Abstract
Background
Many of present chemotherapeutics are inadequate and also resistant against visceral leishmaniasis (VL), an immunosuppressive ailment caused by Leishmania donovani. Despite the interest in plant-based drug development, no antileishmanial drugs from plant source are currently available. Glinus oppositifolius had been reported in favor of being immune modulators along with other traditional uses. Novel anti-VL therapies can rely on host immune-modulation with associated leishmanicidal action.
Objective
Discovery of novel plant-based antileishmanial compound from G. oppositifolius having permissible side effects.
Methods
With this rationale, an n-BuOH fraction of the methanolic extract of the plant and obtained triterpenoid saponin Spergulin-A were evaluated against acellular and intracellular L. donovani. Immunostimulatory activity of them was confirmed by elevated TNF-α and extracellular NO production from treated MФs and was found nontoxic to the host cells. Identification and structure confirmation for isolated Spergulin-A was performed by ESI-MS,13C, and 1H NMR.
Results
Spergulin-A was found ineffective against the acellular forms while, against the intracellular parasites at 30 μg/mL, the reduction was 92.6% after 72 hrs. Spergulin-A enhanced ROS and nitric oxide (NO) release and changes in Gp91-phox, i-NOS, and pro and anti-inflammatory cytokines elaborated its intracellular anti-leishmanial activity.
Conclusion
The results supported that G. oppositifolius and Spergulin-A can potentiate new lead molecules for the development of alternative drugs against VL.
Introduction
Visceral leishmaniasis (VL) considered as the most severe form of leishmaniasis caused by Leishmania donovani and untreated patients nearly always die.Citation1 Leishmaniasis is endemic in nearly 100 countries with approximately 350 million people are at risk with an estimated yearly incidence of 500,000 cases and almost 70,000 deaths. Leishmaniasis is liable for the ninth most substantial infectious diseases burden, however, is mostly disregarded among tropical disease priorities.Citation2 Sodium antimony gluconate, Miltefosine, Pentamidine, and Amphotericin B are the primary therapeutics though associated with toxicity or resistance.Citation3
Pathogen recognition by neutrophils, macrophages, dendritic cells, and natural killer cells activates intracellular signaling pathways leading into inflammatory responses like inflammasome activation and IL-1β production, which is not the case for leishmanial infection.Citation4 L. donovani infection is characterized by the parasite-induced active subversion of the host immune system and also immune deviation which additively favors infection establishment.Citation5 Additionally, Leishmania prevents inflammatory response by the impaired release of different pro-inflammatory cytokines (IL-1, TNF-α, IL-12) and enhanced releases of immunosuppressive signaling molecules, such as arachidonic acid metabolites and the cytokines TGF-β and IL-10.Citation6 Involvement of co-stimulatory molecule B7-CTLA-4 is associated with increased TGF-β production in VL with increased apoptosis of CD4+ T cells and decreased macrophage apoptosis.Citation7 Leishmanial infection also undermines a generation of microbicidal macrophage nitric oxide (NO) and reactive oxygen species (ROS) with the hindrance of antigenic peptide display to T cells, and permeation of IL-10 producing T regulatory cells.Citation8 In recent years, increased instances of VL have been reported in connection with immune-suppressed AIDS patients.Citation8
Discovery of novel compounds which intercedes host immune-modulation associated with leishmanicidal function having permissible side effects is a precise research objective.Citation9,Citation10 It is also of vital importance that the drug required for parasite elimination in immune-stimulated cells was significantly less than the immune-suppressed ones.Citation11
The anti-parasitic effectiveness of plant extracts majorly relies upon secondary metabolites of diverse chemical groups including alkaloids, polyphenol, flavonoids, terpenoids, phenylpropanoids, etc.Citation12 For isolation and characterization of a herbal extract or an active compound, different research strategies can be employed mainly in the extraction steps. However, bioactivity-guided fractionation considered as simple, rapid, cost-effective and reproducible.Citation1 For obtaining a potent anti-leishmanial agent with immune-modulation, different studies were conducted with compounds like aslicarin A, niranthrin, skimmianine, quassin, tannins, linalool, etc., nevertheless with varying degree of effectiveness and satisfaction.Citation8
Aerial parts of Glinus oppositifolius (Family Molluginaceae) are used for treating abdominal pain and jaundice, while decoction is used against malaria.Citation13 Plants of this genus were previously documented for the presence of triterpenoid saponins,Citation14 and isolated pectin polysaccharides are antiprotozoalCitation15 and immunomodulators.Citation14 G. oppositifolius is indicated for wound healing and used in traditional medicine for treating diarrhea, joint pains, inflammations, intestinal parasites, fever, boils and skin disorders.Citation16 Some triterpenoid saponins, 3-O-(β-D-xylopyranosyl)-spergulagenin-A, Spergulacin, Spergulacin-A Spergulin-A, and Spergulin-B had been isolated from G. oppositifolius.Citation17 Application of plant-based immunomodulators is smart as they mediate their effectiveness by enhancing the inherent host-derived protective machinery without the involvement of specific microbicidal agents, namely antibiotics.Citation13
Most of the available antimalarial drugs are plant-derived; regrettably, there is no anti-leishmanial drug present which is of plant origin. The recent efforts to achieve this goal also were restricted against the promastigotes.Citation18 In the present work, an attempt has been taken to evaluate the intracellular anti-leishmanial activity of this plant and its bioactive component, Spergulin-A emphasizing on the immunostimulatory activity.
