Abstract
Shigella spp. are a common cause of diarrheal disease and have remained an important pathogen responsible for increased rates of morbidity and mortality caused by dysentery each year around the globe. Antibiotic treatment of Shigella infections plays an essential role in reducing prevalence and death rates of the disease. However, treatment of these infections remains a challenge, due to the global rise in broad-spectrum resistance to many antibiotics. Drug resistance in Shigella spp. can result from many mechanisms, such as decrease in cellular permeability, extrusion of drugs by active efflux pumps, and overexpression of drug-modifying and -inactivating enzymes or target modification by mutation. Therefore, there is an increasing need for identification and evolution of alternative therapeutic strategies presenting innovative avenues against Shigella infections, as well as paying further attention to this infection. The current review focuses on various antibiotic-resistance mechanisms of Shigella spp. with a particular emphasis on epidemiology and new mechanisms of resistance and their acquisition, and also discusses the status of novel strategies for treatment of Shigella infection and vaccine candidates currently under evaluation in preclinical or clinical phases.
Introduction
Shigella spp. are a Gram-negative, rod-shaped, immotile, and non-spore–forming bacteria and a causative agent of acute diarrhea that may progress to bloody mucoid diarrhea, also known as bacillary dysentery (or shigellosis).Citation1 Shigella is the most common cause of diarrheal disease and has remained a major pathogen responsible for increased rates of morbidity and mortality caused by dysentery each year around the globe, particularly affecting children aged <5 years in developing countries.Citation2 The four types of Shigella spp. comprise subgroup A (S. dysenteriae), subgroup B (S. flexneri), subgroup C (S. boydii), and subgroup D (S. sonnei). Each subgroup contains several serotypes. Shigellosis can occur in pandemic, epidemic, and sporadic forms. Epidemiological reports have shown that the epidemic subgroup of diarrhea typically occurs as a result of infection with S. flexneri in developing countries and S. sonnei in industrialized countries.Citation1,Citation3 Shigella spp. are categorized by the World Health Organization (WHO) as bacteria mainly causing infections in the community.Citation4 Shigellosis is a great public health threat, because its infective dose is on the order of 10–100 organisms compared to other enteric pathogens (usually it is 105–108 for Salmonella and Vibrio, respectively).Citation5,Citation6
Antibiotic treatment of common bacterial infections plays an important role in reducing prevalence and death rates of the disease. However, incorrect antibiotic use or overuse in treating diarrhea increases antibiotic resistance. Shigella spp. are resistant to most antibiotics, and drug treatment related to these bacteria is costly, time-consuming, and sometimes problematic, particularly in areas with limited medical care.Citation7,Citation8 About half the strains of Shigella in many parts of the world are now resistant to multiple drugs. Recently, various antibiotic-resistance mechanisms have been described by researchers, and these antibiotic-resistance mechanisms limit therapeutic options for treatment of Shigella infections.Citation8,Citation9
Drug resistance in Shigella spp. can result from many mechanisms, such as extrusion of drugs by active efflux pumps, decrease in cellular permeability, and overexpression of drug-modifying and -inactivating enzymes or target modification by mutation.Citation6,Citation10,Citation11 The current study was done to review various antibiotic-resistance mechanisms of Shigella spp., with a particular focus on epidemiology and new mechanisms of resistance and their acquisition, and also to discuss treatment and prevention measures for diseases caused by these organisms.
Search Strategy
We searched the biomedical electronic databases Web of Science, Scopus, PubMed (Medline), Embase, Cochrane Library, and Google Scholar for articles on Shigella published in English between 1990 and May 2019, using the key terms (alone and in combination) “Shigella”, “drug resistance”, “mechanism”, “biofilm”, “efflux pump”, “vaccine”, and “treatment”. We excluded case reports and some studies on the implementation of established techniques. Articles published before 1990 were also excluded, except when necessary. A total of 193 relevant articles were identified from the databases and included in this review.
Drug-Resistance Mechanisms In Shigella Spp
Role Of Outer-Membrane Permeability
Natural resistance to antimicrobial drugs by various mechanisms preventing the drug from being absorbed is capable of transforming the drug, its biotransformation into the cell, or reducing affinity with the drugs’ target.Citation12 Cell walls of microorganisms are the first barrier against penetration of the drug. Some modifications of membrane permeability or changes in the membrane lead to porin loss, which can result in an increase in minimum inhibitory concentration(MIC) for antimicrobial agents.Citation13 Most antibiotics used in treatment of Shigella infection should be able to penetrate the cell membrane to reach intracellular accumulation and target sites. For example, quinolone antibacterial agents, such as nalidixic acid, ofloxacin, and ciprofloxacin, interfere with DNA replication by inhibiting DNA topoisomerase IV and gyrase. Aminoglycoside antibiotics, such as streptomycin and spectinomycin, mediate inhibition of protein synthesis by binding to ribosomal subunits and reaching intracellular targets. β-Lactam antibiotics, eg, penicillin and cephalosporin, are a class of antibiotics containing a β-lactam ring in their molecular structures and inhibit cell-wall biosynthesis by targeting penicillin-binding proteins. Mutation or absence of ∼39 kDa porin in the membrane of such Gram-negative bacteria as Shigella spp. mainly influences susceptibility to slow penetration of β-lactams, such as aztreonam and dianionic moxalactam, and also low permeability of hydrophilic antibiotics, such as penicillin and piperacillin.Citation6,Citation13 Indeed, resistance toward β-lactam antibiotics is associated with modification of the outer-membrane porins OmpF (∼38 kDa) and OmpC (∼42 kDa) and cytosolic proteins of ∼26 kDa, OmpR as a transcriptional regulator.Citation6 In a study, three imipenem-resistant mutants of S. dysenteriae were obtained from India and showed lower levels of both major OMPs (∼38 and ∼43 kDa). Increasing imipenem resistance in mutants was associated with permeability of outer-membrane proteins.Citation14 Lipopolysaccharides (LPSs) have been recognized as an essential outer-membrane component needed for assembly of trimeric PhoE porin and confer colicin E2 resistance in S. flexneri strains,Citation15 and have also been reported to be linked with the rise in resistance toward imipenem in S. dysenteriae.Citation14 Some outer-membrane components, such as IcsA molecules, are not only associated with bile salts resistance but are also related to promotion in development of biofilm by mediating bacterial cell–cell interactions. Consequently, they produce resistant phenotypes.Citation16
Efflux Systems
Active efflux pumps play a significant role in antibiotic-resistance phenotypes of Gram-negative bacteria and expelling toxic compounds from their cells. Efflux systems are grouped into five families: the major facilitator superfamily (MFS), resistance–nodulation–division family, small multidrug resistance (MDR) family, ATP-binding cassette superfamily, and multidrug and toxic compound extrusion family.Citation17 AcrAB–TolC pump is involved in antibiotic resistance phenotype of Escherichia spp., Enterobacter spp., Salmonella spp., and Shigella spp isolates. The AcrAB–TolC system is a tripartite complex comprising TolC (outer-membrane channel), AcrB (inner-membrane transporter protein), and periplasmic AcrA involved in assembly and maintenance of these two integral membrane proteins. AcrAB–TolC belongs to resistance–nodulation–division family of efflux pumps, associated with efflux of quinolones, and one of factors responsible for development of resistance among Shigella isolates. Indeed, overexpression of AcrAB–TolC results in overall decreased accumulation of quinolones inside bacterial cells, also resulting in reduced susceptibility to them.Citation18 AcrAB-associated to bile-salt resistance has been found in some strains of S. flexneri. Their expression has been shown to increase after exposure to bile salts, and enabled Shigella to resist bactericidal effects of bile. Researchers believe that this phenomenon may confer resistance to other antimicrobial agents. Furthermore, overexpression of AcrB has been found to be linked to multiple drug-resistance phenotypes in some Gram-negative bacteria.Citation19
Synergistic action regarding activation of acrAB–tolC efflux pumps has been shown to decrease expression of outer-membrane porins, gyrase, and topoisomerase target–gene mutations toward fluoroquinolone resistance in Shigella isolates.Citation20,Citation21 Drug-efflux pumps, such as marA, tolC, ydhE, and mdfA, confer quinolone resistance. Kim et al demonstrated that resistance to fluoroquinolone is due to increased expression level of MdfA efflux pump in Shigella spp. This efflux is a member of the MFS antibiotic-efflux system, and MdfA efflux pump–mediated fluoroquinolone resistance was first identified among MDR Escherichia coli.Citation22 Tetracycline efflux and resistance is associated with the MFS antibiotic–efflux system encoded by various tet genes in Gram-negative bacteria, such as Shigella spp. and Klebsiella spp. Among tet efflux systems, it seems that tetA and tetB are mediated by resistance to tetracycline in S. sonnei and S. flexneri, respectively (for more details, refer to “Tetracycline Resistance” section).Citation11
Resistance To β-Lactam Antibiotics
Class A β-Lactamases
Class A β-lactamases can hydrolyze narrow-spectrum penicillin, but not carbapenems or cephalosporins, and are inhibited by tazobactam and clavulanic acid. Extended-spectrum β-lactamases (ESBLs) belong to Ambler class A. ESBLs conferring resistance to third-generation cephalosporins have been found in Shigella isolates. The first report documenting identification of ESBL-producing Shigella strains was from Bangladesh in 2004.Citation23 Emergence of ESBLs in Shigella spp. is a global major health threat affecting both developed and developing countries. Different β-lactamases belonging to Ambler class A have been reported among Shigella isolates, such as TEM, SHV, and CTX-M enzymes. The first isolate of S. flexneri producing an ESBL and harboring a plasmid encoding the blaSHV-2 gene was reported from France in 1995.Citation24
To date, several reports from Argentina, Israel, Canada, Turkey, Lebanon, Japan, Iran, South Korea, China, and other various regions in Asia have identified Shigella spp. harboring different types of ESBL genes ().Citation23,Citation25–Citation32 Although most ESBLs are derivatives of TEM and SHV β-lactamase families, which were first identified, Shigella spp. can also express the CTX-M family, among which CTX-M-15 is one of the most relevant findings associated with the current epidemiology of ESBLs, which has been predominantly identified in commensal and pathogenic ESBL-producing Shigella isolates around the world. Despite many surveillance studies and investigations, the reason for this epidemiological shift remains unknown.Citation24,Citation33 These enzymes are responsible for selective hydrolysis of ceftriaxone and cefotaxime and even more distinctly for ceftazidime, although some types of CTX-M, such as CTX-M-15, may hydrolyze ceftazidime.Citation33 In general, CTX-M-15 has been found to have great catalytic efficiency (high kcat/Km) against piperacillin, benzylpenicillin, ceftriaxone, and cefotaxime, as reported for other of CTX-M-types, such as CTX-M-3, CTX-M-16, and CTX-M-18.
Table 1 Prevalence Of Antimicrobial Resistance Genes In Shigella Spp. Isolated From Different Regions Of The World
To date, CTX-M-15 has been detected in Shigella isolates from various countries across the world, including Canada, Russia, Poland, the UK, France, Bulgaria, Turkey, and Iran.Citation33,Citation34 CTX-M-type β-lactamases contain at least 40 enzymes, and these can be readily transferred among Shigella isolates by conjugative plasmids belonging to IncF, IncZ, and Incl groups.Citation34,Citation35 Li et al revealed that ISEcp1 was present adjacently to all blaCTX-M genes in Shigella strains, meaning that it plays an important role in mobilizing blaCTX-M genes ().Citation36 A large study conducted in Vietnam analyzed IncI1 plasmid pKHSB1 carrying blaCTX-M-15 collected from a clonal population of S. sonnei.Citation37 Another study reported full sequence of IncI1 plasmid pSH4469 carrying blaCTX-M-15 in a clinical isolate of S. sonnei isolated from an outbreak in South Korea.Citation34 The overall structure of plasmid pSH4469 was nearly identical to pKHSB1, and it seemed that both were responsible for dissemination of blaCTX-M-15 among Shigella isolates ().
Figure 1 Schematic representation of blaCTX-M-55, blaCTX-M-15, and blaTEM-1 genes in different types of plasmid. Arrows indicate positions and directions of different genes and IRL, terminal inverted repeats at the left, IRR, terminal inverted repeats at the right.
