Abstract
Background and aim
Hepatitis C virus (HCV)–HBV coinfection is a significant health problem with rapid progression of liver disease without precise diagnosis and treatment. We aimed in this study to identify if there were any role of HBV antiviral therapy in patients with HBV reactivation after direct-acting antiviral therapy in HCV–HBV coinfected patients.
Methods
A prospective random study was carried out on 140 patients presenting with chronic HCV and chronic HBV coinfection. All patients had pretreatment HBeAg seroconversion, HBV DNA <2,000 IU/mL, normal liver enzymes, and F0/F1 hepatic fibrosis. They treated with sofosbuvir 400 mg and daklatasvir 60 mg once daily for 3 months. All patients underwent pretreatment hepatic fibrosis assessment using Fibro Scan and laboratory investigations: platelet count, liver-function tests, quantitative HCV PCR, HBsAg, HBc IgG, HBeAg, and HBeAb. All patients were followed up at 1, 3, 6, and 12 months from the start of HCV therapy.
Results
The study enrolled 140 HCV–HBV coinfected patients: 55% were F0 and the rest F1. All our patients had negative HCV PCR at 1 month posttreatment and had achieved sustained virologic response with negative HCV PCR 3 months after treatment end. Four patients showed HBV reactivation with raised HBV DNA PCR and liver enzymes. Their mean age was 23.7±2.7 years, and three were male. Regarding patients with HBV reactivation, at 12 months posttreatment they showed significant decreases in liver enzymes, bilirubin, and INR, with increased platelet count (P=0.001), each with undetectable HBV PCR (P=0.001).
Conclusion
HBV–HCV coinfected patients with no/mild hepatic fibrosis, HBeAg seroconversion, and HBV DNA <2,000 IU/mL can complete direct-acting antiviral therapy without HBV antiviral treatment with close monitoring.
Introduction
Hepatitis B virus (HBV) infection is still a global health affair. Patients who progress to chronic HBV can early develop liver cirrhosis and hepatocellular carcinoma, with an accession risk of morbidity and mortality.Citation1 HBV and HCV infections share the same transmission routes, so the presence of HBV coinfection in patients with HCV infection is frequent.Citation2 The presence of HBV surface antigens in patient blood is known as overt HBV infection. However, in the absence of an HBsAg test, detection of HBV DNA is recognized as occult HBV infection, and is commonly found in patients with chronic HCV infection.Citation3
Coinfection with HBV and HCV is common in Southeast Asia and the Mediterranean.Citation4 HBV–HCV coinfection is associated with higher incidence of cirrhosis, hepatic decompensation, and development of primary liver malignancy (hepatocellular carcinoma) compared with monoinfected individuals.Citation5 The true prevalence of HBV–HCV coinfection is unknown.Citation6 HBsAg-positive patients have been excluded from clinical trials during treatment of patients with chronic HCV with direct-acting antivirals (DAAs).Citation7 This was confirmed by a different study wherein interaction between HBV and HCV in coinfected patients led to suppression of HBV replication.Citation8,Citation9 However, severe cases of chronic HBV reactivation after HCV clearance have been reported after DAA therapy, which led to US Food and Drug Administration concern about the risk of HBV reactivation with DAA therapy.Citation10
Aim
The aim of this work was to identify if there was any role of HBV antiviral therapy following DAA treatment of chronic HCV infection in HBV reactivation in patients with pretreatment HBeAg seroconversion, HBV DNA <2,000 IU/mL, normal liver enzymes, and F0/F1 hepatic fibrosis.
Methods
This prospective randomized study was carried out on 140 patients presenting with chronic HCV–HBV coinfection who had been referred to the outpatient clinic of the Tropical Medicine and Gastroenterology Department of Qena University Hospital. The study period was from August 1, 2016 to January 1, 2019. HBV PCR ≤2,000 IU/mL, normal liver enzymes, HBeAg seroconversion with formation of HBeAb before treatment, and F0/F1 hepatic fibrosis were the inclusion criteria. Patients with worse than F1 hepatic fibrosis, previous HBV antiviral treatment, absence of HBeAg seroconversion, had failed previous HCV therapy, and malignancy anywhere in the body, including hepatocellular carcinoma and laboratory parameters of hepatic decompensation, pregnancy, autoimmune disease, and uncontrolled diabetes mellitus were excluded from the study.