Materials and methods
Chemicals and reagents
Cell culture media, serum, antibiotics, HEPES (Gibco, USA), CFSE, DAPI (Invitrogen, USA), MTT, Miltefosine, DMSO (Sigma Chemical Co. USA), cytokine Assay kit (Thermo Scientific, USA), DAF-2 DA, nitric oxide assay kit, DCFDA (Calbiochem, USA) and all other chemicals were of the highest grade commercially available. Primary and secondary antibodies were obtained from Santa Cruz Biotechnology (USA) or Cell Signaling Technologies (USA).
Isolation of methanolic fractions from G. oppositifolius
The aerial parts of G. oppositifolius were shade dried (1 kg) and were first defatted using petroleum ether (60–80°C, 3.5 L × 3) at room temperature for 48 hrs. The marc was then subjected to extraction using MeOH (3.5 L × 3) at room temperature for 48 hrs. The extract was then filtered and MeOH was evaporated under reduced pressure and finally was lyophilized to obtain the crude MeOH extract (13 g). A part (10 g) of ME was then suspended in milli-Q water and partitioned sequentially with EtOAc and n-BuOH. Each fraction was evaporated under vacuum and lyophilized to yield the EtOAc fraction (EAF; 3.6 g), n-BuOH fraction (NBF; 4.1 g) and aqueous fraction (AF; 2.3 g). All the fractions were stored at 4°C till further use.
Among these four fractions, n-BuOH showed promising anti-leishmanial activity. Around 4 g of NBF was subjected to column chromatography of Diaion HP 20 (100 g) and the column washed with water followed by 30%, 40%, 60%, 80%, and 100% of MeOH to obtain a total of six fractions. Fractions eluted with 50% MeOH showed similar spots on TLC and were mixed and then re-chromatographed on Dianion HP 20 column to furnish 7 mg of Spergulin-A. The compound isolation procedures were performed as followed by Kumar et al.Citation19
Isolation and characterization of Spergulin-A
ESI Mass spectra were recorded on an Agilent 6545 Q-TOF mass spectrometer System. 1H and 13C NMR were recorded on a Bruker Ultrashield NMR (600 MHz) in pyridine-d5 with TMS as an internal standard. Diaion HP 20 was used for column chromatography; silica gel (60 F254) was used for TLC and spots were visualized by spraying with Lieberman–Burchard reagent followed by heating.
Parasite culture and maintenance
Leishmania donovani strain Ag83 [MHOM/IN/1983/AG83] was used for the experiments. Promastigotes obtained from transforming amastigote from infected BALB/c mice spleen were maintained in M199 supplemented with 10% FBS, pH 7.4, 100 U/mL penicillin G-sodium, 100 μg/mL streptomycin sulfate, 25 mM HEPES at 22 ºC. For infection, mice were inoculated with 2×107 promastigotes in 0.5 mL of saline. Axenic amastigote from late logarithmic phase cultures of Ag83 promastigotes were prepared as described by Saar et al.Citation20 Pathogen- and animal-related work has been carried out within prior permission from Institutional Biosafety Committee, CSIR-Indian Institute of Chemical Biology (CSIR IICB/IBSC/CERT-32/18-19) and CSIR-IICB-Animal Ethics Committee (IICB/AEC/Meeting/Oct/2017) according to the Committee for the Purpose of Control And Supervision of Experiments on Animals (CPCSEA) guidelines, respectively.
Macrophage culture, parasite infection, and treatment
RAW 264.7 MФ cell line was obtained from the ATCC and maintained in complete RPMI 1640 (10% FBS, 100 μg/mL streptomycin sulfate, 100 U/mL penicillin G sodium, 0.2% sodium bicarbonate, 25 mM HEPES) in a humidified atmosphere and 5% CO2 at 37°C.
Infection of MФs with L. donovani promastigotes was performed in a ratio of 1:10 (MΦ: parasite) for 4 hrs then washed twice with media. Normal and parasitized macrophages were treated with G. oppositifolius fractions or Spergulin-A at required concentrations up to 72 hrs. Infection was measured by counting the intracellular parasites and expressed as parasite count/20 MΦs. Miltefosine served as positive anti-leishmanial reference.