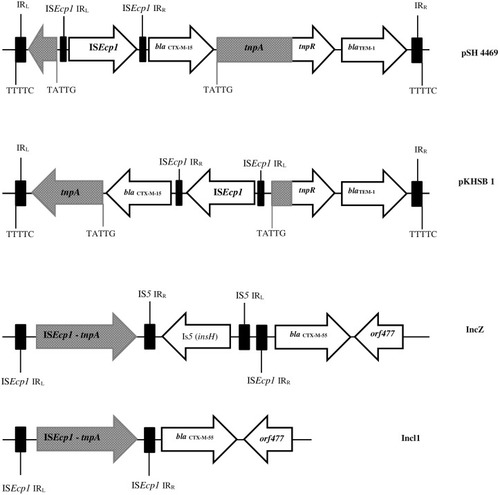
Recently, a new hybrid of CTX-M-9 and CTX-M-1 β-lactamases named CTX-M-123 was identified among S. flexneri isolates from patients in China. In this study, blaCTX-M-123 was carried by two conjugative plasmids named IncHI2 and IncF, and these conjugatable plasmids were responsible for dissemination of blaCTX-M-123 in S. flexneri isolates.Citation38 Moreover, several new β-lactamase subtypes — CTX-M-79, CTX-M-27, CTX-M-24, CTX-M-15, CTX-M-14, CTX-M-64, CTX-M-65, CTX-M-55, and CTX-M-3 — have been found in clinical Shigella strains isolated from different provinces in China.Citation36,Citation39,Citation40 ESBL genes among Shigella isolates might be transferred from E. coli isolates to Shigella spp., especially S. sonnei isolates, through conjugation in human gut.Citation41,Citation42 The increase in MDR and emergence of ESBL in Shigella spp. may be the cause of treatment failures and accordingly limitation in therapeutic options.Citation41
Class B β-Lactamases
Class B β-lactamase enzyme can hydrolyze carbapenem and other β-lactams, except for aztreonam, and classical β-lactamase inhibitors, such as tazobactam and clavulanic acid, do not inhibit them. Metallo-β-Lactamase was first detected in a transferable plasmid from Pseudomonas aeruginosa, and also IMP-1 was first identified from many kinds of Gram-negative rods in Japan.Citation43,Citation44 O’Hara et al reported a novel type of metallo-β-lactamase named MET-1 mediated by a S. flexneri plasmid. They believed that MET-1 was a derivative of IMP-1 β-lactamase.Citation44 This plasmid conferred resistance against sulfonamide and kanamycin, in addition to β-lactamase.Citation44 Lyobe et al found that MET-1 experienced two amino-acid changes from IMP-1. The gene was renamed IMP-3, and thus IMP-3 could be considered an ancestor of IMP-1 β-lactamase. They also showed that this gene was located on a cassette inserted in a class I integron, widely disseminated among other species of Shigella, and conferred resistance to almost all β-lactam antibiotics.Citation45
Carbapenem resistance conferred by blaVIM and blaIMP genes has recently been detected in S. sonnei and S. flexneri isolates from pediatric patients with diarrhea in the Andaman and Nicobar islands in India.Citation43 In this study, after analysis of nucleotide sequencing of blaIMP and blaVIM genes by BLAST, 100% similarity with sequences of these genes isolated from Acinetobacter baumannii and P. aeruginosa available at the NCBI database was confirmed. After spread of carbapenem resistance to Shigella in another part of the world, an indication of a potential public health challenge, treatment options will be limited, and infection-control measures remain of high importance.Citation43 Finally, although class A β-lactamase KPC is one of the most commonly identified carbapenemases among other Gram-negative bacteria in some parts of the world, it has not yet been identified in Shigella isolates, except for a single blaKPC-carrying S. flexneri strain isolated from the National Senegalese Enterobacteriaceae Center located at the Pasteur Institute in Dakar.Citation46
Class C β-Lactamases
Ceftriaxone is recommended for treatment of ciprofloxacin-resistant Shigella isolates. However, today some strains of Shigella spp. have a resistance gene to cephalosporins. Class C β-lactamases, also known as AmpC-type enzymes, confer high-level resistance against cephalosporins. AmpC β-lactamase is encoded by both plasmid and chromosomal genes, and the first report of plasmid-encoded CMY-2-type AmpC β-lactamase was detected among ceftriaxone-resistant S. sonnei isolates obtained from an outbreak of bacillary dysentery in Taiwan.Citation47 CMY-2 enzymes have been reported in China, Taiwan, Costa Rica, Iran, and India from several epidemic strains.Citation47–Citation50 Zhang et al found two AmpC β-lactamase producers with blaCMY-2 and blaDHA-1 in Shigella strains recovered from diarrhea patients in China.Citation51
Tajbakhsh et al reported on the first AmpC β-lactamase (blaCMY-2) producers in S. sonnei isolated from patients in Tehran, Iran.Citation48 In recent years, other studies conducted in Iran have indicated spread of resistance to extended-spectrum cephalosporins among Shigella isolates.Citation31,Citation50 In a similar study carried out in Iran, studying cephalosporin-resistant Shigella isolates, the researchers identified gene CMY-59 in one S. sonnei isolate from pediatric patients aged <12 years.Citation31 However, the majority of AmpC-positive isolates studied in other parts of the world belonging to the CMY-2 genotype and other AmpC genes (blaMOX, blaFOX, blaMIR(ACT-1), blaCIT, and blaACC) have been identified in Shigella isolates; however, so far there has not a study on them.Citation52,Citation53 Indeed, few reports have described the presence of AmpC β-lactamases among Shigella isolates worldwide (see for more details).Citation47,Citation48
Class D β-Lactamases
Class D β-lactamases or OXA-type β-lactamases confer resistance to ampicillin and cephalothin and can hydrolyze oxacillin and cloxacillin, as well as benzylpenicillin, but they are not inhibited by tazobactam or other inhibitors.Citation52,Citation54 Initially, blaOXA β-lactamases were reported among P. aeruginosa isolates, although now blaOXA genes have been identified in integrons and plasmids in many Gram-negative bacteria.Citation55 In Shigella spp., resistance to ampicillin is mainly associated with an OXA-type β-lactamase.Citation54,Citation56 blaOXA-30 was initially described in ampicillin-resistant S. flexneri strains from China in 2000.Citation57 Results of some studies have shown that S. flexneri isolates are a probable host specific for blaOXA-type β-lactamase.Citation52,Citation58 Another study from Iran showed that all blaOXA-positive isolates carried blaOXA-1 and many of them were present in S. flexneri, suggesting an individual host preference of these enzymes in S. flexneri isolates.Citation50
blaOXA-1 and blaOXA-30 genes, containing Tn2603 and Tn1409 transposons, respectively, differ from each other by having one mutation at codon 131. A gene encoding blaOXAβ-lactamases is carried on integron.Citation58 Furthermore, other studies have shown that blaOXA-30 and aadA1 are located in the gene cassettes of class 1 integrons (). Therefore, class 1 integrons carry resistance traits for β-lactams (blaOXA) and trimethoprim (dfrA1).Citation52
Quinolone And Fluoroquinolone Resistance
Resistance To (Fluoro)quinolones Due To Chromosomal Target–Site Mutations
Corresponding subunits for DNA gyrase and topoisomerase IV are gyrA, gyrB, parC, and parE, encoded by genes gyrA, gyrB, parC, and parE genes, respectively. DNA gyrase consists of two gyrA subunits and two gyrB subunits, and topoisomerase IV contains two parC and two parE subunits. The most mutations have been found in a small region near the start of the gyrA gene termed a quinolone resistance–determining region (QRDR), between Ala67 and Gln107, and as reported in several studies (), most frequently mutations occur at codons 83, 87, and 211, while mutations in gyrB were detected with lower frequencies in different studies.Citation59,Citation60 Some researchers believe that when a single mutation occurs in gyrA, it may confer resistance to quinolones, but for decreased susceptibility to fluoroquinolones, a number of further mutations in parC and gyrA regions are needed.Citation61,Citation62 parC gene mutations most frequently occur at codons 80 among Shigella isolates. gyrA gene mutations have been confirmed to be much more prevalent than mutations in the gyrB gene.Citation63,Citation64 Most nucleotide and amino-acid changes in QRDRs of gyrA, gyrB, parC, and parE among Shigella spp. are shown in . Novel mutations in QRDRs have also been identified from different regions of the world. Two novel mutations at codons 86 and 129 in parC and a mutation in codon 211 of gyrA were first reported in S. sonnei strains recovered from China in 2009.Citation65
Table 2 Frequency Of Amino-Acid And Nucleotide Changes In The Quinolone Resistance–Determining Regions Of ShigellaIsolates In Different Parts Of The World
Finally, two novel mutations at codons 408 and 458 in parE have recently been discovered among Shigellaspp., isolated in India in 2013 and Jiangsu Province in China in 2016.Citation10,Citation63 Mutation in codon 458 is believed to result in resistance to ciprofloxacin and nalidixic acid, while a single isolate with a mutation at codon 408 in parE is related to resistance to nalidixic acid, but susceptible to ciprofloxacin. It seems that both the novel mutations in parE of S. flexneri isolates may be correlated with the increased MIC for ciprofloxacin and mediate fluoroquinolone resistance. Also, in neither study were parE mutations identified among quinolone-sensitive isolates.Citation10,Citation63
Data presented in show that mutation patterns in gyrA and parC genes, particularly common mutations, are similar to those reported by other studies, reflecting a universal pattern among Shigella spp. A direct contribution to quinolone and fluoroquinolone resistance by each of these mutations in chromosomal target–site mutations (QRDRs) remains unknown. However, other mechanisms may be present in Shigella isolates, and further investigations are needed.
Resistance To (Fluoro)quinolones Due To Plasmid-Mediated Resistance Mechanisms
Distribution of plasmid genes called plasmid-mediated quinolone-resistance regions (PMQRs) namely qnr (qnrA, qnrB, qnrC, qnrD, qnrS, qep, aac[6ʹ]-lb-cr) genes is the main reason for resistance to quinolones among Shigella isolates, and they are usually associated with transposable or mobile elements on plasmids.Citation64,Citation66 qnr genes, which are often incorporated into integrons, may allow for dissemination among Shigella and possibly other members of the Enterobacteriaceae family, and then quinolone-resistance isolates may spread across geographic regions and even across countries with population mobility.Citation65,Citation67 The aac(6′)-Ib-cr gene encodes an acetyltransferase associated with reduced quinolone activity, and is identified in many members of the Enterobacteriaceae family.Citation68 PMQRs have been identified widely among human and animal isolates, and have become a pressing issue worldwide. In a study conducted in China, fluoroquinolone-resistance rates in animal isolates of S. flexneri were reported to be higher than those in human strains.Citation68
In the US, the Shigella resistance rate to fluoroquinolones reached 87% during 2014–2015.Citation69 Resistance of Shigella isolates to fluoroquinolone is mainly due to mutational alterations in QRDRs of DNA gyrase and topoisomerase IV genes, but PMQRs may facilitate in selection of isolates exhibiting higher levels of resistance through extrachromosomally encoded mechanisms and confer reduced susceptibility to quinolones (or fluoroquinolones).Citation68 aac(6′)-Ib-cr and qnrS genes were first identified in isolates of S. flexneri 2a in 1998 and S. flexneri serotype 1a in 2002, respectively.Citation70,Citation71 Furthermore, the qnrS gene was identified in isolates of Shigella flexneri 2b in 2005 in Japan.Citation70 Also, as reported in two previous studies, aac(6′)-Ib-cr and qnrS were predominant PMQR determinants across two provinces in China, conferring high levels of fluoroquinolone resistance.Citation72,Citation73 These studies indicated that aac(6′)-Ib-cr–positive Shigella isolates have been present in China for many years.Citation70,Citation72,Citation73 S. flexneri serotypes 1a, 2a, 2b, 4c, and S. sonnei carrying the qnrS gene have been reported worldwide.Citation67
Importantly, qnrS-positive isolates of Shigella, especially S. flexneri strains, show high-level resistance to fluoroquinolones, and many researchers from different part ofs the world suggest that the plasmid-mediated quinolone-resistance gene qnrS plays an essential role in reduced susceptibility of Shigella strains to fluoroquinolones.Citation67,Citation72,Citation73 Indeed, qnrS plasmid could change fluoroquinolone susceptibility of S. flexneri isolates containing both gyrA83 and parC80 mutations into ciprofloxacin-resistant isolates.Citation74 Overall, aac(6ʹ)-Ib-cr is the most prevalent gene, followed by qnrS detected in isolates of S. flexneri from the US, India, Japan, China, and Iran, and also most studies have highlighted an increased prevalence of PMQR determinants through the years.Citation50,Citation70,Citation75–Citation77 A recent study conducted in China reported that aac(6)-Ib-cr–positive isolates and qepA-positive isolates expressed high levels of quinolone resistance. This finding indicates that other mechanisms, such as reduced outer-membrane permeability, active efflux pumps, and harboring of different resistance genes, may be responsible for resistance to quinolones.Citation18,Citation77
Fosfomycin Resistance
Fosfomycin (Fom) is a broad-spectrum antibiotic inhibiting bacterial cell-wall biogenesis by inactivating the MurA enzyme.Citation38,Citation78 Despite the use of Fom in treatment of microbial infections for four decades, Fom has remained effective against common uropathogens, and Fom resistance has remained rare throughout the world.Citation79 However, Fom resistance was observed among E. coli strains to harbor novel transferable fosfomycin-resistance determinants named FosC2 and FosA3.Citation80 Two primary resistance mechanisms have been described for fosfomycin resistance: mutations in uhpA/T and glpT genes encoding proteins for two carrier-dependent systems responsible for fosfomycin uptake, and attainment of fosfomycin-modifying enzymes containing two kinases, FomA and FomB, and three types of metalloenzymes: FosX, FosA, and FosB.Citation81
Fosfomycin-modifying enzymes were discovered for the first time among S. flexneri strains isolated from patients in China.Citation38 Some studies have suggested that increasing prevalence of fosA3 was due to dissemination of IncN and IncI plasmids, facilitating its quick dispersal.Citation38,Citation80 Indeed, ESBL (blaCTX-M-123, blaCTX-M-55, or blaCTX-M-15) and fosA3 genes were cocarried by transconjugant plasmids from diverse incompatibility groups, and all of them contained determinants encoding resistance to cefotaxime, ceftriaxone, and fosfomycin.Citation38,Citation80 In this regard, conjugatable plasmids are likely to play an essential role in dissemination of fosA3 and ESBL genes among Shigella isolates with high clonal diversity, and they should be closely monitored.Citation38
Aminoglycoside Resistance
Aminoglycosides are used to treat a wide range of infections. Aminoglycosides mediate inhibition of protein synthesis.Citation82 Resistance to aminoglycosides is associated with enzymatic inactivation, ribosomal modification, and active efflux pumps. Among these mechanisms, aminoglycoside-modifying enzymes are the most common in the clinical setting.Citation83–Citation85 These enzymes are activated through three general reactions, resulting in adenylation, acetylation, or phosphorylation. Aminoglycoside adenyltransferase (aadA gene cassettes) are very common in Enterobacteriaceae, especially among Salmonella and Shigella isolates, conferring resistance to streptomycin and spectinomycin.Citation84,Citation86 Indeed, streptomycin resistance is strongly associated with integrons because of the high prevalence of aadA gene cassettes within class 1 and 2 integrons. A typical class 2 integron has a gene cassette of 2.2 kb with a resistance-gene arrangement (dfrA1–sat–aadA1) conferring resistance to trimethoprim, streptothricin, and spectinomycin/streptomycin, respectively, while aadA1 was absent in atypical class 2 integrons.Citation87,Citation88 Class 2 integrons have been identified in transposon Tn7 and predominantly inserted into chromosomes with high frequency.Citation89 An atypical class 1 integron with an unusual 3ʹ conserved sequence carrying a estX–psp–aadA2–cmlA-aadA1–qacH cassette array has been detected among different Gram-negative species (E. coli, Shigella, and Salmonella) from different hosts (human, animal, and food), periods and geographical regions. Accordingly, horizontal transfer of these integrons by plasmids promotes spread of multiple-resistance genes in sporadic and outbreak isolates of Shigella.Citation89–Citation91
Many types of aadA gene cassettes have been identified among Enterobacteriaceae, but types aadA1 and aadA2 have high prevalence among Shigella isolates.Citation84,Citation89,Citation92 Aminoglycoside phosphotransferases encoded by strA and strB are the most common genes dispersed among Shigella isolates by plasmids, such as IncFII and pNV-Y394.Citation83,Citation87,Citation93,Citation94 The gene encoding strA has been identified in 42.1% of Shigella isolates recovered from diarrheal patients in Pakistan.Citation95 In a study conducted in India, 100% and 88% of S. dysenteriae type 1 and S. sonnei strains harbored strA genes, encoding resistance to streptomycin.Citation96 The majority of Shigella isolates harbored strA and strB, along with unrelated resistance determinants, which are coded by blaTEM, blaCTX-M, qnrS, aadA1, tet(A), tet(B), catA, and catP.Citation95,Citation96 A 6.3 kb plasmid has been detected in the S. flexneri 3a strain and is involved in a streptomycin-resistance phenotype. This plasmid is likely to cause acquired resistance to streptomycin.Citation97 Also, a study conducted in South Korea showed that resistance to streptomycin was mediated by strA or strB among S. sonnei isolates obtained there and revealed that tetA, strA–strB, and sul1 were encoded and present in 8.4 kb of untransferable R plasmid.Citation98
Occurrence of aminoglycoside-resistance genes among Shigella isolates does not occur, because these drugs have long been excluded for treatment of shigellosis in different geographical areas.Citation85 However, there is still a concern, because class 1 integrons containing the gene-cassette array of blaOXA-30 + aadA1 with complete 3′CS have been reported on plasmids in Shigella spp. isolates, Salmonella enterica serovar Typhimurium and E. coli strains, and transferable plasmids may enhance spread of resistance genes within integrin, establishing arole of plasmids in horizontal transfer of resistance genes.Citation99
Tetracycline Resistance
Tetracyclines are used against a wide variety of diseases in humans and animals.Citation100 Tetracycline-resistant bacteria are found in opportunistic pathogens and normal flora species. According to the study by RobertsCitation101 and nomenclature for tetracycline resistance genes(https://faculty.washington.edu/marilynr), five tetracycline-efflux genes — tet(A), tet(B), tet(C), tet(D), and tet(G) — and one ribosomal protection protein encoded by tet(M) have been identified among Shigella isolates, most of which are encoded in transmittable elements, with extensive dissemination in different groups of bacteria. In a study, a 20.4 kb genomic island was identified encoding MDR genes, such as a wide variety of tet genes flanked by transposases.Citation102 This identical MDR cassette was first identified in S. flexneri serotype 2a strain YSH6000 and was referred to as Shigella resistance locus–pathogenicity island.Citation103 Interestingly, these MDR genes have recently been found in an E. coli plasmid, pRSB225, with a similar arrangement.104 The IncB/O/K/Z-type plasmid, termed p866, carrying resistance genes tet(A) and tet(B) have been identified in S. sonnei strains.105 These findings suggest that tet genes might be dispersed among other species by horizontal gene transfer.