All included patients were treated with sofosbuvir 400 mg and daklatasvir 60 mg once daily for 3 months following the Egyptian protocol for treatment of chronic HCV infection, which is based on the European Association for the Study of the Liver guidelines for treatment of HCV infection.Citation11 All patients underwent pretreatment complete history taking, full clinical examination, and laboratory investigations, including liver-function tests, platelet count, prothrombin time, prothrombin concentration, and INR. Quantitate HCV PCR, HBsAg, HBc IgG, HBeAg and HBeAb were also assessed. Also, all patients underwent pretreatment hepatic fibrosis assessment using a FibroScan FS502. Hepatic scans were performed after overnight fasting. Liver stiffness correlate directly with wave velocity. FibroScan results were correlated with the METAVIR histological staging system. Cutoff values in our study were 5.4–6.9 kPa.Citation12 All patients were followed up at 1 month, 3 months (end of HCV treatment), 6 months (to confirm HCV sustained virologic response), and 12 months (to confirm absence of HBV reactivation) from the start of HCV therapy.
Blood Sampling
Venous blood (8 mL) was collected from each patient under aseptic precautions and divided in four tubes: two tubes containing EDTA, one for platelet count used directly and the other for HBV DNA, HBc IgG, HBeAg, and HBeAb centrifuged, separated in sterile tubes, and frozen at −20°C until assay; a plain glass tube for liver-function tests, after clotting the tube being centrifuged at 3,000 rpm for 5 minutes at room temperature and then serum was separated to be used for estimation of liver function (including liver enzymes, albumin, and bilirubin); and a sodium citrate–containing tube for prothrombin time, prothrombin concentration, and INR.
Laboratory Tests
Venous blood (5 mL) was obtained from each subject in a plain tube, centrifuged, and then the serum separated for laboratory tests. Platelet counts were done with a Celtak hematology analyzer (Nihon Kohden. Liver-function tests (total and direct bilirubin, serum albumin, AST, and ALT) were done with a BT 1500 fully automated chemistry analyzer (Biotecnica). Prothrombin time, concentration, and INR were assessed using Thromborel S kits (Siemens) using a BE coagulator. HBV DNA, HBc IgG, HBeAg, and HBeAb were assessed with a StepOnePlus real-time PCR system (Thermo Fisher Scientific) using TaqMan PCR master mix (Thermo Fisher Scientific) after DNA extraction with a Qiagen kit and according to the manufacturer’s pamphlet. HCV PCR and HBsAg were measured with a MiniVidas fully automated immunoassay system (Biomérieux).
Statistical Analysis
Data were analyzed using SPSS version 15.0. Quantitative data are expressed as means ± SD. Qualitative data are expressed as frequencies and percentages. Independent-sample t-tests were used when comparing two means, χ2 when comparing nonparametric data, and one-way ANOVA when comparing more than two means. Significance was established at P<0.05.
Ethical Considerations
The study protocol was approved by the Medical Ethics Committee of South Valley University, and the study was performed in accordance with clinical ethics guidelines, the Declaration of Helsinki, and rules of good clinical practice. All patients provided informed consent for participation in the study. No specific medical intervention was conducted specifically.