ELISA assay
Cell-free supernatants from MФs (1×105 cells/well) were collected, and the concentration of pro-inflammatory TNF-α, IL-6, IL-12, IL-12β, IL-1β and anti-inflammatory IL-10 and TGF-β cytokines was estimated by sandwich ELISA, using a commercially available assay kit.Citation21
MTT assay
20 μL of MTT (5 mg/mL in PBS) was added to each well of the control and treated MФs (4×103) and 5×103 parasites (promastigote and axenic amastigote) in a 96 well plate and incubated for 4 hrs at 37°C and the formazan crystals were dissolved in 150 μL of DMSO. Absorption was measured at 595 nm by an ELISA reader.Citation22
Measurement of extracellular NO
The collected supernatants from control, infected and treated MФs were incubated with equal volumes of Griess reagent, NED (0.1% in distilled water) and sulphanilamide (1% in 5% H3PO4) at room temperature for 10 mins. The absorbance was measured at 540 nm on a microplate reader. NO concentration was determined using a dilution of sodium nitrite as the standard.Citation21
FACS and confocal microscopy with CFSE-tagged L. donovani
MФs were parasitized with CFSE-tagged (25 μM/1×106 promastigote in 1 mL media for 30 mins) promastigotes followed by Spergulin-A (10, 20, 30 μg/mL) treatment for 24 hrs.
For confocal microscopy, MФs (1×105/glass coverslips) after infection and treatment, washed twice with PBS, fixed in chilled 70% ethanol and nuclei were stained with DAPI and observed under Olympus Fluoview FV10i confocal microscope with 60× objective lens. For FACS, MФs (1×105/well in a six-well plate) after infection and treatment, washed twice with PBS then harvested by scraping and re-suspended in 400 μL PBS and analyzed by BD FACS LSR Fortessa with excitation at 494 nm and emission at 518 nm.
FACS analyses of intracellular ROS and NO
The level of intracellular ROS was determined based on the change in fluorescence of H2DCFDA. DAF-2 DA was used for detecting intracellular NO.3 Briefly, after infection and treatment, MФs were scrapped and incubated in PBS containing DAF-2DA (7.0 μM) at 37°C for 30 mins or 20 μM DCF-DA at 37°C for 15 mins and then analyzed by BD FACS LSR Fortessa with excitation at 480 nm and emission at 515 nm for both.
Western blot analysis
40 µg of proteins harvested from MФs were electrophoretically separated in SDS-PAGE and transferred to PVDF membrane, blocked with BSA and incubated with respective primary antibodies overnight. The membranes were then incubated with HRP conjugated secondary antibodies, and immunoreactive bands were visualized by adding proper substrates. β-Actin was used as loading controls.Citation23
Statistical analysis
All values were expressed as mean ± SEM obtained from at least three replicate experiments. Statistical significance and differences among groups were assessed with one-way ANOVA followed by Dunnett’s test. P-values ≤0.05 (*) or ≤0.01 (**) were considered as indicative of significance.
Results
Immunostimulatory effect of methanolic extract of G. oppositifolius
Of the aqueous, ethyl acetate and n-BuOH (50:50) fractions of G. oppositifolius methanolic extract, only the n-BuOH fraction (50 μg/mL) showed a considerable increase in TNF-α and extracellular NO production (Figure S1) from the treated (24 hrs) MФs. The six sub-fractions (50 μg/mL) of the n-BuOH fraction were also checked for the same parameters (), and highest augmentation was noticed in fraction 4 for being a worthy immunostimulatory agent and the lead fraction ().
Figure 1 Evaluation of immunostimulation by subfractions of n-BuOH fraction of G. oppositifolius MeOH extract. (A) The subfractions (50 μg/mL) were checked for altered TNF-α production after 24 hrs treatment of RAW 264.7 MФs from the culture supernatant. (B) Extracellular NO production was monitored in the culture supernatant of treated (six subfractions) and control after 24 hrs by Griess reagent. All values are expressed as mean ± SEM from triplicate assays from three independent experiments (P-values ≤0.05 (*) or ≤0.01 (**) vs control).