Among 154 tetracycline-resistant isolates recovered as confirmed causes of traveler’s diarrhea in Spain, 79.2% (n=122) harbored at least tet(A) or tet(B). Combinations of tet(A) + tet(B), tet(A) + tet(G), and tet(B) + tet(G) were found in five, one, and seven isolates, respectively.Citation106 Results of two studies revealed that tet(A) was more frequent among S. sonnei strains, whereas tet(B) was more frequent among S. flexneri strains.Citation106,Citation107 Also, S. sonnei and S. flexneri differed from each other in terms of prevalence of plasmid Inc groups.Citation106 In 50 isolates of Shigella spp. identified from stool samples collected from children with diarrhea in Iran, 90% and 18% of isolates carried tetA and tetB, respectively, and no positive results were identified for tet(C) or tet(D) in this study.Citation11 Results of the same study conducted in Iran revealed that tet(A) and tet(B) were present in 75.7% and 21.42% of Shigella spp. and that tet(A) was more frequent in S. flexneri and S. sonnei populations.Citation108
A study carried out in Mexico identified genes tet(A), tet(B), and tet(C) in 1, 6, and 18 S. sonnei isolates, and 2, 7, and 1 S. flexneri isolates, respectively, whereas tet(D) was observed only in S. sonnei isolates (8%).Citation109 Among 20 S. dysenteriae isolates from dysentery outbreaks obtained from different parts of India, tet(B) was more common (90%) than tet(A) (10%). In the same study, genes tet(A) and tet(B) were detected in 15% and 79% of Shigella isolates, respectively, samples of which were obtained from children with diarrhea in southern Mozambique.Citation107 Based on recent studies,Citation107,Citation108,Citation110 it seems that efflux-mediated tetracycline resistance to tetracycline in S. sonnei and S. flexneri strains may be related to expression of tet(A) and tet(B), respectively. According to tetracycline resistance–gene nomenclature (https://faculty.washington.edu/marilynr), tet(B) is able to confer resistance to minocycline, while other efflux pumps encoded in a transferable tet gene do not have such a property and may cause clonal dissemination of tet(B)-positive Shigella isolates worldwide. In general, tet(A) and tet(B) are the most prevalent tetracycline-resistance genes in Shigella spp. Other tetracycline-efflux genes, such as tet(C) or tet(D), are rarely detected alone.
Phenicol Resistance
Phenicols have used to treat Shigella infections during the past few years around the world, and application of them led to strong selective pressure for resistance to these antibiotics.Citation111 Resistance in Shigella is associated with enzymatic inactivation of unfluorinated phenicols by chloramphenicol acetyltransferase genes encoded by variants of catA (catA1, catA2, catA3) and catB (catB2, catB3, catB7, catB8), as well as active efflux by cmlA (cmlA1, cmlA4, cmlA9) and/or fluorinated and unfluorinated phenicols (floR) by major facilitator–superfamily proteins.Citation112
Chloramphenicol resistance in Shigella isolates is mainly associated with the presence of cat genes. Among 95 Shigella isolates collected from diarrheal patients in Pakistan, at least 69 (72.6%) were resistant to chloramphenicol. catA and catP were detected in 32 (33.68%) and 24 (25.26%) isolates, respectively, from chloramphenicol-resistant Shigella isolates.Citation95 Among 103 S. sonnei isolates associated with several waterborne outbreaks in Taiwan, in 84% of sporadic isolates, tetB and catA genes were transferred along with 130 kb plasmids.Citation89 The presence of catA encoding chloramphenicol O-acetyltransferase was detected and confirmed in S. flexneri strains with strA or aadA1 genes or both.Citation96
Colistin Resistance
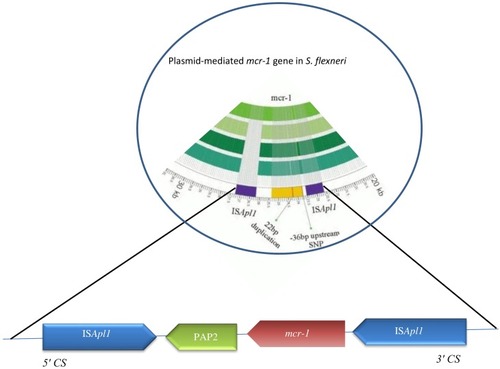
Colistin (polymyxin E) is a polypeptide antimicrobial agent interacting with outer membranes of Gram-negative bacteria. Since the first report of the plasmid-mediated polymyxin-resistance gene mcr-1 was published in an E. coli isolate in November 2016 in China,Citation113 this gene has also been identified in Salmonella enterica and Klebsiella pneumoniae, but is rarely reported in other Enterobacteriaceae members here.Citation114 This gene has now been identified in other Enterobacteriaceae genera, such as Shigella, Cronobacter, Kluyvera, and Enterobacter isolated from vegetables, the environment, food, animals, and human beings.Citation115,Citation116 The mechanism of resistance of mcr-1 is produced by a phosphatidylethanolamine transferase, leading to modification of lipid A present as a result of addition of phosphoethanolamine to lipid A in cell membranes of Gram-negative bacteria, resulting in more cationic LPSs and lower affinity for colistin and related polymyxins and consequently reduced antimicrobial activity.Citation117 The presence of mcr-1 in Shigella isolates leads to a four- to eightfold increase in the MIC of polymyxin B.Citation118 mcr-1 is located on various plasmid backbones, including IncI2, IncFI, IncHI2, IncFIB, IncP, IncY, and IncX4, sized 58–252 kb ().Citation114 ESBL-encoding genes or other resistance genes might coexist with it. Also, plasmid-mediated colistin-resistant S. flexneri isolates recovered from animal feces on a farm showed that it might be circulated via the fecal–oral route, at least between animals on that farm, and possibly distributed via the food-production network.Citation118 In most reports, mcr-1 is known to be the only resistance gene for related plasmids, indicating that selective pressure associated with polymyxin is responsible for mcr-1 acquisition.Citation114,Citation118 It means that other plasmids conferring MDR phenotypes can be achieved from resistant S. flexneri strains. Mobile elements, such as IS and integrons, could also help isolates acquiring other resistance elements from the environment.Citation118 A novel transposon, Tn6390 has been detected in S. flexneri C960, in which two copies of ISApl1 bracketed to the mcr-1 gene play a pivotal role in transposition of mcr-1.Citation118 Recently, mcr-1 was identified in S. sonnei isolates from Shanghai (2010–2012) with polymyxin B resistance (MIC 4–8 μg/mL).Citation118
Sulfonamide And Trimethoprim Resistance
After the spread of trimethoprim–sulfonamide resistance in different parts of the world, these agents are currently considered ineffective for empirical therapy of shigellosis.Citation119 Acquired resistance mechanisms have frequently been identified, mostly due to mutational or recombinational changes in target enzymes (dihydropteroate synthase and dihydrofolate reductase, respectively) or acquired resistance by drug-resistant target enzymes, such as acquired sul genes coding for drug-resistant dihydropteroate synthases or dfr genes coding for drug-resistant dihydrofolate reductases.Citation120,Citation121
Resistance To Trimethoprim
At least 42 dfr genes conferring trimethoprim resistance have been detected in different groups of bacteria worldwide, 12 of which have been identified in Shigella spp. Resistance to trimethoprim might be explained by the presence of integron-borne dfr genes (). Gene cassettes within class 1 integrons detected on plasmids or chromosomes in Shigella isolates often encode resistance to trimethoprim (dfrA), streptomycin (aadA), and ampicillin (oxa-1).Citation58,Citation85 Class 2 integrons borne on Tn7 have often been found in Shigella spp., and gene-cassette arrays of them usually contain dfrA1, sat1, and aadA1.Citation122
Figure 4 Variable regions of class 1 (A) and class 2 (B) integrons reported in different geographic area. Horizontal arrows indicate transcriptional orientation of genes.
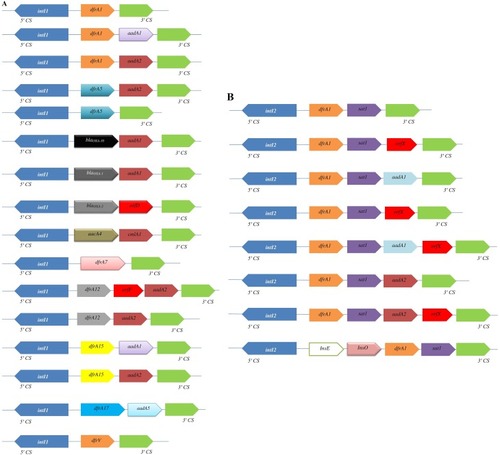
The presence of dfrA1 genes among Shigella isolates is the main mechanism of trimethoprim resistance, occurring in a cassette in both class 1 and class 2 integrons (). Two types of class 2 integrons among S. sonnei strains have been identified in Japan. One of them was typical type of class 2 integrons (2,158 bp) with dfrA1, sat1, and aadA1 cassette arrays, and the other was an atypical type of class 2 integrons (1,313 bp) carried only two gene-cassette arrays with dfrA1 and sat1.Citation123 This integron‐associated antibiotic resistance can be transferred to other species via plasmid conjugation. dfr12–orfF–aadA2, dfr17–aadA5, and aadA1 cassette arrays carried by class 1 integrons have been recognized in S. sonnei isolates recovered from South Korea, China, Vietnam, and Australia.Citation84,Citation85,Citation99 Also, dfrA1–sat1–aadA1–orfX, free aadA1, or free orfX cassette arrays carried by class 2 integrons have been detected in Senegalese Shigella spp. isolates.Citation92 In contrast to dfrA genes, dfrB genes have not been identified among Shigella isolates. Recently, trimethoprim-resistance clones were sequenced by primer walking, and a native 6,779 bp plasmid was identified with presence of the dfrA14 gene in a sul2–strA’–dfrA14–‘strA–strB gene arrangement in S. sonnei strains, suggesting that dfrA14 was associated with a small nonconjugative plasmid.Citation124 Class 2 integrons within a dfr1–sat2–aadA1 cassette array were predominant in S. sonnei isolates from outbreaks cases in Taiwan, while class 2 integrons were absent in sporadic cases.Citation89
A large study conducted in South Korea analyzed 122 S. sonnei isolates collected from stool samples in different parts of the country from 1991 to 2000. Resistance to trimethoprim was associated with dfrA1 and dfrA12. dfrA1 was found as a gene cassette of Tn7 located in chromosomes, while dfrA12 was located in conjugative R plasmids as a gene cassette of class 1 integrons. Tn7 was not detected in S. sonnei isolates recovered from the 1980s, while in this study Tn7 was found in all S. sonnei isolates. The authors proposed that S. sonnei isolates carrying Tn7 were responsible for outbreaks of shigellosis in different parts of South Korea in the 2000s.Citation98
Resistance To Sulfonamides
Since the first report of resistance to sulfonamides was found in both S. sonnei and S. flexneri isolates recovered from the early 1970s in South Korea,Citation98,Citation125 resistance to this antibiotic has been identified in 94 % of S. sonnei strains from the 1980s and 100% of isolates from the 2000s and 2010s in different parts of the world.Citation2,Citation50,Citation118 Sulfonamide resistance is mediated by sul1, sul2, and sul3 genes, and they are common in Shigella.Citation89 The sul1 gene is highly frequent among Shigella isolates, because it is part of the 3ʹ-conserved sequence region of class 1 integrons. Also, sul3, linked to an unusual 3ʹ-conserved segment, is associated with class 1 integrons.Citation89,Citation126 According to one study, sul2 is one of the three sulfonamide-resistance genes, and is usually located on large transmissible plasmids or small non-conjugative plasmids and was first detected on a small nonconjugative plasmid of E. coli.Citation90 Since then, this gene has been found mostly on plasmids in Shigella isolates from humans in South Korea,Citation98 Taiwan,Citation89 Australia,Citation127 and Bangladesh.Citation128
Several studies have suggested that sul genes are linked to other resistance genes. The sul1 gene is often identified together with other antimicrobial-resistance genes located on gene-cassette arrays in variable regions of class 1 integrons. Class 1 integrons differ from class 2 in their excise gene cassettes, capacity to integrate, and presence of sul1 in 3′ conserved region (3′CS). In Brazil, sul1 (sulfonamide-resistant) was identified in two (3%) MDR Shigella samples, which were also positive for class 1 integrons.Citation122 In previous studies on MDR S. sonnei isolates obtained in South Korea, resistance to sulfamethoxazole was mainly associated with sul1, located in 8.4 kb of nonconjugative R plasmid.Citation98
Antibiotic-resistance gene clusters containing strA, strB, and sul2 are widespread among Gram-negative bacteria, particularly in Shigella isolates.Citation124 Iqbal et alCitation128 found sul1, sul2, sul3, integron 1, and integron 2 genes in all MDR S. flexneri 2a strains, and also found that sul2 was absent in all sulfamethoxazole-sensitive strains (n= 54), while it was present in all sulfamethoxazole-resistant strains (n=146). However, in this study, no change was observed in expressions of sul1, sul3, integron 1, or integron 2 genes in sulfamethoxazole-resistant and -sensitive S. flexneri 2a strains. Interestingly, curing of this 4.3 MDa plasmid resulted in loss of sul2 and susceptibility to sulfamethoxazole in paired strains, suggesting involvement of sul2 and this plasmid in resistance to sulfamethoxazole. In the same study, 24 S. sonnei isolates were detected to carry an atypical class 1 integron associated with sul3. The estX–psp–aadA2–cmlA1–aadA1–qacH cassette array of sul3-associated class 1 integron has been found to encode an esterase, a lipase, putative phosphoserine phosphatase/resistance to streptomycin, chloramphenicol, and quaternary amines.Citation23
Macrolide Resistance
The American Academy of Pediatrics and the Infectious Diseases Society of America have recommended azithromycin as a medication for treatment of shigellosis in children, and also the WHO introduced it as second-line treatment in adults.Citation129 The Centers for Disease Control and Prevention has observed resistance to azithromycin in approximately 3% of Shigella cases tested. Resistant outbreaks involving Shigella spp. isolates with reduced susceptibility to azithromycin (RSA) are more recent phenomena and continually detected in Asia, North America, the US, AustraliaCitation130,Citation131 and other geographic regions.Citation132–Citation134 According to updated Clinical and Laboratory Standards Institute guidelines, if MICmeasured by broth microdilution is ≤16 and ≤8 µg/mL, then epidemiological cutoff values denote susceptibility for S. sonnei and S. flexneri wild-type, and if MIC is ≥32 and ≥16 µg/mL, then susceptibility is confirmed for non–wild-type, respectively.Citation135 Recently, several reports have suggested that resistance to azithromycin in Shigella spp. isolates is associated with presence of mphA or ermB plasmid-mediated genes or by both genes.Citation133,Citation136 Macrolide resistance is mediated by four main mechanisms: enzymatic inactivation by phosphotransferases encoded by mph genes or esterases encoded by ere determinants; target-site modification by an rRNA methylase encoded by erm genes; punctual mutations in rplV encoding L22 ribosomal protein, rplD encoding L4 ribosomal protein, and rrlH (23S rRNA); and drug-resistance mediated by efflux pumps, such as OmpA, OmpW, mefA, and msrA.Citation137,Citation138 All macrolide-resistance mechanisms can mediate resistance to azithromycin and erythromycin. The mph(A) gene was first identified in an E. coli isolate from Japan.Citation139 Since then, this gene has been recognized among Pseudomonas spp., Aeromonas spp., Stenotrophomonas spp., Shigella spp., and other enteropathogens.Citation137 Dissemination and acquisition of macrolide mphA resistance mechanisms in Shigella spp. has been shown to be mainly due to spread of plasmids from E. coli.Citation140 All the discovered mphA-associated plasmids have been identified in E. coli isolates, indicating their role as a repository from which antimicrobial resistance to Shigella spp. may appear.Citation135 This phenomenon has been previously been described in E. coli donating mphA to S. sonnei.Citation141