Results
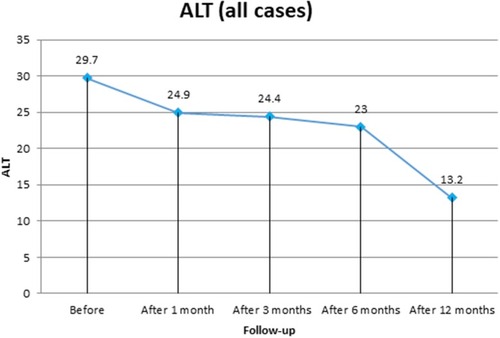
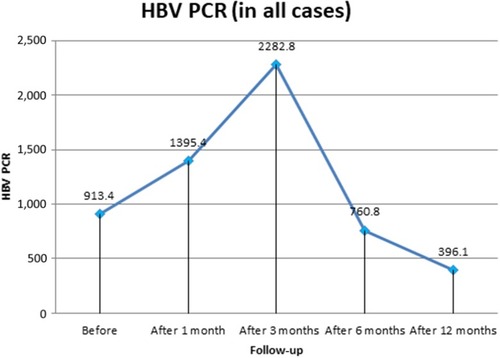
The study enrolled 140 patients coinfected with HCV–HBV. All patients had HBV PCR ≤2,000 IU/mL, normal pretreatment liver enzymes, and showed HBeAg seroconversion with development of HBeAb. In sum, 77 (55%) were F0 and 63 (45%) F1, mean age was 32.4±8.02 years, and 96 (68.6%) were male and 44 (31.4%) female. Four patients (2.86%, three male, mean age 23.7±2.7 years) showed HBV reactivation in the form of raised liver enzymes and HBV DNA PCR 1 month after DAA therapy (). On liver-function tests, all patients showed decreased mean ALT at 1 month, 3 months, 6 months, and 12 months post:treatment: 24.9± 14.2, 24.4±10.8, 23±8.2, and 13.2±3.5 U/L, respectively (P=0.001). Significant improvement in AST levels was also detected, reaching 13.7±3.5 U/L at 1 year posttherapy. (P=0.001). Bilirubin, INR, and platelet counts showed significant improvement at 1 year posttreatment (, and ). Patients showed decreased mean HBV PCR levels: from 913.4±1,362.4 IU/mL (before treatment) to 760.8±379.02 IU/mL (6 months after starting treatment) and 396.1±124.8 IU/mL (12 months after starting treatment).
Table 1 Demographic Data Of Studied Patients
Table 2 Comparisons Of ALT, AST, And HBV PCR (Before Chronic Hepatitis C Virus Treatment, 1 Month, 3 Months, And 6 Months After Therapy)
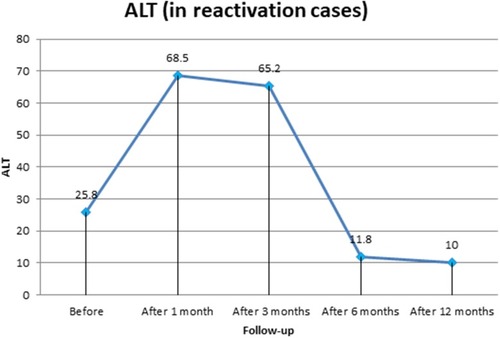
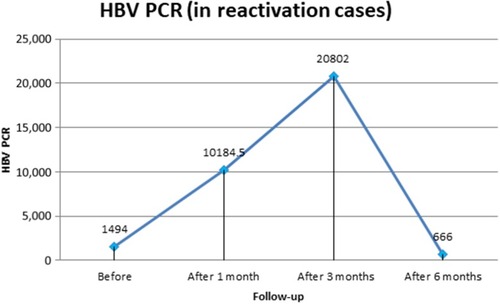
For liver-function tests in patients with HBV reactivation, AST levels before treatment and at 1 month, 3 months, 6 months, and 12 months after starting DAA therapywere 29.7±2.2, 60.3±4.8, 71.5±5.8, 23.3±0.5, and 11.5±3.3 U/L, respectively, with a significant decrease at 12 months posttreatment (P=0.001). ALT was 25.8±5.5, 68.5±5.1, 65.2±8.3, 11.8±4.9, and 10.0±1.4 U/L before treatment and at 1 month, 3 months, 6 months, and 1 year after DAA therapy, respectively, with significant improvement (P=0.001). Bilirubin and INR both showed significant 1-year posttreatment improvement (P=0.001). Quantitative HBV DNA PCR in patients with HBV reactivation during DAA therapy was raised at 1 month (10,184.5±1,857.2 IU/mL) and 3 months (20,802±1,059.7 IU/mL) after starting DAAs. HBV PCR then gradually decreased (without HBV antiviral therapy) until finally becoming undetectable 1 year posttherapy (6 months after the end of DAA therapy, P=0.001). At 1 year posttreatment, platelet counts showed significant increase: from 183±4.2×103/µL (before treatment) to 202.3±2.2×103/µL (1 year posttreatment; P=0.001; , and ). All patients had negative HCV PCR at 1 month posttreatment, and all had achieved sustained virologic response with negative HCV PCR 3 months after the end of treatment.
Discussion
The reactivation of HBV means detection of de novo HBV DNA not previously detected, a 1–2 log IU/mL increase in serum HBV DNA, or lasty seroconversion in individuals with HBc Ab.Citation13 Studies concerned with the reactivation of HBV after DAA treatment for HCV have given different results over the past few years. It is postulated that HCV infection suppresses HBV replication through many HCV core proteins, such as NS5Aa and NS2.Citation14–Citation19 This is why suppression of HCV by DAA is postulated to increase HBV replication. This was manifested in our data, where after 1 month of treatment there was marked elevation in HBV DNA.