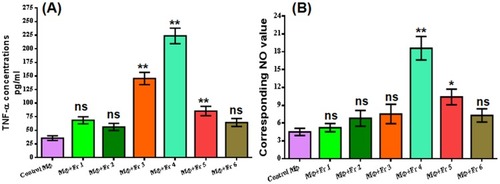
Figure 2 Dose-dependent evaluation of the immunostimulatory effect of the n-BuOH lead fraction and impact on RAW 264.7 MФ survival. (A) TLC profile of n-BuOH lead fraction. (B) The dose-dependent release of TNF-α was monitored in treated (24 hrs) RAW 264.7 MФs, and highest increment was found in 30 μg/mL. (C) Survival of RAW 264.7 MФs at different doses of an n-BuOH lead fraction after 24, 48 and 72hrs exposure measured by MTT assay. (D) Dose-dependent evaluation of extracellular NO production was monitored in the culture supernatant of 24 hrs treated RAW 264.7 MФs by Griess reagent. All values are denoted as mean ± SEM from triplicate assays from three independent experiments (P-values ≤0.05 (*) or ≤0.01 (**) vs control).
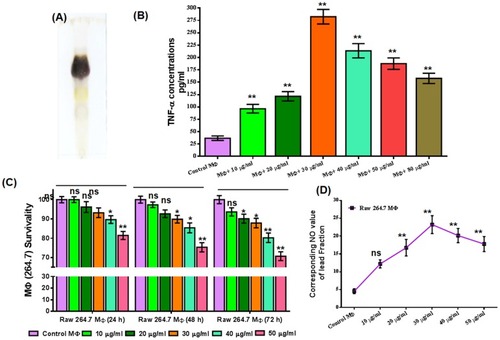
Dose-dependent immunostimulatory effect of the lead fraction
A dose-dependent increase in TNF-α production was monitored in treated (24 hrs) MФs in two sets (). In first set the lead fraction was applied at the doses of 10, 20, 40 and 80 μg/mL () and in the second set at the doses of 20, 30, 40 and 50 μg/mL and most increments were noticed at 30 μg/mL () and selected as the significant dose for this study.
Assessment of macrophage survival and extracellular NO release
Survival of the MФs after exposing them to increasing concentrations (10, 20, 30, 40 and 50 μg/mL) of the lead fraction for 24, 48 and 72 hrs was monitored by MTT assay (). The lead fraction was found safe for this dose range, and survival was about 81.45% even at 50 μg/mL after 24 hrs. And, at 30 μg/mL survival of exposed MФs was found to be 93.22%, 89.87% and 87.88%, respectively, after 24, 48 and 72 hrs. Most increment (5-fold) in extracellular NO was noticed for 30 μg/mL of the lead fraction (). The increase was also significant for most of the doses.
Identification and characterization of Spergulin-A
Among those six n-BuOH bioactive sub-fractions, fraction 4 has shown the most significant upliftment in TNF-α and extracellular NO production. After purification of this fraction by using column chromatography, a white amorphous compound was obtained. Molecular formula C35H58O11S () was assigned from the ESI-MS (Figure S2). Structural elucidation of this compound was achieved by critical analysis of the 1H NMR, 13C NMR and 13C NMR DEPT results (Supplementary results and Figures S3–S6). All the spectral data of this compound were found to be in complete agreement with reported ones and structure was then confirmed as Spergulin-A.Citation24
Figure 3 Anti-leishmanial activity of Spergulin-A. (A) Structure of the triterpenoid saponins Spergulin-A. (B) Dose-dependent effects of Spergulin-A against acellular stages, namely the promastigotes and axenic amastigote of Leishmania donovani after 24, 48 and 72 hrs interval. (C) Dose-dependent effect of Spergulin-A against the intracellular amastigote of L. donovani after 24 hrs of treatment and expressed as number of parasites/20 MФs. (D) 24 hrs time lapse evaluation of anti amastigote effect of Spergulin-A at 30 μg/mL for 72 hrs. (E) Miltefosine LC50 and LC90 values against promastigote, axenic amastigote and intracellular amastigote forms of L. donovani at 24, 48 and 72 hrs time lapse. All values are expressed as mean ± SEM from triplicate assays from three independent experiments (P-values ≤0.05 (*) or ≤0.01 (**) vs control). #Statistical significance of treated macrophages with different time lapses with respect to 0 hrs. ¥Statistical significance of the differences between the infected macrophages and treated macrophages at the same time.
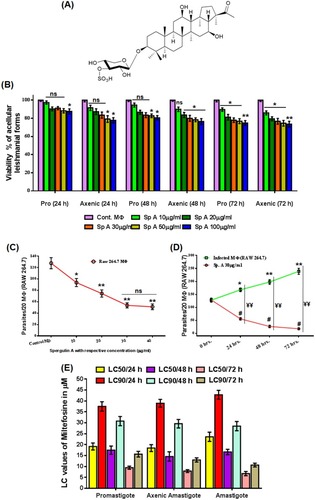
Assessment of anti-leishmanial activity of Spergulin-A
Effect on promastigote and axenic form of L. donovani
Anti-leishmanial effect of Spergulin-A (10, 20, 30, 50, 100 μg/mL) was first evaluated by MTT assay against promastigote and axenic amastigote, the acellular forms () at 24 hrs interval up to 72 hrs. Viability was 87.6% and 77.8%, respectively, for promastigote and axenic forms even at 100 μg/mL after 24 hrs and 74.79% and 73.55% even after 72 hrs at the exposed dose of 100 μg/mL. At the lower doses (10, 20 and 30 μg/mL) the viability reduction was found to be non-significant ().