Additionally, Shigella strains with RSA have been found mostly in strains recovered from men who have sex with men (MSM; 68.8% or higher) from the Montreal region. In this study, complete sequence analysis of six selected plasmids from different serotypes of S. flexneri and S. sonnei emphasized the role of IS26 in dispersal of RSA.Citation130 Also, in a study conducted in Taiwan, a series of clonally related azithromycin insusceptible Shigella spp. isolates was reported in relation to MSM.Citation136 Various gene-transfer systems (mobile genetic element acquisitions) are involved in acquiring antibiotic-resistance genes, such as transposons, integrons, and conjugative plasmids. The IS26–mphA–mrx–mphR (A)–IS6100 gene cassette has been characterized in a clinical strain of S. boydii carrying the p2246–CTXM plasmid. Insertion sequences of IS6100 and IS26 have been found in the neighborhood of mphA in an S. sonnei strain recovered from France. Indeed, in addition to plasmid mobilization, dissemination, and acquisition of RSA among Shigella spp. and serotypes has also potentially occurred through IS26 mobilization.Citation130,Citation137
IncFI and IncFII plasmids have been shown to carry the azithromycin-resistance gene erm.Citation135 The IncFI plasmid containing an ISCR3 insertion sequence surrounds both ermC and ermB genes and carries a blaCTX-M-24 gene downstream of an ISEcp1 element. The IncFII plasmid carries ermB and ermC genes downstream of an IS6 transposase. Both plasmids share significant DNA homology with other previously sequenced plasmids found in S. sonnei and S. flexneri serotype 3a isolates associated with development of the disease in MSM.Citation135
Recently, a plasmid carrying azithromycin-resistance genes, namely pKSR100 (conjugative R-plasmid) in S. flexneri serotype 3a has been described to be involved in intercontinental spread of RSA among MSM-associated outbreak lineage.Citation142 pKSR100-like plasmids have been found to be predominantly related to MSM-associated outbreak lineage in Australia and elsewhere.131 There are a considerable number of studies on the prevalence of RSA among Shigella isolates throughout the world, particularly in MSM, and demonstrated global dissemination of a multi-resistant plasmid, highly associated with MSM, which is present across different continents.Citation131,Citation142
Biofilm Formation–Mediated Resistance
Recently, much attention has been given to biofilm formation in bacteria, because microbial cells grown in biofilms are less sensitive to antimicrobial agents and more resistant to environmental stress such as dehydration and oxidation. Microbial infections caused by biofilm-associated Shigella spp. are global health challenges.Citation143 Biofilm formation is regulated by a multifactorial process, with cellular adherence, exopolysaccharide secretion, and numerous gene regulations controlling detachment of bacteria from mature biofilm, and is mainly related to quorum sensing and social networking in the microbial world.Citation144,Citation145 Ellafi et al investigated biofilm formation by Shigella strains grown in different NaCl concentrations, and they showed that all the isolates produced biofilm. According to their study, biofilm formation is a protective system under different environmental stress conditions.146 Recent studies have demonstrated that bile salts increase the capacity of S. flexneri strains to adhere to and penetrate epithelial cells.Citation147,Citation148 Indeed, extended exposure of Shigella to bile salts occurred in cases of increased biofilm formation, and thus it is an important resistance mechanism for Shigella sp. Similar biofilm phenotypes have been observed for Campylobacter, Listeria, and Vibrio, demonstrating that bile salt–induced biofilm production is conserved among members of the Enterobacteriaceae family.Citation19,Citation148 Also, biofilm formation has been shown to require the presence of glucose, whereas biofilm diffusion requires the elimination of bile salts from the medium.Citation19 Bacteria in the form of biofilm can be 100,000 times more resistant to antimicrobial agents than planktonic forms of bacteria in the same species.Citation143 During biofilm formation, the effect of shf, mdoH, VpsT, and LuxR-like genes and OpgH protein expression has been confirmed among enteric bacteria, as well as Shigella.Citation143,Citation145 Another study described biofilm-formation potentials and pathological behaviors of various mutants S. flexneri strains with an incomplete inner core of LPS containing only Kdo moieties.
Interestingly, 1rfaC (also called waaC) mutant, with an incomplete inner core of LPS due to deficiency in Hep biosynthesis, shows strong biofilm-formation ability and considerably high invasiveness and adhesiveness to human epithelial cells compared LPS-mutant strains. However, this strategy is successful in conferring high-level resistance only in bacterial species with a deficiency in Hep synthesis of LPS.Citation149 The relationship between biofilm formation and pathogenicity, as well as virulence factors and antimicrobial properties, has not been thoroughly studied in Shigella spp., and further studies are needed.
Therapeutics
Treatment for Shigella infections is recommended to prevent spread of infection to others and to shorten disease duration. According to current WHO guidelines and a systematic review, the use of fluoroquinolones (first-line, preferably ciprofloxacin), cephalosporins (second-line), and β-lactams (second-line) for 7–10 days is recommended for treatment of shigellosis.Citation4,Citation111 In regions known to have high rates of resistance to ciprofloxacin, azithromycin may be considered appropriate second-line therapy. Cefixime is also a good alternative, although its use should be balanced with respect to risk of developing antimicrobial resistance and spread of ESBL.Citation111 Conventional antibiotic therapeutics against shigellosis have become increasingly inefficient, due to the increase in number of MDR strains. However, no well-designed in vitro or in vivo combinations of antimicrobial agents have been performed to evaluate different antibiotic-class regimens for treating infections caused by Shigella. There has only been a related study on an antimicrobial-sensitivity case series reporting resistance in different regions and treatment outcomes for infections caused by Shigella.Citation4,Citation111 In general, it has been observed that there is a decrease in susceptibility to first- and second-line agents. As such, there is an urgent need for development of novel therapeutic strategies for treatment of MDR Shigella infections.Citation111
Alternative Therapeutics
Natural And Organic Products
Natural products are small molecules produced naturally by microbial agents, plants, and animals that have been demonstrated to be useful in treating Shigella infections. Biotherapeutic agents (preferably probiotics) have been suggested in prevention of antibiotic-induced diarrhea, and are also an alternative therapeutic choice for treatment of gastroenteritis infectious.Citation150 Bacteria and yeast are the most frequent microorganisms used as probiotics. Several mechanisms have been suggested for antimicrobial activity of bacteria toward enteric bacterial pathogens, including production of undissociated organic acids, organic acid molecules, and bacteriocin, competition for adhesion sites, and coaggregation with pathogens.Citation151
Pretreatment of cells with bacterial components and products obtained from Lactobacillus rhamnosus and L. acidophilus results in interference with Shigella adherence and internalization into host cells and leads to an absence of IL8 expression, substantiating attenuation of inflammatory response during aggregate pretreatment. Lactobacilli have been shown to regulate cytokine production and stimulate the immune system.Citation152 Time-kill methodology has shown that viability of S. sonnei decreased after contact with cell-free culture supernatants of lactobacilli, which could be potentially used as probiotic strains in food industry.151 Zhang et al selected a total of 91 lactobacilli for antimicrobial activity against Shigella isolates, among which 16 lactobacilli displayed potent antibacterial activity against S. sonnei strains. The nature of these antimicrobial agents was studied and found to be dependent on production of organic acids.153 Also, other studies have indicated that lactobacilli and lactic and acetic acid bacteria possess high activity against MDR Shigella pathogenic strains, and that they can be the best candidate for probiotics.Citation152,Citation154,Citation155 Saccharomyces boulardii is a thermophilic and nonpathogenic yeast showing antagonistic activity against several bacterial pathogens, such as enterohemorrhagic and enteropathogenic E. coli, Vibrio cholera, Salmonella typhimurium, and S. flexneri.156
In one study, the effect of aqueous ethanol extract of Euphorbia prostrata was investigated in vitro and in vivo on bacterial growth of S. dysenteriae type 1 and found to be effective against Shigella isolates, with MIC and minimal bactericidal concentration of 3,500–12,000 µg/mL.Citation157 The preventive role of orally administered Aloe barbadensis Miller (Aloe vera)–supplemented probiotic lassi (APL) was determined for S. dysenteriae infection in mice, with a significant (P<0.05) decrease found in Shigella counts (log CFU/mL) and immunoprotective effects of APL against S. dysenteriae.Citation158 Antivirulence activity of a boiling black tea (Camellia sinensis) extract was shown to reduce expression of virulence traits by S. dysenteriae, as shown by decreased bacterium-survival strategies, and also an enhancement was found in innate immunoresponse against Shigella isolates.Citation159 Antishigellosis activity of Picralima nitida Stapf (Apocynaceae) extract has been found to be effective against S. dysenteriae type I strains, and MIC and minimal bactericidal concentration were 800 and 6,400 μg/mL, respectively.Citation160 In vitro antibacterial activity of methyl gallate isolated from Terminalia chebula has been shown to cause total disintegration of outer and inner membranes and leakage of cytoplasmic contents of MDR S. dysenteriae. Viable intracellular S. dysenteriae reduced in a time-dependent manner in the presence of methyl gallate and decreased to zero within 20 hours.Citation161 In another study, antimicrobial activity of thyme oil and ciprofloxacin and their synergistic effects were evaluated, and the combination displayed differing degrees of effects on microbial cell formation based on results obtained from scanning electron microscopy and transmission electron microscopy. In vitro and in vivo synergy between them showed maximum growth inhibition in S. flexneri. Bacterial loads in infected colons reduced as a result of treatment with thyme oil, while the conventional drug failed to heal colon ulcers. Also, it decreased penetration of lamina propria by inflammatory cells.Citation162
Antishigellosis activity of a Crinum jagus water–ethanol extract was found to be effective against S. flexneri, with inhibition of diameter by 18.90 (0.39 mg/mL) and 25.36 (200 mg/mL) mm, respectively. Indeed, Crinum jagus extract drastically decreased (P<0.01) diarrheal stool emission and microbial load and also reduced IFNγ, IL2, IgM, IgA, and motilin blood levels in S. flexneri–induced diarrheic rats.Citation163 An in vitro study on bovine lactoferrin recognized it as a coadministered adjuvant therapeutic in antibiotic therapy against Shigella isolates. Some strains of Shigella show a twofold or more decrease in their ampicillin MIC values in the presence of bovine lactoferrin.Citation164 In vitro data showed that antibacterial activity of gallic acid inhibited the effect on biofilm formation and reduced the number of viable S. flexneri strains. Indeed, gallic acid inhibited biofilm formation in S. flexneri by regulating expression of the mdoH gene. mdoH is essential for glucosyltransferase activity and osmoregulated periplasmic glucans synthesis, as they both contribute to biofilm formation and develop antibiotic resistance in pathogenesis. Inhibition of mdoH can help in treatment of S. flexneri biofilm.Citation143 Organic acids, such as citric, acetic, lactic, and malic acid, are natural substances categorized as “generally recognized as safe” according to the US Food and Drug Administration. They have antimicrobial activity and are widely used to inactivate bacteria. Results of a study showed that organic acids, carvacrol, and their combination were useful against S. sonnei. However, S. sonnei was shown to decrease to 4.53 and 3.25 log CFU/mL using 0.5% w:v malic, lactic acid, respectively, indicating the synergistic effect of combination therapy.Citation165 A previous study reported the effects of dithiocarbamate transition-metal complexes on survival and recovery of pathogenic bacteria, and they are very attractive and novel pharmaceutical targets for control and management of antibiotic-resistant bacteria, as well as Shigella isolates.Citation166
Novel Therapeutic Strategies For Shigella Treatment
Nanoparticles
Nanoparticles (NPs) have gained growing importance in recent years and shown broad-spectrum antibacterial activity against pathogenic bacteria, due to their bactericidal characteristics.Citation167 NPs usually destroy bacterial targets with a damaging effect on membrane load cells and their integrity, along with generation of free oxygen radicals. Commonly, they can be delivered efficiently as antimicrobial agents. Recently, copper oxide NPs have been recognized as an antimicrobial agent for treatment of Shigella. MIC and minimum bactericidal concentration of copper oxide NPs were 2,500 μg/mL and ≤5,000 IU/mL, respectively, in treatment of S. sonnei using 33 nm NPs. The study also showed that smaller copper oxide NPs had stronger antibacterial effects than larger NPs at a specific time and concentration.Citation168 Iron is a biocompatible element, and can be directly used for treatment of many types of microbial pathogens. In one study, antimicrobial properties of Fe2O3 and Ag–Fe2O3 NPs against S. dysenteriae strains was evaluated, and both Fe2O3 and Ag–Fe2O3 NPs were shown to have antimicrobial effects, with antimicrobial activity of Ag–Fe2O3 NPs much more than that of Fe2O3 NPs alone.Citation169 The bactericidal effect of iron oxide NPs has been determined, with values of 50–100 μg/mL against Gram-positive and Gram-negative bacteria, as well as S. dysenteriae and E. coli.Citation167 The use of nanoantibiotic formulations is another strategy for treatment of drug-resistant Shigella. Mukherjee et al reported on the synthesis of a nanosized form of tetracycline by loading it in calcium phosphate NPs and showed that this treatment significantly decreased incidence of colon-length shortening, mushy-stool excretion, weight loss, and microbial colonization in gastrointestinal tracts of Shigella-infected mice. Immunohistological research has shown that as a result of tetracycline–calcium phosphate NP treatment, changes in morphology and level of inflammatory cytokines IL1β, IFNγ, and TNFα in intestinal tissue of mice caused by shigellosis were reverted to almost normal characteristics.Citation170 Silver NPs (AgNPs) are characterized by their broad-spectrum bactericidal toxic effects against broad-spectrum bacterial pathogens. Omara et al tested AgNPs against pathogenic Salmonella and Shigella strains recovered from layer-poultry farms. AgNPs at a concentration of 16 μg/mL were found to have both bacteriostatic and bactericidal effects against Salmonella and Shigella isolates.Citation171 Although NPs have shown high antibacterial activity during in -vitro and in -vivo experiments, future studies and judiciously performed clinical trials are required to achieve a better understanding of their potential side effects and clear regulatory guidelines.
