Abstract
Purpose
To describe trends and correlation between antibacterial exposure and bacterial resistance from hospitalized patients in a hospital in southern China.
Patients and methods
This study used hospital-wide data regarding antimicrobial resistance and consumption between January 1, 2014 and December 31, 2018. Antibacterial consumption was expressed as antimicrobial use density (AUD). The changes in trends and associations between antibacterial utilization and resistance were analyzed using linear regression and time series analysis.
Results
The total AUD of all antimicrobials decreased year by year (50.66 in 2014 vs 44.28 in 2018, P=0.03). The annual use of antimicrobials, such as penicillins, monobactams, aminoglycosides, macrolides, and lincosamides, significantly decreased (P<0.05), while the annual use of quinolones and tetracyclines significantly increased (P<0.05). Among the top ten isolated bacteria, antimicrobial resistance trends of Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Staphylococcus aureus, and Staphylococcus epidermidis significantly decreased (P<0.05). Significant positive correlation was found between AUD of carbapenems and resistance rate of Acinetobacter baumannii to imipenem (β=32.87, P<0.01), as well as the correlation between AUD of quinolones and resistance rate of Enterococcus faecium to levofloxacin (β=104.40, P<0.01).
Conclusion
The consumption of antibiotics and antibiotic resistance has been significantly improved in this tertiary hospital. Additionally, the efforts of China’s antibiotic management may be suggested by the relationship between indicated antibiotic resistance and consumption. However, overall AUD levels and poor control of the use of antibiotics, such as quinolones and tetracyclines, still require strengthened management.
Introduction
After more than 70 years of widespread use of antibiotics in treating infectious diseases, antibiotic resistance is now being considered as a worldwide problem.Citation1 In September 2016, antibiotic resistance became the fourth health issue after HIV, non-communicable diseases, and Ebola to be discussed by the United Nations General Assembly.Citation2 In most cases, antibiotic-resistant infections lead to longer hospital stays with costlier treatments and a need for more toxic antibiotics, as well as resulting in greater disability and death compared with infections that are easily treatable with antibiotics.Citation3,Citation4 According to reports, current worldwide deaths attributable to antibiotic resistance, including antimalarial and antiviral resistance, have been estimated to be about 700,000 per year, with as high as $20 billion in excess direct health care costs.Citation5 If the current trend continues, deaths will reach 10 million per year by 2050.Citation2,Citation6
Similarly, antibiotic resistance is receiving increasing attention from government and medical institutions in China. The total usage of antibiotics was approximately 162,000 tons, accounting for approximately half of the antibiotic usage worldwide, including human use (48%) and use in animals (52%) in China in 2013. The per-capita usage of antibiotics in China is more than 5 times that in Europe and the United States.Citation7 Statistical data from antibacterial resistance investigation collected by the China Antimicrobial Surveillance Network (CHINET), which involved 34 hospitals from 25 provinces covering 960 million people, showed that gram-negative bacilli have higher antimicrobial resistance in China.Citation2 The problem of antibiotic resistance, for example, the carbapenem-resistant Klebsiella pneumonia (K. pneumoniae), is becoming increasingly prominent globally as well as in China. In accordance with a European Center for Disease Prevention and Control report, significant increasing trends of carbapenem-resistant K. pneumoniae were discovered in 5 of the 24 reporting countries between 2009 and 2012.Citation8 Moreover, carbapenem-resistant Klebsiella pneumonia was significantly different in China, increasing from 3.0% in 2005 to 20.9% in 2017. The problem of gram-negative bacilli resistance to carbapenems has attracted great attention in China. However, antibiotic resistance results from nationwide studies were variable at different time periods in China. For instance, in distinct studies from CHINET, Acinetobacter baumannii, which showed high resistance rates against 5 commonly used antimicrobials, had different probabilities of resistance in 2005–2014 and 2014–2017.Citation9,Citation10 The resistance rate of imipenem increased from 31% in 2005 to 62.4% in 2014, while the latest results show that the resistance rate reached 70.7% in 2017. As for amikacin, the resistance levels decreased between 2005 and 2014 and then increased from 2014 to 2017. Therefore, antibiotic resistance surveillance is a long-term repetitive process. It is necessary to understand the trends of antibiotic resistance that continue over time to develop a better management strategy for the use of antibiotics. Moreover, health authorities of China have been taking steps to address the serious problem of antibiotic resistance. In 2016, 14 ministries led by the National Health Commission of China jointly issued the National Action Plan for Containing Antibacterial Resistance (2016–2020).Citation11 Strengthening the supervision of antibiotic usage is one of the major tasks indicated for dealing with antibacterial resistance in this action plan.