After that, the effect of Spergulin-A was evaluated against the MФ internalized parasites (). A significant reduction of intracellular L. donovani was monitored in 10 μg/mL of Spergulin-A exposure and parasite count further reduced with the increment of doses (). However, above 30 μg/mL MФ internalized the anti-leishmanial effect of Spergulin-A had reached a plateau, and thus further experiments were mostly conducted with 30 μg/mL.
Time-dependent intracellular leishmanicidal effect
Efficacy of Spergulin-A (30 μg/mL) against the intracellular parasites was evaluated at every 24 hrs interval up to 72 hrs (). Inside the infected MФs parasite count increased significantly. The treated MФs exhibited significantly reduced numbers of parasites from the initial infection as well as the corresponding observation point of infected macrophages (). The parasite reduction at 72 hrs was 86.2% less than the initial point of infection and 92.6% less than the 72 hrs count of infected MФs. Miltefosine was used in parallel as a positive reference anti-leishmanial compound and LC50 and LC90 values of it at 24 hrs time interval for 72 hrs against these three forms were expressed as histogram (). LC50 values of Spergulin-A against amastigote stage of L. donovani was found to be 15.15, 9.32 and 6.22 μg/mL after 24, 48 and 72 hrs, respectively, as compared to Miltefosine LC50 doses of 23.59, 16.64 and 6.73 μg/mL after the same time intervals of 24, 48 and 72 hrs.
Dose-dependent quantitative and qualitative anti-leishmanial effect
After infecting the MФs with CFSE-tagged promastigotes and treatment with Spergulin-A (10, 20 and 30 μg/mL, 24 hrs), the cells were evaluated by FACS in FITC filter (.i–v). Highest intensity was noticed in the infected panels (.ii) and with the increment of Spergulin-A doses, the intensity gradually reduces which is reciprocal to the reduced parasite count (.iii–v). Interestingly, no significant changes in number of parasitized MФs observed within the treatment panel (.vi) and what changes were the intensity denoted the parasite count (.vii). Thus, possibly Spergulin-A reduced the parasite by immune-stimulating the host MФs.
Figure 4 Elaboration of the anti-leishmanial activity of Spergulin-A. (A) Quantitative dose-dependent assessment of intracellular CFSE-stained antileishmanial activity of Spergulin-A by flow cytometry after 24 hrs treatment. Changes in MФ population harboring Leishmania donovani parasites seem insignificant in respect to the decrease of the ultimate parasite count inside MФs (vi) reciprocal to the tagged CFSE fluorescence signaling (vii). (B) Confocal laser-scanning micrographs to evaluate the reduction in intracellular CFSE-stained L. donovani after 24 hrs of Spergulin-A treatment (magnification 120×). (C) FACS monitored the changes in ROS production in control, infected, and treated (30 μg/mL Spergulin-A) MФs with H2DCFDA. (D) The changes in intracellular NO production were monitored by FACS with fluorescent probe DAF-2 DA. (E) Levels of cytokines, pro-inflammatory (IL-6, IL-12, IL-12β and IL-1β) and anti-inflammatory (IL-10 and TGF-β) were measured in control, infected, Spergulin-A treated MФ panels. (F, G, H) Changes in the expression of Gp91-Phox, i-NOS and cytokines, namely pro-inflammatory TNF-α, IL-12β and anti-inflammatory IL-10 and TGF-β with β-actin as loading control and densitometry analyses were provided after band normalization. Images are representative of three separate experiments, and all values are expressed as mean ± SEM from triplicate assays from three independent experiments (P-values ≤0.05 (*) or ≤0.01 (**) vs control).
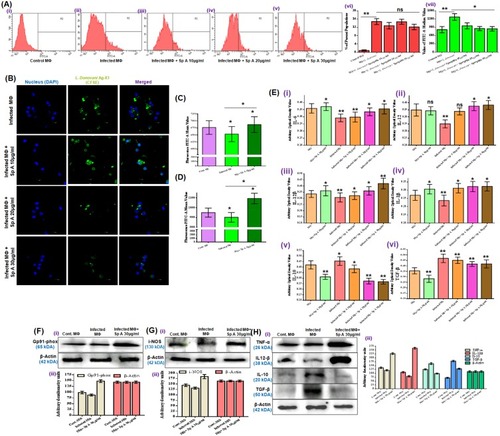
Confocal micrographs depicted the reduction in parasite emitting green fluorescence with the increment of Spergulin-A doses. Moreover, most decreases were noticed when the parasitized MФs were treated with 30 μg/mL of it ().