Phage Therapy
Bacteriophages (phages) are the most abundant organisms killing bacteria a through lysis mechanism. They can be recovered from various environments. Phage therapy has earned increasing attention due to several advantages, including high specificity to target bacteria without effects on normal microflora of the human body, replication at infection site, bactericidal activity against antibiotic-resistant bacteria, and fewer side effects than other therapies. Phages are self-limiting, because phages remain at a shallow level on target sites after killing bacterial targets.Citation172 Phage therapy of shigellosis was discovered by a French microbiologist named d'Herelle, who described efficacy of phages in curing symptoms of dysentery.Citation173 Use of phages for treatment of MDR S. dysenteriae recovered from wastewater has been investigated as an alternative to antibiotics.Citation172 Another study showed that a virulent phage named pSb1 was able to infect all the S. boydii strains and had productive lytic activity against them. Also, results indicated that pSb1 might be a member of an N4-like phage group and might have potential applications as an alternative option for treatment of shigellosis.Citation174 Regular targeting of only a subgroup of strains within one bacterial species or closely related species without causing distortions in the gut microbiota is one of the benefits of phage treatment over antibiotic therapy in treatment of shigellosis.Citation175 Numerous animal have studies demonstrated that phages are able to survive in experimental animals with dysentery. Despite no reports on significant undesirable reactions during the long history of phage therapy in humans,Citation176 phage treatments still need to overcome admission constraints in the main medical repertoire.
Vaccine Strategies
Varieties of candidate vaccines have been developed to prevent infection by Shigella spp., most of which are currently under evaluation for safety and immunogenicity. However, there is no licensed vaccine available against this pathogen. At present, studies in humans and animals have shown that protection by vaccination is possible. Potential candidates for Shigella vaccines include glycoconjugate vaccines, such as recombinant glycoconjugate, synthetic glycoconjugate, O-polysaccharide covalently linked to immunogenic carrier proteins,Citation177,Citation178 virG-based live attenuated (WRSS1, WRSs3, WRSf3, WRSf2G12, WRSf2G15and WRSd1),Citation179,Citation180 recombinant outer-membrane proteins,Citation181 live attenuated vaccines,Citation182,Citation183 invasion-plasmid antigens B, C, and D,Citation184 DNA-based vaccines, Ty21a typhoid vaccine expressing Shigella LPS,Citation185 recombinant probiotic–based candidates,Citation186 and whole-cell-killed and Shigella trivalent inactivated whole-cell and heat-killed multiserotype Shigella,Citation187,Citation188 as well as novel antigen candidates, such as triacylated S-LPS, subcellular complexes purified from virulent cultures (Invaplex),Citation189 GMMA protein particles,Citation190 and an OMV-NP vaccine ().Citation191
Table 3 Overview Of Shigella Vaccines In A Aifferent Phase
Live attenuated strains such as S. dysenteriae type 1 WRSd1, S. sonnei WRSS1, WRSs2, WRSs3, and some whole-cell-killed and novel antigen candidates are now developing, and were safe and immunogenic in a phase I trial (more details are presented in ). Among possible Shigella candidate vaccines, GMMA protein particles, live attenuated Shigella flexneri 2a SC602, and S. dysenteriae type 1 SC599 strains have entered phase II clinical evaluation, and only glycoconjugate candidates have already undergone Phase III trials, with other formulations still under development in patients with shigellosis.Citation192,Citation193 As shown in , many studies have demonstrated humoral response as a main value of an immunoresponse to vaccination, and also fever and transient diarrhea have been repaorted as the most frequent complications in relation to some vaccine candidates in clinical investigations (). Finally, it seems that development and evaluation of multivalent candidates may provide a means for protection against serogroups/serotypes of Shigella in future.
Conclusion
In this review, antibiotic-resistance mechanisms and therapeutic strategies have been summarized regarding Shigella infection. Antimicrobial resistance in Shigella spp. is multifactorial in that it can occur through innate, acquired, or adaptive mechanisms, and infections resulting from it are exceedingly difficult to treat. Drug resistance among Shigella spp. occurs as a result of selective pressure and horizontal resistance-gene transmission. Accordingly, there is an urgent need to comprehensively learn and understand mechanisms of drug resistance among Shigella isolates, in order to develop antishigellosis drugs. The multifarious nature of antibiotic-resistance mechanisms contributes in an increase in the number of MDR strains and causes conventional antibiotic therapeutics to be highly inefficient against shigellosis. Despite intensive research efforts in the last few decades related to determination of antimicrobial resistance, researchers have not been able to find the best solution to control MDR isolates. Also, direct contributions to antibiotic resistance by many antimicrobial-resistant mechanisms remains unknown, showing a need for continuous monitoring of a broader range of associated mechanisms contributing to development of MDR strains. Most candidate vaccines cause an improvement in host immunity along with prevention of infection, but some have low efficiency and host challenge, which must be thoroughly overcome before being used against Shigella infection. More understanding on the host–microbe relationship is needed to develop novel therapeutic strategies to fight shigellosis. Development of innovative therapeutic and alternative strategies is also required for prevention and treatment of Shigella infections.
Author Contributions
Both authors conceptualized and designed the review, contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Kahsay AG, Muthupandian S. A review on sero diversity and antimicrobial resistance patterns of Shigella species in Africa, Asia and South America, 2001–2014. BMC Res Notes. 2016;9(1):422. doi:10.1186/s13104-016-2236-727576729
- Puzari M, Sharma M, Chetia P. Emergence of antibiotic resistant Shigella species: a matter of concern. J Infect Public Health. 2018;11(4):451–454. doi:10.1016/j.jiph.2017.09.02529066021
- Qu F, Bao C, Chen S, et al. Genotypes and antimicrobial profiles of Shigella sonnei isolates from diarrheal patients circulating in Beijing between 2002 and 2007. Diagn Microbiol Infect Dis. 2012;74(2):166–170. doi:10.1016/j.diagmicrobio.2012.06.02622858547
- Organization WH. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014.
- Grimont F, Lejay-Collin M, Talukder KA, et al. Identification of a group of shigella-like isolates as Shigella boydii 20. J Med Microbiol. 2007;56(6):749–754. doi:10.1099/jmm.0.46818-017510258
- Raja SB, Murali MR, Devaraj SN. Differential expression of ompC and ompF in multidrug-resistant Shigella dysenteriae and Shigella flexneri by aqueous extract of Aegle marmelos, altering its susceptibility toward beta-lactam antibiotics. Diagn Microbiol Infect Dis. 2008;61(3):321–328. doi:10.1016/j.diagmicrobio.2008.02.00618358664
- Taneja N, Mewara A. Shigellosis: epidemiology in India. Indian J Med Res. 2016;143(5):565–576. doi:10.4103/0971-5916.18710427487999
- Qiu S, Wang Y, Xu X, et al. Multidrug-resistant atypical variants of Shigella flexneri in China. Emerg Infect Dis. 2013;19(7):1147. doi:10.3201/eid1909.13068223763754
- Traa BS, Walker CLF, Munos M, Black RE. Antibiotics for the treatment of dysentery in children. Int J Epidemiol. 2010;39(suppl_1):i70–i84. doi:10.1093/ije/dyq02420348130
- Bhattacharya D, Bhattacharya H, Thamizhmani R, et al. Shigellosis in Bay of Bengal Islands, India: clinical and seasonal patterns, surveillance of antibiotic susceptibility patterns, and molecular characterization of multidrug-resistant Shigella strains isolated during a 6-year period from 2006 to 2011. Eur J Clin Microbiol Infect Dis. 2014;33(2):157–170. doi:10.1007/s10096-013-1937-223990135
- Shahsavan S, Owlia P, Lari AR, Bakhshi B, Nobakht M. Investigation of efflux-mediated tetracycline resistance in Shigella isolates using the inhibitor and real time polymerase chain reaction method. Iran J Pathol. 2017;12(1):53.29760753
- Poole K. Outer membranes and efflux: the path to multidrug resistance in gram-negative bacteria. Curr Pharm Biotechnol. 2002;3(2):77–98. doi:10.2174/138920102337845412022261
- Kar AK, Ghosh AS, Chauhan K, et al. Involvement of a 43-kilodalton outer membrane protein in beta-lactam resistance of Shigella dysenteriae. Antimicrob Agents Chemother. 1997;41(10):2302–2304.9333070
- Ghosh AS, Kar AK, Kundu M. Impaired imipenem uptake associated with alterations in outer membrane proteins and lipopolysaccharides in imipenem-resistant Shigella dysenteriae. J Antimicrob Chemother. 1999;43(2):195–201. doi:10.1093/jac/43.2.19511252324
- Tran EN, Papadopoulos M, Morona R. Relationship between O-antigen chain length and resistance to colicin E2 in Shigella flexneri. Microbiology. 2014;160(Pt 3):589–601. doi:10.1099/mic.0.074955-024425769
- Koseoglu VK, Hall CP, Rodriguez-Lopez EM, Agaisse H. The autotransporter IcsA promotes Shigella flexneri biofilm formation in presence of bile salts. Infect Immun. 2019. doi:10.1128/iai.00861-18
- Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453(2):254–267. doi:10.1016/j.bbrc.2014.05.09024878531
- Yang H, Duan G, Zhu J, et al. The AcrAB-TolC pump is involved in multidrug resistance in clinical Shigella flexneri isolates. Microb Drug Resist. 2008;14(4):245–249. doi:10.1089/mdr.2008.084719035770
- Nickerson KP, Chanin RB, Sistrunk JR, et al. Analysis of Shigella flexneri resistance, biofilm formation, and transcriptional profile in response to bile salts. Infect Immun. 2017;85(6). doi:10.1128/iai.01067-16
- Kim JY, Kim SH, Jeon SM, Park MS, Rhie HG, Lee BK. Resistance to fluoroquinolones by the combination of target site mutations and enhanced expression of genes for efflux pumps in Shigella flexneri and Shigella sonnei strains isolated in Korea. Clin Microbiol Infect. 2008;14(8):760–765. doi:10.1111/j.1469-0691.2008.02033.x18727800
- Taneja N, Mishra A, Kumar A, Verma G, Sharma M. Enhanced resistance to fluoroquinolones in laboratory-grown mutants & clinical isolates of Shigella due to synergism between efflux pump expression & mutations in quinolone resistance determining region. Indian J Med Res. 2015;141(1):81–89.25857499
- Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 1997;179(7):2274–2280. doi:10.1128/jb.179.7.2274-2280.19979079913
- Rahman M, Shoma S, Rashid H, Siddique AK, Nair GB, Sack DA. Extended-spectrum beta-lactamase-mediated third-generation cephalosporin resistance in Shigella isolates in Bangladesh. J Antimicrob Chemother. 2004;54(4):846–847. doi:10.1093/jac/dkh41315329365
- Fortineau N, Naas T, Gaillot O, Nordmann P. SHV-type extended-spectrum beta-lactamase in a Shigella flexneri clinical isolate. J Antimicrob Chemother. 2001;47(5):685–688. doi:10.1093/jac/47.5.68511328785
- Matar GM, Jaafar R, Sabra A, et al. First detection and sequence analysis of the bla-CTX-M-15 gene in lebanese isolates of extended-spectrum-beta-lactamase-producing Shigella sonnei. Ann Trop Med Parasitol. 2007;101(6):511–517. doi:10.1179/136485907x19386017716434
- Sabra AH, Araj GF, Kattar MM, et al. Molecular characterization of ESBL-producing Shigella sonnei isolates from patients with bacilliary dysentery in Lebanon. J Infect Dev Ctries. 2009;3(4):300–305.19759494
- Alici O, Acikgoz ZC, Gocer S, Gamberzade S, Karahocagil MK. [Short communication: prevalence of extended spectrum beta-lactamases in gram negative rods: data of 2001–2004 period]. Mikrobiyol Bul. 2006;40(4):355–361.17205693
- Kim S, Kim J, Kang Y, Park Y, Lee B. Occurrence of extended-spectrum beta-lactamases in members of the genus Shigella in the Republic of Korea. J Clin Microbiol. 2004;42(11):5264–5269. doi:10.1128/jcm.42.11.5264-5269.200415528724
- Xiong Z, Li T, Xu Y, Li J. Detection of CTX-M-14 extended-spectrum beta-lactamase in shigella sonnei isolates from China. J Infect. 2007;55(5):e125–e128. doi:10.1016/j.jinf.2007.07.01717767959