The widespread use of antibiotics, especially use of broad-spectrum antibiotics which are effective against a variety of bacteria, is the most important factor leading to antibiotic resistance.Citation12 Additionally, from 2000 to 2010, Brazil, Russia, India, China, and South Africa accounted for three-quarters of the increase in antibiotic consumption.Citation13 Although increasing antibiotic use makes greater resistance inevitable, it is unclear whether reducing the use of antibiotics will lead to a certain reduction in antibiotic resistance,Citation14–Citation19 and antibiotic resistance rates will change with time and management policies. Since the implementation of antibacterial management in a tertiary-care teaching hospital in northwest China in 2009, studies have evaluated the trend and correlation of antibacterial resistance and usage from 2009 to 2013 in this hospital.Citation20 The purpose of this study was to describe the trends and correlation between antibacterial exposure and bacterial resistance from hospitalized patients in a comprehensive hospital from 2014 to 2018 and further to evaluate the effectiveness of the latest antibacterial use management in China.
Materials and methods
Setting and study design
The study protocol was approved by the Research Ethics Committee of Xiangya Hospital (2018121128). This study was conducted at Xiangya Hospital, a tertiary comprehensive academic hospital, located in the southern region of China. The hospital has over 3500 beds with over 3 million emergency department visits and over 130,000 patient discharges annually. This study used hospital-wide data regarding antimicrobial resistance and consumption of both adult and child patients for 5 years between January 1, 2014 and December 31, 2018. The data were obtained from the Intravenous Infusion Safety Evaluation Center of Hunan Province in Changsha, Hunan.
Antibacterial utilization
Antibiotics were classified based on the Anatomical Therapeutic Chemical (ATC) classification system proposed by the WHO.Citation21 The defined daily dose (DDD), which was developed using the ATC index, is the assumed average maintenance dose per day for a drug used for its main indication in adults. According to the 2019 version of the ATC/DDD classification, antibacterial consumption was defined as the number of defined daily doses/100 patient-days (DDDs/100 PDs) and expressed as antimicrobial use density (AUD), which is positively correlated with antibacterial consumption.Citation22 Prophylactic usage and therapeutic medication were not distinguished in this study.
Inclusion and exclusion criteria of microbiology data
A total of 49,123 cases with data of bacterial resistance were recruited from hospitalized patients of this tertiary comprehensive hospital, including all positive clinical specimens (blood, sterile fluid, sputum, urine, wound, and anaerobic specimens) between 2014 and 2018. Microbiological isolates from community samples, outpatient clinics, or the emergency room were excluded. Among these patients, susceptibilities of isolates in 48,732 cases to all antibacterials tested were integrally recorded as susceptible, intermediate, or resistant as per the latest Clinical and Laboratory Standards Institute document. Eight thousand six hundred seven cases with the same susceptibility results for duplicate samples from the same patient were excluded, and 40,125 cases with complete, nonrepetitive susceptibility results were included in our clinical study (Figure S1). The resistance rate of those 40,125 cases was defined as the percentage of total isolates that were resistant to the selected antibacterial agents. Isolates with intermediate susceptibility were not included in the analysis.
Statistical analysis
The changes in trends of antimicrobial usage and resistance during the study period were evaluated by the linear regression. The linear trend by year is defined as the slope of the response over time, each expressed by a coefficient (β). The trends and associations between antibacterial utilization and acquisition of resistance were analyzed using time series analysis. The relationship between the monthly use of specific antibacterial classes and antibacterial resistance over time was evaluated by the autoregressive integrated moving average (ARIMA) models. The β-value indicates the change of the dependent variable when the independent variable changes by one unit within a uniform time interval, for instance, 1 month. All statistical analyses were conducted with SPSS software, version 18.0 (SPSS, Inc.). P-values of two-tailed tests less than 0.05 were the threshold for statistical significance.