In a cohort study on chronic HCV patients with positive HBsAg who underwent DAAs in Egypt, the risk of reactivation in the absence of HBV treatment was 28.6% (95% CI 15.6%–46.4%) and the risk of hepatitis in the patients who experienced reactivation 10.0% (95% CI 0.9%–57.8%). Also, the pooled risk of reactivation in HBsAg-negative anti-HBc-positive patients was negligible (0.1%, 95% CI 0–0.3%), irrespective of the presence of anti-HBs.Citation20
We studied patients that were coinfected with HCV–HBV. All patients had HBV PCR <2,000 IU/mL and showed HBeAg seroconversion with development of HBeAb. From the 140 patients studied, only four showed HBV reactivation, representing 2.8% of the studied group. When we followed all patients after DAA therapy, there were significant decreases in liver enzymes and HBV DNA. This was similar to a study that showed a decrease in liver enzymes after treatment of HCV, but without the statistical significance found in our study. Also, that study counted on quantitative HbcAb to diagnose the condition, while we tested patients for seroconversion of HbeAg, but not quantitative HbcAb.Citation21 It is known that the HBeAg seroconversion is associated with low levels of HBV DNA, with clinical improvement in liver disease in the majority of patients.Citation22–Citation25 This was the case in our patients, where a significant decrease was observed in liver enzymes that was associated with a decrease in HBV DNA.
In a meta-analysis, Chen et al compared the rate of reactivation in patients with chronic HBV versus those with occult infection. One of the results of the study was that HBV reactivation and hepatitis were less common among individuals with occult HBV infection.Citation26 This was in concordance with our study. In another study investigating 848 individuals treated with DAAs, only eight patients showed detectable HBV DNA with titers <20 IU/mL at the end of treatment, and no HBV reactivation was observed in HBsAg-negative but anticore-positive patients.Citation27
HBV reactivation was manifested in our study by elevated liver enzymes and increasing HBV DNA in four patients. This rise had resolved at 6-month of follow up without adding antiviral treatment for HBV. This was the case in another study where elevated ALT was present in 18 patients during follow-up of 108 patients coinfected with HCV–HBV.Citation28 In a 72-year-old woman, HBV reactivation was observed 4 weeks after the end of treatment with sofosbuvir and ribavirin with resolved HBV.Citation29 This was similar to our study, where resolution of the four reactivated cases occurred with no treatment.
In our study, we conclude that patients with F0 or F1, HBeAg seroconversion, and HBV DNA <2,000 IU/mL can complete without treatment with close monitoring. Also, the risk of HBV reactivation is low in patients treated with DAAs. Being a prospective study with close monitoring of the patients gives strength to our work. Still, we need longer follow-up and larger samples.
Disclosure
The authors report no conflicts of interest in this work.
References
- Bartholomeusz A, Locarnini SA. Antiviral drug resistance: clinical consequences and molecular aspects. Semin Liver Dis. 2006;26(2):162–170. doi:10.1055/s-2006-93975816673294
- Chen LW, Chien RN, Yen CL, Chang JJ, Liu CJ, Lin CL. Therapeutic effects of pegylated interferon plus ribavirin in chronic hepatitis C patients with occult hepatitis B virus dual infection. J Gastroenterol Hepatol. 2010;25(2):259–263. doi:10.1111/j.1440-1746.2009.06006.x19817959
- Mrani S, Chemin I, Menouar K, et al. Occult HBV infection may represent a major risk factor of nonresponse to antiviral therapy of chronic hepatitis C. J Med Virol. 2007;79(8):1075–1081. doi:10.1002/jmv.2094317596829
- Crespo J, Lozano JL, de la Cruz F, et al. Prevalence and significance of hepatitis C viremia in chronic active hepatitis B. Am J Gastroenterol. 1994;89:1147–1151.8053425
- Pol S, Haour G, Fontaine H, et al. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther. 2017;46:1054–1060. doi:10.1111/apt.1435228994127
- Tyson GL, Kramer JR, Duan Z, Davila JA, Richardson PA, El-Serag HB. Prevalence and predictors of hepatitis B virus co-infection in a United States cohort of hepatitis C virus-infected patient. Hepatology. 2013;58:538–545. doi:10.1002/hep.2640023505059
- AASLD/IDSA. Recommendations for testing, managing and treating hepatitis C. Joint panel from the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America. Available from: www.Hcvguidelines.org/. Accessed 1211, 2017.