- Vasilev V, Japheth R, Yishai R, et al. Extended-spectrum beta-lactamase-producing Shigella strains in Israel, 2000–2004. Eur J Clin Microbiol Infect Dis. 2007;26(3):189–194. doi:10.1007/s10096-007-0263-y17265070
- Ranjbar R, Ghazi FM, Farshad S, et al. The occurrence of extended-spectrum beta-lactamase producing Shigella spp. in Tehran, Iran. Iran J Microbiol. 2013;5(2):108–112.23825726
- Andres P, Petroni A, Faccone D, et al. Extended-spectrum beta-lactamases in Shigella flexneri from Argentina: first report of TOHO-1 outside Japan. Int J Antimicrob Agents. 2005;25(6):501–507. doi:10.1016/j.ijantimicag.2005.02.01615878653
- Bialvaei AZ, Pourlak T, Aghamali M, Asgharzadeh M, Gholizadeh P, Kafil HS. The prevalence of CTX-M-15 extended-spectrum beta-lactamases among Salmonella spp. and Shigella spp. isolated from three Iranian hospitals. Eur J Microbiol Immunol (Bp). 2017;7(2):133–137. doi:10.1556/1886.2017.0000428690880
- Kim JS, Kim J, Jeon SE, et al. Complete nucleotide sequence of the IncI1 plasmid pSH4469 encoding CTX-M-15 extended-spectrum beta-lactamase in a clinical isolate of Shigella sonnei from an outbreak in the Republic of Korea. Int J Antimicrob Agents. 2014;44(6):533–537. doi:10.1016/j.ijantimicag.2014.08.00725446906
- Kim JS, Kim S, Park J, et al. Plasmid-mediated transfer of CTX-M-55 extended-spectrum beta-lactamase among different strains of Salmonella and Shigella spp. in the Republic of Korea. Diagn Microbiol Infect Dis. 2017;89(1):86–88. doi:10.1016/j.diagmicrobio.2017.03.01428689895
- Li J, Li B, Ni Y, Sun J. Molecular characterization of the extended-spectrum beta-lactamase (ESBL)-producing Shigella spp. in Shanghai. Eur J Clin Microbiol Infect Dis. 2015;34(3):447–451. doi:10.1007/s10096-014-2244-225252628
- Holt KE, Thieu Nga TV, Thanh DP, et al. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc Natl Acad Sci U S A. 2013;110(43):17522–17527. doi:10.1073/pnas.130863211024082120
- Liu Y, Cheng Y, Yang H, et al. Characterization of extended-spectrum beta-lactamase genes of Shigella flexneri isolates with fosfomycin resistance from patients in China. Ann Lab Med. 2017;37(5):415–419. doi:10.3343/alm.2017.37.5.41528643490
- Zhang R, Zhou HW, Cai JC, et al. Serotypes and extended-spectrum beta-lactamase types of clinical isolates of Shigella spp. from the Zhejiang province of China. Diagn Microbiol Infect Dis. 2011;69(1):98–104. doi:10.1016/j.diagmicrobio.2010.08.02721146721
- Liu G, Qian H, Tang B, et al. Prevalence and characterisation of third-generation cephalosporin-resistant Shigella flexneri isolates from Jiangsu Province, China, 2013–2015. J Glob Antimicrob Resist. 2018;15:283–287. doi:10.1016/j.jgar.2018.08.01230144637
- Rashid H, Rahman M. Possible transfer of plasmid mediated third generation cephalosporin resistance between Escherichia coli and Shigella sonnei in the human gut. Infect Genet Evol. 2015;30:15–18. doi:10.1016/j.meegid.2014.11.02325461693
- Qu F, Ying Z, Zhang C, et al. Plasmid-encoding extended-spectrum beta-lactamase CTX-M-55 in a clinical Shigella sonnei strain, China. Future Microbiol. 2014;9(10):1143–1150. doi:10.2217/fmb.14.5325405884
- Thamizhmani R, Rhagavan R, Sugunan AP, Vijayachari P. VIM- and IMP-type metallo-beta-lactamase-producing Shigella spp. in childhood diarrhea from Andaman Islands. Infect Dis (Lond). 2015;47(10):749–750. doi:10.3109/23744235.2015.102287425768231
- O’Hara K, Haruta S, Sawai T, Tsunoda M, Iyobe S. Novel metallo beta-lactamase mediated by a Shigella flexneri plasmid. FEMS Microbiol Lett. 1998;162(2):201–206. doi:10.1111/j.1574-6968.1998.tb12999.x9627953
- Iyobe S, Kusadokoro H, Ozaki J, et al. Amino acid substitutions in a variant of IMP-1 metallo-beta-lactamase. Antimicrob Agents Chemother. 2000;44(8):2023–2027. doi:10.1128/aac.44.8.2023-2027.200010898670
- Sambe-Ba B, Seck A, Wane AA, Fall-Niang NK, Gassama-Sow A. [Sensitivity to antibiotics and genetic support to resistance of Shigella flexneri strains isolated in Dakar from 2001 to 2010]. Bull Soc Pathol Exot. 2013;106(2):89–94. doi:10.1007/s13149-013-0283-z23483461
- Huang IF, Chiu CH, Wang MH, Wu CY, Hsieh KS, Chiou CC. Outbreak of dysentery associated with ceftriaxone-resistant Shigella sonnei: first report of plasmid-mediated CMY-2-type AmpC beta-lactamase resistance in S. sonnei. J Clin Microbiol. 2005;43(6):2608–2612. doi:10.1128/jcm.43.6.2608-2612.200515956372
- Tajbakhsh M, Garcia Migura L, Rahbar M, et al. Antimicrobial-resistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother. 2012;67(5):1128–1133. doi:10.1093/jac/dks02322345385
- Ayala AT, Acuna HM, Calvo MT, Morales JL, Chacon EC. [Emergence of CMY-2-type plasmid-mediated AmpC beta-lactamase in Shigella sonnei and Salmonella spp. in Costa Rica, 2003–2015]. Rev Panam Salud Publica. 2016;40(1):70–75.27706388
- Zamanlou S, Ahangarzadeh Rezaee M, Aghazadeh M, Ghotaslou R, Babaie F, Khalili Y. Characterization of integrons, extended-spectrum beta-lactamases, AmpC cephalosporinase, quinolone resistance, and molecular typing of Shigella spp. from Iran. Infect Dis (Lond). 2018;50(8):616–624. doi:10.1080/23744235.2018.145522229595080
- Zhang CL, Liu QZ, Wang J, Chu X, Shen LM, Guo YY. Epidemic and virulence characteristic of Shigella spp. with extended-spectrum cephalosporin resistance in Xiaoshan District, Hangzhou, China. BMC Infect Dis. 2014;14:260. doi:10.1186/1471-2334-14-26024886028
- Cui X, Wang J, Yang C, et al. Prevalence and antimicrobial resistance of shigella flexneri serotype 2 variant in China. Front Microbiol. 2015;6:435. doi:10.3389/fmicb.2015.0043525999941
- Anandan S, Muthuirulandi Sethuvel DP, Gajendiren R, Verghese VP, Walia K, Veeraraghavan B. Molecular characterization of antimicrobial resistance in clinical Shigella isolates during 2014 and 2015: trends in South India. Germs. 2017;7(3):115–122. doi:10.18683/germs.2017.111628932711
- Toro C, Farfán M, Contreras I, et al. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect. 2005;133(1):81–86. doi:10.1017/S095026880400304815724714
- Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300(6):371–379. doi:10.1016/j.ijmm.2010.04.00520537585
- Cui X, Yang C, Wang J, et al. Antimicrobial resistance of Shigella flexneri serotype 1b isolates in China. PLoS One. 2015;10(6):e0129009. doi:10.1371/journal.pone.012900926039698
- Siu LK, Lo JY, Yuen KY, Chau PY, Ng MH, Ho PL. beta-lactamases in Shigella flexneri isolates from Hong Kong and Shanghai and a novel OXA-1-like beta-lactamase, OXA-30. Antimicrob Agents Chemother. 2000;44(8):2034–2038. doi:10.1128/aac.44.8.2034-2038.200010898672
- Navia MM, Capitano L, Ruiz J, et al. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J Clin Microbiol. 1999;37(10):3113–3117.10488163
- Azmi IJ, Khajanchi BK, Akter F, et al. Fluoroquinolone resistance mechanisms of Shigella flexneri isolated in Bangladesh. PLoS One. 2014;9(7):e102533. doi:10.1371/journal.pone.010253325028972
- Hu LF, Li JB, Ye Y, Li X. Mutations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in clinical strains of fluoroquinolone-resistant Shigella in Anhui, China. J Microbiol. 2007;45(2):168–170.17483803
- Ramana J, Ramana J. Structural Insights into the fluoroquinolone resistance mechanism of Shigella flexneri DNA gyrase and topoisomerase IV. Microb Drug Resist. 2016;22(5):404–411. doi:10.1089/mdr.2015.001826859259
- Nuesch-Inderbinen M, Heini N, Zurfluh K, Althaus D, Hachler H, Stephan R. Shigella antimicrobial drug resistance mechanisms, 2004–2014. Emerg Infect Dis. 2016;22(6):1083–1085. doi:10.3201/eid2206.15208827191035
- Xue C, Cai J, Kang H, et al. Two novel mutations in parE among Shigella flexneri isolated from Jiangsu Province of China, 2016. Ann Transl Med. 2018;6(15):306. doi:10.21037/atm.2018.07.1130211194
- Dutta S, Kawamura Y, Ezaki T, Nair GB, Iida K, Yoshida S. Alteration in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant Shigella dysenteriae serotype 1 clinical isolates from Kolkata, India. Antimicrob Agents Chemother. 2005;49(4):1660–1661. doi:10.1128/aac.49.4.1660-1661.200515793166
- Gu B, Qin TT, Fan WT, et al. Novel mutations in gyrA and parC among Shigella sonnei strains from Jiangsu Province of China, 2002–2011. Int J Infect Dis. 2017;59:44–49. doi:10.1016/j.ijid.2017.03.02328392317
- Das A, Natarajan M, Mandal J, Herman C. The emergence of quinolone resistant Shigella sonnei, Pondicherry, India. PLoS One. 2016;11(8):e0160290. doi:10.1371/journal.pone.016029027494616
- Zhang WX, Chen HY, Tu LH, Xi MF, Chen M, Zhang J. Fluoroquinolone resistance mechanisms in Shigella isolates in Shanghai, China, between 2010 and 2015. Microb Drug Resist. 2019;25(2):212–218. doi:10.1089/mdr.2018.011330307807
- Zhu Z, Cao M, Zhou X, Li B, Zhang J. Epidemic characterization and molecular genotyping of Shigella flexneri isolated from calves with diarrhea in Northwest China. Antimicrob Resist Infect Control. 2017;6:92. doi:10.1186/s13756-017-0252-628878891
- Bowen A, Hurd J, Hoover C, et al. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin - United States, May 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(12):318–320.25837241
- Pu XY, Pan JC, Wang HQ, Zhang W, Huang ZC, Gu YM. Characterization of fluoroquinolone-resistant Shigella flexneri in Hangzhou area of China. J Antimicrob Chemother. 2009;63(5):917–920. doi:10.1093/jac/dkp08719297378
- Hata M, Suzuki M, Matsumoto M, et al. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother. 2005;49(2):801–803. doi:10.1128/aac.49.2.801-803.200515673773
- Qin T, Qian H, Fan W, et al. Newest data on fluoroquinolone resistance mechanism of Shigella flexneri isolates in Jiangsu Province of China. Antimicrob Resist Infect Control. 2017;6:97. doi:10.1186/s13756-017-0249-128932390
- Pu XY, Pan JC, Zhang W, Zheng W, Wang HQ, Gu YM. Quinolone resistance-determining region mutations and the plasmid-mediated quinolone resistance gene qnrS played important roles in decreased susceptibility to fluoroquinolones among Shigella isolates in southeast China between 1998 and 2013. Int J Antimicrob Agents. 2015;45(4):438–439. doi:10.1016/j.ijantimicag.2014.12.00425593013
- Pu XY, Gu Y, Li J, Song SJ, Lu Z. Characterization of the complete sequences and stability of plasmids carrying the genes aac(6ʹ)-Ib-cr or qnrS in Shigella flexneri in the Hangzhou area of China. World J Microbiol Biotechnol. 2018;34(6):72. doi:10.1007/s11274-018-2454-329777316
- Ferjani S, Saidani M, Amine FS, Boutiba-Ben Boubaker I. Prevalence and characterization of plasmid-mediated quinolone resistance genes in extended-spectrum beta-lactamase-producing Enterobacteriaceae in a Tunisian hospital. Microb Drug Resist. 2015;21(2):158–166. doi:10.1089/mdr.2014.005325247633
- Yaghoubi S, Ranjbar R, Soltan Dallal MM, Shirazi MH, Sharifi-Yazdi MK. Frequency of mutations in quinolone resistance-determining regions and plasmid-mediated quinolone resistance in Shigella isolates recovered from pediatric patients in Tehran, Iran: an Overlooked Problem. Microb Drug Resist. 2018;24(6):699–706. doi:10.1089/mdr.2017.015529148915
- Yang H, Duan G, Zhu J, Zhang W, Xi Y, Fan Q. Prevalence and characterisation of plasmid-mediated quinolone resistance and mutations in the gyrase and topoisomerase IV genes among Shigella isolates from Henan, China, between 2001 and 2008. Int J Antimicrob Agents. 2013;42(2):173–177. doi:10.1016/j.ijantimicag.2013.04.02623796894
- Perea EJ, Torres MA, Borobio MV. Synergism of fosfomycin-ampicillin and fosfomycin-chloramphenicol against Salmonella and Shigella. Antimicrob Agents Chemother. 1978;13(5):705–709. doi:10.1128/aac.13.5.705666297
- Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10(1):43–50. doi:10.1016/s1473-3099(09)70325-120129148
- Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother. 2010;54(7):3061–3064. doi:10.1128/aac.01834-0920404116
- Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med. 2017;7(2):a025262. doi:10.1101/cshperspect.a02526228062557
- Fourmy D, Yoshizawa S, Puglisi JD. Paromomycin binding induces a local conformational change in the A-site of 16 S rRNA. J Mol Biol. 1998;277(2):333–345. doi:10.1006/jmbi.1997.15519514734
- Shaw PC, Liang AC, Kam KM, Ling JM. Presence of strA-strB gene within a streptomycin-resistance operon in a clinical isolate of Shigella flexneri. Pathology. 1996;28(4):356–358. doi:10.1080/003130296001693449007957
- McIver CJ, White PA, Jones LA, et al. Epidemic strains of Shigella sonnei biotype g carrying integrons. J Clin Microbiol. 2002;40(4):1538–1540. doi:10.1128/jcm.40.4.1538-1540.200211923391