Intracellular ROS and NO status due to infection and treatment
The fluorescence intensity for H2DCFDA reciprocal to ROS production was found to be minimal for the infected panel () and significantly higher in the treated panel (). The fluorescence intensity of DAF-2DA for NO was also found to be highest for the treated panel () compared to the control and infected MФs () (additional information can be obtained from Figure S7).
Estimation of alteration of different cytokines and immunostimulators
Levels of cytokines, both pro-inflammatory and anti-inflammatory were measured in control, infected, Spergulin-A treated MФ panels (.i–vi). As expected pro-inflammatory cytokine levels, namely IL-6, IL-12, IL-12β and IL-1β were decreased significantly due to L. donovani infection compared to control MФ (.i–iv). Reverse was observed for anti-inflammatory cytokines, i.e., IL-10 and TGF-β as their levels were significantly upregulated in the infection scenario (.v–vi). Spergulin-A exposure to the uninfected MФs was found to upregulated the pro-inflammatory cytokines in general and at the same time down-regulated the levels of anti-inflammatory cytokines. Dose-dependent exposure of Spergulin-A (10, 20 and 30 μg/mL) towards the infected MФs increased the pro-inflammatory cytokines and also decreased the anti-inflammatory ones (.i–vi).
In Western blot analysis, it was observed that Gp91-phox, the key component among the six subunits of NADPH oxidase liable for anti-microbial ROS production in MФs got up regulated in treated panel (.i-ii). Level of i-NOS which regulates anti-leishmanial NO production also increased with Spergulin-A treatment compared to control and infected MФs (.i–ii).
Leishmania inside the host cells can survive in cells via downregulating the pro-inflammatory cytokines while increasing the anti-inflammatory cytokine. Expression of pro-inflammatory TNF-α and IL-12β got down-regulated with infection and considerably up-regulated in treatment panel (.i–ii). Anti-inflammatory IL-10 and TGF-β expression were up-regulated in infected MФs compared to the control, and with the treatment of Spergulin-A (30 μg/mL) their appearance got normalized like that of control (TGF-β) or even less (IL-10) (.i–ii).
Discussion
Precisely an immune-suppressive ailment, VL inhabit and modulate the microbicidal function of the macrophages and create a microenvironment favoring parasite growth inside the visceral organs by modulating pro and anti-inflammatory cytokines and impairing ROS and NO release.Citation6 The present in vitro study aimed to assess the immunostimulatory property of G. oppositifolius and isolation of a compound Spergulin-A which can reduce the intracellular parasites by immune-stimulation within a safe dose range for the host cells. Primary appraisal of an extract that can have an immunostimulatory effect was based upon the increased release of TNF-α and extracellular NO from treated MФs. It was observed that at an applied same dose (50 μg/mL, 24 hrs) only the n-BuOH fraction showed a considerable increase in TNF-α and extracellular NO production. The selection of TNF-α and extracellular NO as the potent immunostimulatory index in the present context is vital because endogenous TNF-α produced by infected macrophages can elicit the release of L-arginine-derived nitrogen intermediates detrimental for the intracellular parasites.Citation9 The six n-BuOH sub-fractions were again verified for immune-stimulation, and fraction 4 emerged as the most promising one. The fraction was also considered to be safe for the MФs even at 50 μg/mL (81.5% viability) having CC50 well above 100 μg/mL.
