activity, while fluconazole had the least. The early diagnosis of Candida BSI and its resistance profile are essential to improve outcomes, especially in critically ill neonates and children of preschool age.
Limitations
The lack of availability of advanced molecular techniques in the present study limited the ability to identify the genetic basis of the antifungal resistance and the determination of the degree of genetic relatedness of the isolated Candida species. CHROM agar was challenged by lacking ability to discriminate some non-C. albicans species, which all give similar beige/brown/yellow colors, due to the mixture of natural pigmentation and some alkaline phosphatase enzyme activity.
Disclosure
The authors report no conflicts of interest in this work.
References
- Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis. 2012;73(1):45–48. doi:10.1016/j.diagmicrobio.2012.02.00122578938
- Bajwa S, Kulshrestha A. Fungal infections in intensive care unit: challenges in diagnosis and management. Ann Med Health Sci Res. 2013;3:238. doi:10.4103/2141-9248.11366923919197
- Hesstvedt L, Gaustad P, Andersen CT, et al. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect. 2015;21:938–945. doi:10.1016/j.cmi.2015.06.00826093076
- Ericson EL, Klingspor L, Ullberg M, et al. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73:153–156. doi:10.1016/j.diagmicrobio.2012.02.02022494558
- Zaki SM, Denning DW. Serious fungal infections in Egypt. Eur J Clin Microbiol Infect Dis. 2017;36(6):971–974. doi:10.1007/s10096-017-2929-428213689
- Chow JK, Golan Y, Ruthazer R, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46:1206–1213. doi:10.1086/52943518444857
- Lyon GM, Karatela S, Sunay S. Antifungal susceptibility testing of Candida isolates from the Candida surveillance study. J Clin Microbiol. 2010;48(4):1270–1275. doi:10.1128/JCM.02363-0920129963
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent problem. Clin Microbiol Rev. 2007;20(1):133–163. doi:10.1128/CMR.00029-0617223626
- BioMérieux product list. 2012 Available from: www.bioMérieux.com. doi:10.1094/PDIS-11-11-0999-PDN. Accessed July 2016.
- Oxoid product list. 2012 Available from: www.oxoid.com. doi:10.1094/PDIS-11-11-0999-PDN. Accessed July 2016.
- Cheesbrough M. Microbiological tests In: Cheesbrough M, editor. District Laboratory Practice in Tropical Countries. 2nd ed. 2009 Cambridge University Press: Part II, Chapter (7). 1–266.
- Lee SC, LO HJ, Fung CP, Lee N, See LC. Disk diffusion test and E-test with enriched Mueller-Hinton agar for determining susceptibility of Candida species to voriconazole and fluconazole. J Microbiol Immunolo Infect. 2009;42(2):148–153.
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2012 (Document M27-S4).
- Alastruey-Izquierdo A, Melhem MSC, Bonfietti LX, Rodriguez-Tudela JL. Susceptibility test for fungi: clinical and laboratorial correlations in medical mycology. Rev Inst Med Trop Sao Paulo. 2015;57(19):57–64. doi:10.1590/S0036-46652015000700011
- Fu J, Ding Y, Wei B, et al. Epidemiology of Candida albicans and non- C. albicans of neonatal candidemia at a tertiary care hospital in western China Journal List. BMC Infect Dis. 2017;17(1):329. doi:10.1186/s12879-017-2757-228477628
- Hegazi M, Abdelkader A, Zaki M, El-Deek B. Characteristics and risk factors of candidemia in pediatric intensive care unit of a tertiary care children’s hospital in Egypt. J Infect Dev Ctries. 2014;8(5):624–634. doi:10.3855/jidc.418624820467
- Gupta P, Prateek S, Chatterjee B, Kotwal A, Singh AK, Mittal G. Prevalence of candidaemia in ICU in a tertiary care hospital in North India. Int J Curr Microbiol Sci. 2015;4(6):566–575.
- Fraimow HS, Tsigrelis C. Antimicrobial resistance in the intensive care unit: mechanisms, epidemiology, and management of specific resistant pathogens. Crit Care Clin. 2011;27(1):163–205. doi:10.1016/j.ccc.2010.11.00221144992
- Dutta A, Palazzi DL. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population. Pediatr Infect Dis J. 2011;30(8):664–668. doi:10.1097/INF.0b013e318213da0f21372750
- Rajendran RL, Sherry CJ, Nile A, et al. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—scotland, 2012–2013. Clin Microbiol Infect. 2016;22(1):87–93. doi:10.1016/j.cmi.2015.09.01826432192
- Tumbarello M, Fiori B, Trecarichi EM, et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE. 2012;7(3):e33705. doi:10.1371/journal.pone.003370522479431
- Furlaneto MC, Rota JF, Quesada RM, et al. Species distribution and in vitro fluconazole susceptibility of clinical Candida isolates in a Brazilian tertiary-care hospital over a 3-year period. Rev Soc Bras Med Trop. 2011;44(5):595–599.22031076
- Caggiano G, Coretti C, Bartolomeo N, Lovero G, De Giglio O, Montagna MT. Candida bloodstream infections in Italy: changing epidemiology during 16 years of surveillance. Biomed Res Int. 2015;2015:256580. doi:10.1155/2015/25658026064890
- Capoor MR, Nair D, Deb M, Verma PK, Srivastava L, Aggarwal P. Emergence of non-albicans Candida species and antifungal resistance in tertiary care hospital. Jpn J Infect Dis. 2005;58(6):344–348.16377864
- Mota AJ, Graziella N, Back-Brito GN, Nobrega FG. Molecular identification of Pichia guilliermondii, Debaryomyceshansenii and Candida palmioleophila. Genet Mol Biol. 2012;35:122–125.22481884
- Shields RK, Nguyen MH, Clancy CJ. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis. 2015;28(6):514–522. doi:10.1097/QCO.000000000000021526524326
- Garnacho-Montero J, Díaz-Martín A, García-Cabrera E, et al. Risk factors for fluconazole-resistant candidemia. Antimicrob Agents Chemother. 2010;54:3149–3154. doi:10.1128/AAC.00479-1020498325
- Liao X, Qiu H, Li R, et al. Risk factors of fluconazole-resistant invasive candidiasis in intensive care unit patients. J Crit Care. 2015;30(4):862e1–862e5.