- Iversen J, Sandvang D, Srijan A, Cam PD, Dalsgaard A. Characterization of antimicrobial resistance, plasmids, and gene cassettes in Shigella spp. from patients in vietnam. Microb Drug Resist. 2003;9(Suppl 1):S17–S24. doi:10.1089/10766290332254185614633363
- Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin Microbiol Infect. 2016;22(12):968–974. doi:10.1016/j.cmi.2016.07.03327506509
- Dutta S, Jain P, Nandy S, Matsushita S, Yoshida S. Molecular characterization of serologically atypical provisional serovars of Shigella isolates from Kolkata, India. J Med Microbiol. 2014;63(Pt 12):1696–1703. doi:10.1099/jmm.0.081307-025261061
- Ranjbar R, Aleo A, Giammanco GM, Dionisi AM, Sadeghifard N, Mammina C. Genetic relatedness among isolates of Shigella sonnei carrying class 2 integrons in Tehran, Iran, 2002–2003. BMC Infect Dis. 2007;7:62. doi:10.1186/1471-2334-7-6217587439
- Chang CY, Lu PL, Lin CC, Lee TM, Tsai MY, Chang LL. Integron types, gene cassettes, antimicrobial resistance genes and plasmids of Shigella sonnei isolates from outbreaks and sporadic cases in Taiwan. J Med Microbiol. 2011;60(Pt 2):197–204. doi:10.1099/jmm.0.022517-020947666
- Antunes P, Machado J, Sousa JC, Peixe L. Dissemination of sulfonamide resistance genes (sul1, sul2, and sul3) in Portuguese Salmonella enterica strains and relation with integrons. Antimicrob Agents Chemother. 2005;49(2):836–839. doi:10.1128/aac.49.2.836-839.200515673783
- Bischoff KM, White DG, Hume ME, Poole TL, Nisbet DJ. The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple-drug resistance in swine Escherichia coli. FEMS Microbiol Lett. 2005;243(1):285–291. doi:10.1016/j.femsle.2004.12.01715668031
- Gassama Sow A, Aidara-Kane A, Barraud O, Gatet M, Denis F, Ploy MC. High prevalence of trimethoprim-resistance cassettes in class 1 and 2 integrons in Senegalese Shigella spp isolates. J Infect Dev Ctries. 2010;4(4):207–212.20440057
- Muthuirulandi Sethuvel DP, Anandan S, Devanga Ragupathi NK, et al. IncFII plasmid carrying antimicrobial resistance genes in Shigella flexneri: vehicle for dissemination. J Glob Antimicrob Resist. 2019;16:215–219. doi:10.1016/j.jgar.2018.10.01430342929
- Parajuli P, Deimel LP, Verma NK. Genome analysis of Shigella flexneri serotype 3b strain SFL1520 reveals significant horizontal gene acquisitions including a multidrug resistance cassette. Genome Biol Evol. 2019;11(3):776–785. doi:10.1093/gbe/evz02630715343
- Tariq A, Haque A, Ali A, et al. Molecular profiling of antimicrobial resistance and integron association of multidrug-resistant clinical isolates of Shigella species from Faisalabad, Pakistan. Can J Microbiol. 2012;58(9):1047–1054. doi:10.1139/w2012-08522906205
- Pazhani GP, Niyogi SK, Singh AK, et al. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. J Med Microbiol. 2008;57(Pt 7):856–863. doi:10.1099/jmm.0.2008/000521-018566144
- Barman S, Chatterjee S, Chowdhury G, et al. Plasmid-mediated streptomycin and sulfamethoxazole resistance in Shigella flexneri 3a. Int J Antimicrob Agents. 2010;36(4):348–351. doi:10.1016/j.ijantimicag.2010.06.03720685089
- Seol SY, Kim YT, Jeong YS, et al. Molecular characterization of antimicrobial resistance in Shigella sonnei isolates in Korea. J Med Microbiol. 2006;55(Pt 7):871–877. doi:10.1099/jmm.0.46441-016772414
- Pan JC, Ye R, Meng DM, Zhang W, Wang HQ, Liu KZ. Molecular characteristics of class 1 and class 2 integrons and their relationships to antibiotic resistance in clinical isolates of Shigella sonnei and Shigella flexneri. J Antimicrob Chemother. 2006;58(2):288–296. doi:10.1093/jac/dkl22816766536
- Hartman AB, Essiet II, Isenbarger DW, Lindler LE. Epidemiology of tetracycline resistance determinants in Shigella spp. and enteroinvasive Escherichia coli: characterization and dissemination of tet(A)-1. J Clin Microbiol. 2003;41(3):1023–1032. doi:10.1128/jcm.41.3.1023-1032.200312624025
- Roberts MC. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev. 1996;19(1):1–24. doi:10.1111/j.1574-6976.1996.tb00251.x8916553
- Parajuli P, Rajput MI, Verma NK. Plasmids of Shigella flexneri serotype 1c strain Y394 provide advantages to bacteria in the host. BMC Microbiol. 2019;19(1):86. doi:10.1186/s12866-019-1455-131035948
- Luck SN, Turner SA, Rajakumar K, Sakellaris H, Adler B. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect Immun. 2001;69(10):6012–6021. doi:10.1128/iai.69.10.6012-6021.200111553538
- Wibberg D, Szczepanowski R, Eikmeyer F, Puhler A, Schluter A. The IncF plasmid pRSB225 isolated from a municipal wastewater treatment plant’s on-site preflooder combining antibiotic resistance and putative virulence functions is highly related to virulence plasmids identified in pathogenic E. coli isolates. Plasmid. 2013;69(2):127–137. doi:10.1016/j.plasmid.2012.11.00123212116
- Allue-Guardia A, Koenig SSK, Quiros P, Muniesa M, Bono JL, Eppinger M. Closed genome and comparative phylogenetic analysis of the clinical multidrug resistant Shigella sonnei strain 866. Genome Biol Evol. 2018;10(9):2241–2247. doi:10.1093/gbe/evy16830060169
- Pons MJ, Torrents de la Pena A, Mensa L, et al. Differences in tetracycline resistance determinant carriage among Shigella flexneri and Shigella sonnei are not related to different plasmid Inc-type carriage. J Glob Antimicrob Resist. 2018;13:131–134. doi:10.1016/j.jgar.2017.12.01529307861
- Mandomando I, Jaintilal D, Pons MJ, et al. Antimicrobial susceptibility and mechanisms of resistance in Shigella and Salmonella isolates from children under five years of age with diarrhea in rural Mozambique. Antimicrob Agents Chemother. 2009;53(6):2450–2454. doi:10.1128/aac.01282-0819332670
- Alizadeh-Hesar M, Bakhshi B, Najar-Peerayeh S. Clonal dissemination of a single Shigella sonnei strain among Iranian children during Fall 2012 in Tehran, I.R. Iran. Infect Genet Evol. 2015;34:260–266. doi:10.1016/j.meegid.2015.06.02426117443
- Martinez-Salazar JM, Alvarez G, Gomez-Eichelmann MC. Frequency of four classes of tetracycline resistance determinants in Salmonella and Shigella spp. clinical isolates. Antimicrob Agents Chemother. 1986;30(4):630–631. doi:10.1128/aac.30.4.6303789700
- Ledov VA, Golovina ME, Markina AA, et al. Highly homogenous tri-acylated S-LPS acts as a novel clinically applicable vaccine against Shigella flexneri 2a infection. Vaccine. 2019;37(8):1062–1072. doi:10.1016/j.vaccine.2018.12.06730670300
- Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health. 2018;38(sup1):S50–s65. doi:10.1080/20469047.2017.140945429790845
- Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev. 2004;28(5):519–542. doi:10.1016/j.femsre.2004.04.00115539072
- Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/s1473-3099(15)00424-726603172
- Pham Thanh D, Thanh Tuyen H, Nguyen Thi Nguyen T, et al. Inducible colistin resistance via a disrupted plasmid-borne mcr-1 gene in a 2008 vietnamese Shigella sonnei isolate. J Antimicrob Chemother. 2016;71(8):2314–2317. doi:10.1093/jac/dkw17327246235
- Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother. 2016;71(8):2066–2070. doi:10.1093/jac/dkw27427342545
- Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30(2):557–596. doi:10.1128/cmr.00064-1628275006
- Hinchliffe P, Yang QE, Portal E, et al. Insights into the mechanistic basis of plasmid-mediated colistin resistance from crystal structures of the catalytic domain of MCR-1. Sci Rep. 2017;7:39392. doi:10.1038/srep3939228059088
- Ma Q, Huang Y, Wang J, et al. Multidrug-resistant Shigella sonnei carrying the plasmid-mediated mcr-1 gene in China. Int J Antimicrob Agents. 2018;52(1):14–21. doi:10.1016/j.ijantimicag.2018.02.01929501823
- Niyogi SK. Shigellosis. J Microbiol. 2005;43(2):133–143.15880088
- Skold O. Resistance to trimethoprim and sulfonamides. Vet Res. 2001;32(3–4):261–273. doi:10.1051/vetres:200112311432417
- Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32(11):1608–1614. doi:10.1086/32053211340533
- Peirano G, Agerso Y, Aarestrup FM, dos Prazeres Rodrigues D. Occurrence of integrons and resistance genes among sulphonamide-resistant Shigella spp. from Brazil. J Antimicrob Chemother. 2005;55(3):301–305. doi:10.1093/jac/dki01215681578
- Ahmed AM, Furuta K, Shimomura K, Kasama Y, Shimamoto T. 1Genetic characterization of multidrug resistance in Shigella spp. from Japan. J Med Microbiol. 2006;55(Pt 12):1685–1691. doi:10.1099/jmm.0.46725-017108272
- Miranda A, Avila B, Diaz P, et al. Emergence of plasmid-borne dfrA14 trimethoprim resistance gene in Shigella sonnei. Front Cell Infect Microbiol. 2016;6:77. doi:10.3389/fcimb.2016.0007727489797
- Chun D-K, Seol S-Y. Drug resistance of Shigella and Salmonella and the inhibition and elimination of drug resistance. J Korean Soc Microbiol. 1979;14(1):27–37.
- Summers AO. Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim Biotechnol. 2006;17(2):125–135. doi:10.1080/1049539060095721717127524
- Nigro SJ, Hall RM. GIsul2, a genomic island carrying the sul2 sulphonamide resistance gene and the small mobile element CR2 found in the enterobacter cloacae subspecies cloacae type strain ATCC 13047 from 1890, Shigella flexneri ATCC 700930 from 1954 and acinetobacter baumannii ATCC 17978 from 1951. J Antimicrob Chemother. 2011;66(9):2175–2176. doi:10.1093/jac/dkr23021653606
- Iqbal MS, Rahman M, Islam R, et al. Plasmid-mediated sulfamethoxazole resistance encoded by the sul2 gene in the multidrug-resistant Shigella flexneri 2a isolated from patients with acute diarrhea in Dhaka, Bangladesh. PLoS One. 2014;9(1):e85338. doi:10.1371/journal.pone.008533824416393
- Salah M, Shtayeh I, Ghneim R, et al. Evaluation of Shigella species azithromycin CLSI epidemiological cutoff values and macrolide resistance genes. J Clin Microbiol. 2019;57(4). doi:10.1128/jcm.01422-18
- Yousfi K, Gaudreau C, Pilon PA, et al. Genetic mechanisms behind the spread of reduced susceptibility to azithromycin in Shigella strains isolated from men who have sex with men in Quebec, Canada. Antimicrob Agents Chemother. 2019;63(2). doi:10.1128/aac.01679-18
- Ingle DJ, Easton M, Valcanis M, et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men, Australia. Clin Infect Dis. 2019. doi:10.1093/cid/ciz005
- Borg ML, Modi A, Tostmann A, et al. Ongoing outbreak of Shigella flexneri serotype 3a in men who have sex with men in England and Wales, data from 2009–2011. Euro Surveill. 2012;17(13):20137.22490381
- Gaudreau C, Barkati S, Leduc JM, Pilon PA, Favreau J, Bekal S. Shigella spp. with reduced azithromycin susceptibility, Quebec, Canada, 2012–2013. Emerg Infect Dis. 2014;20(5):854–856. doi:10.3201/eid2005.13096624750584
- Sjolund Karlsson M, Bowen A, Reporter R, et al. Outbreak of infections caused by Shigella sonnei with reduced susceptibility to azithromycin in the United States. Antimicrob Agents Chemother. 2013;57(3):1559–1560. doi:10.1128/aac.02360-1223274665
- Darton TC, Tuyen HT, The HC, et al. Azithromycin resistance in Shigella spp. in Southeast Asia. Antimicrob Agents Chemother. 2018;62(4). doi:10.1128/aac.01748-17
- Liao YS, Liu YY, Lo YC, Chiou CS. Azithromycin-nonsusceptible Shigella flexneri 3a in men who have sex with men, Taiwan, 2015–2016. Emerg Infect Dis. 2016;23(2):345–346. doi:10.3201/eid2302.16126028098533
- Boumghar-Bourtchai L, Mariani-Kurkdjian P, Bingen E, et al. Macrolide-resistant Shigella sonnei. Emerg Infect Dis. 2008;14(8):1297–1299. doi:10.3201/eid1408.08014718680661
- Zhang C, Zhang R, Yu Q, Chu X, Sun J, Liu Q. Decreased susceptibility to azithromycin among clinical Shigella isolates from China. Microb Drug Resist. 2017;23(5):596–601. doi:10.1089/mdr.2016.013427841958
- Healy M, Huong J, Bittner T, et al. Microbial DNA typing by automated repetitive-sequence-based PCR. J Clin Microbiol. 2005;43(1):199–207. doi:10.1128/jcm.43.1.199-207.200515634972
- Mannion AJ, Martin HR, Shen Z, et al. Plasmid-mediated quinolone resistance in Shigella flexneri isolated from Macaques. Front Microbiol. 2018;9:311. doi:10.3389/fmicb.2018.0031129556221
- Phuc Nguyen MC, Woerther PL, Bouvet M, Andremont A, Leclercq R, Canu A. Escherichia coli as reservoir for macrolide resistance genes. Emerg Infect Dis. 2009;15(10):1648–1650. doi:10.3201/eid1510.09069619861064
- Baker KS, Dallman TJ, Ashton PM, et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis. 2015;15(8):913–921. doi:10.1016/s1473-3099(15)00002-x25936611
- Kang J, Liu L, Liu M, Wu X, Li J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control. 2018;94:147–154. doi:10.1016/j.foodcont.2018.07.011
- Agle ME, Blaschek HP. Shigella: Survival on Produce and Biofilm Formation. Oxford: Blackwell Publishing Ltd. 2006:19–46.