Interested by the initial observations on elevated immunostimulation by the n-BuOH sub-fraction 4 and based upon the previous inspection of the presence of triterpenoid saponins in this fractionCitation24 a triterpenoid saponin Spergulin-A was isolated and purified following the well-established method ( and Supplementary information).Citation19,Citation24
Evaluation of the anti-leishmanial property of Spergulin-A was the principal research interest after identification and isolation of it. The possible anti-leishmanial effect was first checked against the acellular forms of the parasite but without major success even at 100 μg/mL (). Therefore, if Spergulin-A has any leishmanicidal effect on the intracellular parasites that will possibly mediate by altering the microenvironment of the host MФs. After 24 hrs of incubation Spergulin-A (30 μg/mL) was found to be pretty useful in reducing the intracellular parasite count and when applied for a prolonged period of 72 hrs reduction of intracellular Leishmania was significant in respect to both the infected MФs of corresponding time point and initial-parasitized MФs (). It is also of great significance that no considerable alteration in number of parasitized MФs was found in treatment panel while reduction of CFSE-stained internalized parasites was notable (). This specific observation strongly advocated in favor of immunostimulation by Spergulin-A which mediated parasite killing. For being leishmanicidal, Spergulin-A should be useful in enhancing the release of NO and ROI, well recognized for their worth against Leishmania.Citation6,Citation25 NO is especially critical for intracellular parasite clearance as it was reported that mice with impaired inducible nitric oxide synthase (i-NOS) and thus restricted production and release of NO are incapable of Leishmania control even for isolated macrophages in vitro.Citation26 At the dose of 30 μg/mL, Spergulin-A showed enhanced production of intracellular NO and ROI () which was found to be directly reciprocal to the reduction of intracellular parasites and signified their connection. In the Western blot analysis also increment in i-NOS production () was noticed in Spergulin-A treated parasitized MФs in amendable enhanced NO production and parasite control. The same interpretation is also pertinent for an increase of Gp91-phox, a subunit of the NADPH oxidase and enhancement of ROI () in Spergulin-A mediated intracellular parasite killing. It was previously established that intracellular leishmanial killing proceeds by NO production from arginine by i-NOS, and superoxide (O2−) generated by the NADPH oxidase.Citation27 Several Leishmania species induce immunosuppressive TGF-β production, and diminution of TGF-β secretion is in direct correlation with enhanced i-NOS productionCitation6 and can lead to internalized parasite removal as found with Spergulin-A treatment. TGF-β also found to increase the VL progression and averts disease cure in murine models.Citation27 IL-10 is another anti-inflammatory cytokine which makes macrophages indifferent to various activation signals and thus causes impairment of intracellular parasite killing also by down-regulating the production of TNF-α and NO.Citation28 IL-10 production increased in infected macrophages in vitro, apparently via interaction with the Fc receptorCitation29 and which down-regulated with Spergulin-A treatment () in connection with parasite reduction. Parasite infection is responsible for the suppression of macrophage microbicidal activity that relies upon NO, ROI and cytokines like IL-1, IL-12β, and TNF-αCitation30 as with infection the levels of TNF-α IL-12β got down-regulated (), but with the application of Spergulin-A, these cytokines significantly elevated which signifies their role in intracellular Leishmania parasite control.
Conclusion
The inconsistency of direct leishmanicidal effect of G. oppositifolius and Spergulin-A isolated from it against promastigotes and axenic amastigotes, on the one hand, and the proved efficacy of them against intracellular parasites was explained by emphasizing host MФ immunostimulation as elaborated precisely in the present study. Bio-guided fractionation was employed to isolate and identify Spergulin-A as the immunostimulant, and its anti-leishmanial property was evaluated at a dose best suited for the research and found to be safe against the host cells assessed in vitro in RAW 264.7 MФs infected with a virulent strain of L. donovani.
Acknowledgment
Sincere thanks are given to Science and Engineering Research Board, Govt. of India (Grant No. PDF/2016/001437 and EMR/2015/001674) for financial assistance. The manuscript has been checked critically by the full version of the Grammarly software and corrected by Dr. Basudeb Achari, ex-scientist of CSIR-Indian Institute of Chemical Biology.
Disclosure
The authors report no conflicts of interest in this work.
References
- Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BP, Nakamura CV. Recent advances in leishmaniasis treatment. Int J Infect Dis. 2011;15:e525–e532. doi:10.1016/j.ijid.2011.03.02121605997
- Alvar J, Ve´lez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi:10.1371/journal.pone.003567122693548