- Sagnelli E, Coppola N, Scolastico C, et al. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology. 2000;32:1106–1110. doi:10.1053/jhep.2000.1928811050062
- Jardi R, Rodriguez F, Buti M, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404–410. doi:10.1053/jhep.2001.2651111481626
- Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration; Adverse Event Reporting System. Ann Intern Med. 2017;166:792–798. doi:10.7326/M17-037728437794
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236. doi:10.1016/j.jhep.2015.03.02525911336
- Mostafa AA, El-Shewi ME, Amin AM, Alibiary SHAA. Assessment of CD163 as a predictor of esophageal varices in patients with liver cirrhosis. N Y Sci J. 2016;9:3747.
- Gonzalez SA, Perrillo R. Hepatitis B virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis. 2016;62:S306–S313. doi:10.1093/cid/ciw04327190320
- Shih CM, Chen CM, Chen SY, Lee Y. Modulation of the trans-suppression activity of hepatitis C virus core protein by phosphorylation. J Virol. 1995;69:1160–1171 [PMC free article] [PubMed].7815494
- Shih CM, Lo SJ, Miyamura T, Chen SY, Lee Y. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67:5823–5832 [PMC free article] [PubMed].8396658
- Chen SY, Kao CF, Chen CM, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591–607 [PubMed]. doi:10.1074/jbc.M20424120012401801
- Schuttler CG, Fiedler N, Schmidt K, Repp R, Gerlich WH, Schaefer S. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J Hepatol. 2002;37:855–862. doi:10.1016/S0168-8278(02)00296-912445429
- Dumoulin FL, von Dem Bussche A, Li J, et al. Hepatitis C virus NS2 protein inhibits gene expression from different cellular and viral promoters in hepatic and nonhepatic cell lines. Virology. 2003;305:260–266 [PubMed]. doi:10.1006/viro.2002.176912573571
- Pan Y, Wei W, Kang L, et al. NS5A protein of HCV enhances HBV replication and resistance to interferon response. Biochem Biophys Res Commun. 2007;359:70–75. doi:10.1016/j.bbrc.2007.05.05217532300
- Kassas ME, Shimakawa Y, Ali-Eldin Z, et al. Risk of hepatitis B virus reactivation with direct-acting antivirals against hepatitis C virus: A cohort study from Egypt and meta-analysis of published data. Liver Int. 2018;38:2159–2169. doi:10.1111/liv.1387429738637
- Bhatia M, Gupta E, Choudhary MC, Jindal A, Sarin SK. Evaluation of impact of occult hepatitis B infection in chronic HCV-infected patients: a retrospective cohort study. J Lab Physicians. 2018;10(3):304–308. doi:10.4103/JLP.JLP_12_1830078967
- Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi:10.1053/jhep.2002.3363812029639
- Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829–834. doi:10.1016/j.amjmed.2003.12.04015178498
- Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepatol. 2007;14:147–152. doi:10.1111/j.1365-2893.2006.00810.x
- Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology. 2007;133:1458–1465. doi:10.1053/j.gastro.2007.08.03917935720
- Chen G, Wang C, Chen J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology. 2017;66:13–26. doi:10.1002/hep.2910928195337
- Mucke VT, Mucke MM, Peiffer KH, et al. No evidence of hepatitis B virus reactivation in patients with resolved infection treated with direct-acting antivirals for hepatitis C in a large real-world cohort. Aliment Pharmacol Ther. 2017;46:432–439. doi:10.1111/apt.1417728627791
- Mücke MM, Mücke VT, Peiffer K-H, et al. Absence of HBV reactivation in patients with resolved HBV infection following DAA therapy for hepatitis C: a 1-year follow-up study. Open Forum Infect Dis. 2019;6(1):1–5. doi:10.1093/ofid/ofy340
- Odolini S, Lanza P, Angiola A, et al. Hepatitis B virus reactivation after effective sofosbuvir and ribavirin treatment in a patient with occult hepatitis B virus infection. New Microbiol. 2017;40:218–220.28513813