- Dorman MJ, Dorman CJ. Regulatory hierarchies controlling virulence gene expression in Shigella flexneri and vibrio cholerae. Front Microbiol. 2018;9:2686. doi:10.3389/fmicb.2018.0268630473684
- Ellafi A, Abdallah FB, Lagha R, Harbi B, Bakhrouf A. Biofilm production, adherence and morphological alterations of Shigella spp. Under salt conditions. Annals of microbiology. 2011;61(4):741–747. doi:10.1007/s13213-010-0190-5
- Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun. 2007;75(5):2626–2629. doi:10.1128/iai.01599-0617296762
- Barta ML, Guragain M, Adam P, et al. Identification of the bile salt binding site on IpaD from Shigella flexneri and the influence of ligand binding on IpaD structure. Proteins. 2012;80(3):935–945.22423359
- Xu D, Zhang W, Zhang B, Liao C, Shao Y. Characterization of a biofilm-forming Shigella flexneri phenotype due to deficiency in Hep biosynthesis. PeerJ. 2016;4:e2178. doi:10.7717/peerj.217827478696
- Filho-Lima JV, Vieira EC, Nicoli JR. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli combinations against experimental infections with Shigella flexneri and Salmonella enteritidis subsp. typhimurium in gnotobiotic mice. J Appl Microbiol. 2000;88(3):365–370. doi:10.1046/j.1365-2672.2000.00973.x10747216
- Zhang Y, Zhang L, Du M, et al. Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res. 2011;167(1):27–31. doi:10.1016/j.micres.2011.02.00621466951
- Moorthy G, Murali MR, Niranjali Devaraj S. Lactobacilli inhibit Shigella dysenteriae 1 induced pro-inflammatory response and cytotoxicity in host cells via impediment of Shigella-host interactions. Dig Liver Dis. 2010;42(1):33–39. doi:10.1016/j.dld.2009.04.02119535308
- Zhang YC, Zhang LW, Ma W, et al. Screening of probiotic lactobacilli for inhibition of Shigella sonnei and the macromolecules involved in inhibition. Anaerobe. 2012;18(5):498–503. doi:10.1016/j.anaerobe.2012.08.00722967793
- Mirnejad R, Vahdati AR, Rashidiani J, Erfani M, Piranfar V. The antimicrobial effect of lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneri in vitro. Iran Red Crescent Med J. 2013;15(2):122–126. doi:10.5812/ircmj.745423682323
- Ayeni AO, Ayeni FA. Antagonistic effects of lactic and acetic acid bacteria on Shigella sp. SS10 in co-culture. TAF Preventive Medicine Bulletin. 2016;15(1):27–31. doi:10.5455/pmb.1-1438753866
- Mumy KL, Chen X, Kelly CP, McCormick BA. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G599–G609. doi:10.1152/ajpgi.00391.200718032477
- Rene K, Hortense G, Pascal W, et al. Activity of aqueous ethanol extract of Euphorbia prostrata ait on Shigella dysenteriae type 1-induced diarrhea in rats. Indian J Pharmacol. 2007;39(5):240–244. doi:10.4103/0253-7613.37275
- Hussain SA, Patil GR, Reddi S, et al. Aloe vera (Aloe barbadensis Miller) supplemented probiotic lassi prevents Shigella infiltration from epithelial barrier into systemic blood flow in mice model. Microb Pathog. 2017;102:143–147. doi:10.1016/j.micpath.2016.11.02327914960
- Kiran S, Ratho RK, Sharma P, Harjai K, Capalash N, Tiwari RP. Effect of black tea (Camellia sinensis) on virulence traits of clinical isolates of Shigella dysenteriae and Escherichia coli EPEC P2 1265 strain. Eur Food Res Technol. 2010;231(5):763–770. doi:10.1007/s00217-010-1328-1
- Kouitcheu LB, Tamesse JL, Kouam J. The anti-shigellosis activity of the methanol extract of picralima nitida on Shigella dysenteriae type I induced diarrhoea in rats. BMC Complement Altern Med. 2013;13:211. doi:10.1186/1472-6882-13-21123957940
- Acharyya S, Sarkar P, Saha DR, Patra A, Ramamurthy T, Bag PK. Intracellular and membrane-damaging activities of methyl gallate isolated from Terminalia chebula against multidrug-resistant Shigella spp. J Med Microbiol. 2015;64(8):901–909. doi:10.1099/jmm.0.00010726272388
- Allam NG, Eldrieny EA, Mohamed AZ. Effect of combination therapy between thyme oil and ciprofloxacin on ulcer-forming Shigella flexneri. J Infect Dev Ctries. 2015;9(5):486–495. doi:10.3855/jidc.630225989168
- Noubissi PA, Fokam Tagne MA, Fankem GO, Ngakou Mukam J, Wambe H, Kamgang R. Effects of crinum jagus water/ethanol extract on Shigella flexneri-induced diarrhea in rats. Evid Based Complement Alternat Med. 2019;2019:9537603. doi:10.1155/2019/953760330992711
- Mosquito S, Zegarra G, Villanueva C, Ruiz J, Ochoa TJ. Effect of bovine lactoferrin on the minimum inhibitory concentrations of ampicillin and trimethoprim-sulfamethoxazole for clinical Shigella spp. strains. Biochem Cell Biol. 2012;90(3):412–416. doi:10.1139/o11-06622397495
- Chai C, Lee S, Kim J, Oh S-W. Synergistic antimicrobial effects of organic acids in combination with carvacrol against Shigella Sonnei. J Food Saf. 2016;36(3):360–366. doi:10.1111/jfs.12251
- Balakrishnan S, Duraisamy S, Kasi M, Kandasamy S, Sarkar R, Kumarasamy A. Syntheses, physicochemical characterization, antibacterial studies on potassium morpholine dithiocarbamate nickel (II), copper (II) metal complexes and their ligands. Heliyon. 2019;5(5):e01687. doi:10.1016/j.heliyon.2019.e0168731193102
- Saqib S, Munis MFH, Zaman W, et al. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc Res Tech. 2019;82(4):415–420. doi:10.1002/jemt.2318230565799
- Babaei S, Bajelani F, Mansourizaveleh O, Abbasi A, Oubari F. A study of the bactericidal effect of copper oxide nanoparticles on Shigella Sonnei and Salmonella Typhimurium. J Babol Univ Med Sci. 2017;19(11):76–81. doi:10.18869/acadpub.jbums.19.11.76
- Kareem ZH, Shareef HK, Alkaim AF. Evaluation of antibacterial activity of Fe2 O3 nanoparticles against Shigella dysenteriae. J Pharm Sci Res. 2018;10(8):1980–1982.
- Mukherjee R, Dutta D, Patra M, Chatterjee B, Basu T. Nanonized tetracycline cures deadly diarrheal disease ‘shigellosis’ in mice, caused by multidrug-resistant Shigella flexneri 2a bacterial infection. Nanomedicine. 2018. doi:10.1016/j.nano.2018.11.004
- Omara ST, Zawrah MF, Samy AA. Minimum bactericidal concentration of chemically synthesized silver nanoparticles against pathogenic Salmonella and Shigella strains isolated from layer poultry farms. J Appl Pharm Sci. 2017;7(8):214–221. doi:10.7324/JAPS.2017.70829
- Jamal M, Chaudhry WN, Hussain T, Das CR, Andleeb S. Characterization of new myoviridae bacteriophage WZ1 against multi-drug resistant (MDR) Shigella dysenteriae. J Basic Microbiol. 2015;55(4):420–431. doi:10.1002/jobm.20140068825557472
- Goodridge LD. Bacteriophages for managing Shigella in various clinical and non-clinical settings. Bacteriophage. 2013;3(1):e25098. doi:10.4161/bact.2509823819110
- Jun JW, Yun SK, Kim HJ, Chai JY, Park SC. Characterization and complete genome sequence of a novel N4-like bacteriophage, pSb-1 infecting Shigella boydii. Res Microbiol. 2014;165(8):671–678. doi:10.1016/j.resmic.2014.09.00625283727
- Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5(4):e1088124. doi:10.1080/21597081.2015.108812426909243
- Tang SS, Biswas SK, Tan WS, Saha AK, Leo BF. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. PeerJ. 2019;7:e6225. doi:10.7717/peerj.622530984476
- Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci U S A. 2007;104(36):14478–14482. doi:10.1073/pnas.070696910417726093
- Phalipon A, Costachel C, Grandjean C, et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J Immunol. 2006;176(3):1686–1694. doi:10.4049/jimmunol.176.3.168616424198
- Ranallo RT, Fonseka S, Boren TL, et al. Two live attenuated Shigella flexneri 2a strains WRSf2G12 and WRSf2G15: a new combination of gene deletions for 2nd generation live attenuated vaccine candidates. Vaccine. 2012;30(34):5159–5171. doi:10.1016/j.vaccine.2012.05.00322658966
- Raqib R, Sarker P, Zaman K, et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum Vaccin Immunother. 2019:1–12. doi:10.1080/21645515.2019.1575165.
- Heine SJ, Diaz-McNair J, Andar AU, et al. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol. 2014;192(4):1630–1640. doi:10.4049/jimmunol.130274324453241
- DeLaine BC, Wu T, Grassel CL, et al. Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. Pathog Dis. 2016;74(5). doi:10.1093/femspd/ftw034
- Toapanta FR, Bernal PJ, Kotloff KL, Levine MM, Sztein MB. T cell mediated immunity induced by the live-attenuated Shigella flexneri 2a vaccine candidate CVD 1208S in humans. J Transl Med. 2018;16(1):61. doi:10.1186/s12967-018-1439-129534721
- Mitobe J, Sinha R, Mitra S, et al. An attenuated Shigella mutant lacking the RNA-binding protein Hfq provides cross-protection against Shigella strains of broad serotype. PLoS Negl Trop Dis. 2017;11(7):e0005728. doi:10.1371/journal.pntd.000572828727722
- Wu Y, Chakravarty S, Li M, Wai TT, Hoffman SL, Sim BK. Development of a live attenuated bivalent oral vaccine against Shigella sonnei Shigellosis and Typhoid Fever. J Infect Dis. 2017;215(2):259–268. doi:10.1093/infdis/jiw52827803169
- Yagnik B, Sharma D, Padh H, Desai P. Immunization with r-Lactococcus lactis expressing outer membrane protein A of Shigella dysenteriae type-1: evaluation of oral and intranasal route of administration. J Appl Microbiol. 2017;122(2):493–505. doi:10.1111/jam.1335327860045
- Kaminski RW, Wu M, Turbyfill KR, et al. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol. 2014;21(3):366–382. doi:10.1128/cvi.00683-1324403527
- Nag D, Sinha R, Mitra S, et al. Heat killed multi-serotype Shigella immunogens induced humoral immunity and protection against heterologous challenge in rabbit model. Immunobiology. 2015;220(11):1275–1283. doi:10.1016/j.imbio.2015.07.00226210044
- Turbyfill KR, Clarkson KA, Vortherms AR, Oaks EV, Kaminski RW, Pasetti MF. Assembly, biochemical characterization, immunogenicity, adjuvanticity, and efficacy of Shigella artificial invaplex. mSphere. 2018;3(2). doi:10.1128/mSphere.00583-17
- Obiero CW, Ndiaye AGW, Scire AS, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a Shigellosis-endemic Country. Front Immunol. 2017;8:1884. doi:10.3389/fimmu.2017.0188429375556
- Camacho AI, Irache JM, de Souza J, Sanchez-Gomez S, Gamazo C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine. 2013;31(32):3288–3294. doi:10.1016/j.vaccine.2013.05.02023727423
- Barel LA, Mulard LA. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: from concept to efficacy in human. Hum Vaccin Immunother. 2019. doi:10.1080/21645515.2019.1606972
- Passwell JH, Ashkenzi S, Banet-Levi Y, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine. 2010;28(10):2231–2235. doi:10.1016/j.vaccine.2009.12.05020056180
- Taneja N, Mewara A, Kumar A, Verma G, Sharma M. Cephalosporin-resistant Shigella flexneri over 9 years (2001-09) in India. J Antimicrob Chemother. 2012;67(6):1347–1353. doi:10.1093/jac/dks06122410619
- Nagano Y, Nagano N, Wachino J, Ishikawa K, Arakawa Y. Novel chimeric beta-lactamase CTX-M-64, a hybrid of CTX-M-15-like and CTX-M-14 beta-lactamases, found in a Shigella sonnei strain resistant to various oxyimino-cephalosporins, including ceftazidime. Antimicrob Agents Chemother. 2009;53(1):69–74. doi:10.1128/aac.00227-0818955524
- Schumacher H, Nir M, Mansa B, Grassy A. beta-lactamases in Shigella. Apmis. 1992;100(10):954–956.1445702
- Qin T, Bi R, Fan W, Kang H, Ma P, Gu B. Novel mutations in quinolone resistance-determining regions of gyrA, gyrB, parC and parE in Shigella flexneri clinical isolates from eastern Chinese populations between 2001 and 2011. Eur J Clin Microbiol Infect Dis. 2016;35(12):2037–2045. doi:10.1007/s10096-016-2761-227620866
- Chowdhury FM, Rahman MZ, Sarkar MMH, et al. Protection against shigellosis caused by Shigella dysenteriae serotype 4 in guinea pigs using Escherichia albertii DM104 as a live vaccine candidate strain. Acta Microbiol Immunol Hung. 2017;64(2):151–164. doi:10.1556/030.64.2017.01528597684
- McKenzie R, Venkatesan MM, Wolf MK, et al. Safety and immunogenicity of WRSd1, a live attenuated Shigella dysenteriae type 1 vaccine candidate. Vaccine. 2008;26(26):3291–3296. doi:10.1016/j.vaccine.2008.03.07918468742
- Rahman KM, Arifeen SE, Zaman K, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29(6):1347–1354. doi:10.1016/j.vaccine.2010.10.03521040694
- Launay O, Sadorge C, Jolly N, et al. Safety and immunogenicity of SC599, an oral live attenuated Shigella dysenteriae type-1 vaccine in healthy volunteers: results of a phase 2, randomized, double-blind placebo-controlled trial. Vaccine. 2009;27(8):1184–1191. doi:10.1016/j.vaccine.2008.12.02119135496
- Noriega FR, Losonsky G, Wang JY, Formal SB, Levine MM. Further characterization of delta aroA delta virG Shigella flexneri 2a strain CVD 1203 as a mucosal Shigella vaccine and as a live-vector vaccine for delivering antigens of enterotoxigenic Escherichia coli. Infect Immun. 1996;64(1):23–27.8557344
- Nag D, Koley H, Sinha R, et al. Immunization of mice with a live transconjugant Shigella hybrid strain induced Th1 and Th17 cell-mediated immune responses and confirmed passive protection against heterologous Shigellae. Scand J Immunol. 2016;83(2):92–101. doi:10.1111/sji.1239426478541
- Formal SB, Hale TL, Kapfer C, et al. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984;46(2):465–469.6389344
- Kim MJ, Moon Y-H, Kim H, et al. Cross-protective Shigella whole-cell vaccine with a truncated O-polysaccharide chain. Front Microbiol. 2018;9:2609. doi:10.3389/fmicb.2018.0260930429838
- Chakraborty S, Harro C, DeNearing B, et al. Evaluation of the safety, tolerability, and immunogenicity of an oral, inactivated whole-cell Shigella flexneri 2a vaccine in healthy adult subjects. Clin Vaccine Immunol. 2016;23(4):315–325. doi:10.1128/cvi.00608-1526865592
- Chitradevi STS, Kaur G, Sivaramakrishna U, Singh D, Bansal A. Development of recombinant vaccine candidate molecule against Shigella infection. Vaccine. 2016;34(44):5376–5383. doi:10.1016/j.vaccine.2016.08.03427591952
- Hatz CF, Bally B, Rohrer S, et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized phase I study. Vaccine. 2015;33(36):4594–4601. doi:10.1016/j.vaccine.2015.06.10226162850
- Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci U S A. 1999;96(9):5194–5197. doi:10.1073/pnas.96.9.519410220442
- Mitra S, Sinha R, Mitobe J, Koley H. Development of a cost-effective vaccine candidate with outer membrane vesicles of a tolA-disrupted Shigella boydii strain. Vaccine. 2016;34(15):1839–1846. doi:10.1016/j.vaccine.2016.02.01826878295