- Banerjee S, Bose D, Chatterjee N, et al. Attenuated Leishmania induce pro-inflammatory mediators and influence leishmanicidal activity by p38 MAPK dependent phagosome maturation in Leishmania donovani co-infected macrophages. Sci Rep. 2016;6:22335. doi:10.1038/srep2233526928472
- Freitas EO, Leoratti FMS, Freire-de-Lima CG, Morrot A, Feijó DF. The contribution of immune evasive mechanisms to parasite persistence in visceral leishmaniasis. Front Immunol. 2016;7:153. doi:10.3389/fimmu.2016.0015327148272
- Bhardwaj S, Srivastava N, Sudan R, Saha B. Leishmania interferes with host cell signaling to devise a survival strategy. J Biomed Biotechnol. 2010;2010:109189. doi:10.1155/2010/10918920396387
- Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi:10.1128/CMR.18.2.293-305.200515831826
- Goto H, Lindoso JAL. Immunity and immunosuppression in experimental visceral leishmaniasis. Braz J Med Biol Res. 2004;37:615–623. doi:10.1590/S0100-879X200400040002015064826
- Islamuddin M, Chouhan G, Want MY, Ozbak HA, Hemeg HA, Afrin F. Immunotherapeutic potential of eugenol emulsion in experimental visceral leishmaniasis. PLoS Negl Trop Dis. 2016;10:e0005011. doi:10.1371/journal.pntd.000501127776125
- Maran N, Gomes PS, Freire-de-Lima L, Freitas EO, Freire-de-Lima CG, Morrot A. Host resistance to visceral leishmaniasis: prevalence and prevention. Expert Rev Anti Infect Ther. 2016;14:435–442. doi:10.1586/14787210.2016.116077926934623
- Flora R, Aghazadeh-Dibavar S, Bandyopadhyay M, Dasgupta S. Immunosuppression during Leishmania donovani infection: a potential target for the development of therapy. Ann Parasitol. 2014;60:239–245.25706420
- Haidaris CG, Bonventre PF. Efficacy of combined immunostimulation and chemotherapy in experimental visceral Leishmaniasis. Am J Trop Med Hyg. 1983;32:286–295. doi:10.4269/ajtmh.1983.32.5126301300
- Mukherjee N, Mukherjee S, Saini P, Roy P, Sinha Babu SP. Phenolics and terpenoids; the promising new search for anthelmintics: a critical review. Mini Rev Med Chem. 2016;16:1415–1441. doi:10.2174/138955751666615112012103626586122
- Inngjerdingen KT, Kiyohara H, Matsumoto T, et al. An immunomodulating pectic polymer from Glinus oppositifolius. Phytochemistry. 2007;68:1046–1058. doi:10.1016/j.phytochem.2007.01.01117337024
- Sahakitpichan P, Disadee W, Ruchirawat S. Kanchanapoom T. L-(-)-(N-trans-cinnamoyl)-arginine, an acylamino acid from Glinus oppositifolius (L.) Aug. DC. Molecules. 2010;15:6186–6192. doi:10.3390/molecules1509618620877215
- Traore F, Faure R, Ollivier E, et al. Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Med. 2000;66:368–371. doi:10.1055/s-2000-855110865459
- Sahu SK, Das D, Tripathy NK, Dinda SC, Sundeep Kumar HK. Evaluation of hypoglycemic activity of Mollugo pentaphylla and Glinus oppositifolius (L). Rasayan J Chem. 2012;5:57–62.
- Kumar D, Shah V, Ghosh R, Pal BC. A new triterpenoid saponins from Glinus oppositifolius with a-glucosidase inhibitory activity. Nat Prod Res. 2013;27:624–629. doi:10.1080/14786419.2012.68690722594571
- Kaur H, Thakur A, Kaur S. Immunoprophylactic potential of a cocktail of three low molecular weight antigens of leishmania donovani along with various adjuvants against experimental visceral leishmaniasis. Iran J Parasitol. 2018;13:11–23.29963081
- Kumar D, Mallick S, Vedasiromoni JR, Pal BC. Anti-leukemic activity of Dillenia indica L. fruit extract and quantification of betulinic acid by HPLC. Phytomedicine. 2010;17:431–435. doi:10.1016/j.phymed.2010.04.00519679456
- Saar Y, Ransford A, Waldman E, et al. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi:10.1016/s0166-6851(98)00096-69763285
- Chatterjee N, Das S, Bose D, et al. Exploring the anti-inflammatory activity of a novel 2-phenylquinazoline analog with protection against inflammatory injury. Toxicol Appl Pharmacol. 2012;264:182–191. doi:10.1016/j.taap.2012.07.03222902631
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi:10.1016/0022-1759(83)90303-46606682
- Sikdar Y, Modak R, Bose D, et al. Doubly chloro bridged dimeric copper(II) complex: magneto-structural correlation and anticancer activity. Dalton Trans. 2015;44:8876. doi:10.1039/C5DT0752F25871579
- Sahu NP, Koike K, Banerjee S, Achari B, Nikaido T. Triterpenoid saponins from Mollugo spergula. Phytochemistry. 2001;58:1177–1182. doi:10.1016/s0031-9422(01)00346-611738403
- Matte C, Maion G, Mourad W, Olivier M. Leishmania donovani-induced macrophages cyclooxygenase-2 and prostaglandin E2 synthesis. Parasite Immunol. 2001;23:177–184. doi:10.1046/j.1365-3024.2001.00372.x11298294
- Wei XQ, Charles IG, Smith A, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi:10.1038/375196b07539113
- Wilson ME, Jeronimo SMB, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathogenesis. 2005;38:147–160. doi:10.1016/j.micpath.2004.11.002
- Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi:10.1016/j.it.2007.07.00417689290
- Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217–222. doi:10.1084/jem.188.1.2179653099
- Kane MM, Mosser DM. Leishmania parasites and their ploys to disrupt macrophage activation. Curr Opin Hematol. 2000;7:26–31. doi:10.1097/00062752-200001000-0000610608501