Results
Antibacterial use
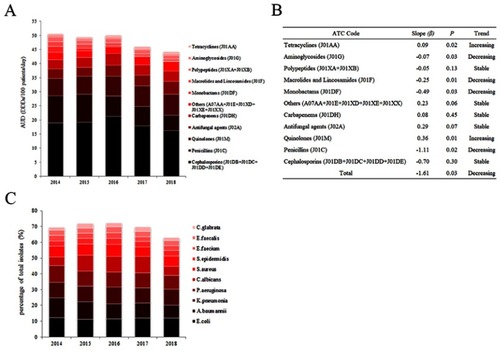
The total AUD of all antimicrobials decreased year by year, from 50.66 daily doses/100 patient-days (DDDs/100 PDs) in 2014 to 44.28 DDDs/100 PDs in 2018 (β=−1.61, P=0.03). The varieties of antibiotics increased from 73 in 2014 to 75 in 2018. The top three departments of the highest antibiotic consumption were hematology (AUD: 26.66), neurosurgery (AUD: 23.88), and geriatrics (AUD: 15.53) from 2014 to 2018 (Table S1). show the AUD and annual usage trends of antibiotics classified according to the Anatomical Therapeutic Chemical (ATC) classification system. Among them, cephalosporins are one of the most consumed varieties, especially the third and fourth generation cephalosporins. The most frequently used is levofloxacin, followed by ceftriaxone. The annual use of penicillins (J01C), monobactams (J01DF), macrolides and lincosamides (J01F), and aminoglycosides (J01G) significantly decreased (P<0.05), while the annual use of quinolones (J01M) and tetracyclines (J01AA) increased significantly (P<0.05), and the annual usage of other types of antibacterials fluctuated or remained stable, but the trend was not statistically significant (P>0.05).
To explore specific antibiotics during clinical application, further linear regression analysis was performed on detailed categories of antibiotics according to ATC. The trends of antibiotic annual usage, including aminoglycosides, macrolides, lincosamides, and monobactams, were significantly decreased, while tetracyclines, second-generation cephalosporins, and others (A07AA + J01XX) were significantly increased ().
Table 1 Annual usage of antibiotic classified by ATC index in this tertiary comprehensive hospital during 2014–2018
Bacterial resistance
Bacterial isolates
A total of 40,125 strains were separated from clinical samples in this study. The top ten frequently isolated species were Escherichia coli (E. coli), Acinetobacter baumannii (A. baumannii), Klebsiella pneumonia (K. pneumonia), Pseudomonas aeruginosa (P. aeruginosa), Candida albicans (C. albicans), Staphylococcus aureus (S. aureus), Staphylococcus epidermidis (S. epidermidis), Enterococcus faecium (E. faecium), Enterococcus faecalis (E. faecalis), and Candida glabrata (C. glabrata). The total rates of top ten bacteria accounted for approximately 70% of all detected bacteria, and 42% were concentrated in gram-negative bacteria (including E. coli, A. baumannii, K. pneumonia, P. aeruginosa), 17% were concentrated in gram-positive bacteria (including S. aureus, S. epidermidis, E. faecium, E. faecalis), and 11% were concentrated in fungus (including C. albicans, C. glabrata). E. coli was the most common strain in the past 3 years. shows the percentage of ten species in all isolated strains per year, and Table S2 displays the numbers and rank order of these species by year.
Antibacterial resistance
Then, we focused on antibacterial resistance problems among the top ten isolated species. Using linear regression, we found that eight isolated species had significantly different rates of resistance to antibiotics (P<0.05), including E. coli, A. baumannii, K. pneumonia, P. aeruginosa, C. albicans, S. aureus, S. epidermidis, and E. faecium (Table S3).
The isolated species which have significant decreasing trends of antimicrobial resistance are as follows (). 1) For E. coli, the resistance rates to amikacin (β=−0.37), aztreonam (β=−3.72), trimethoprim/sulfamethoxazole (β=−2.56), ciprofloxacin (β=−2.72), cefepime (β=−5.19), ceftriaxone (β=−3.57), tobramycin (β=−1.42), and levofloxacin (β=−2.57) were significantly decreased in 5 years. 2) For P. aeruginosa, the resistance rates to gentamicin, ceftazidime, and tobramycin were significantly decreased. The coefficients (β) of P. aeruginosa to gentamicin, ceftazidime, and tobramycin were −4.30, −3.03, and −4.93, respectively. 3) For C. albicans, the resistance rate to fluconazole was significantly decreased (β=−2.74), with a relatively lower resistance rate compared to other isolated species. 4) For S. aureus, the resistance rates of rifampicin (β=−2.96), gentamicin (β=−3.36), and tetracycline (β=−2.97) were significantly decreased. 5) For S. epidermidis, the resistance rate of tetracycline was significantly decreased (β=−2.88).
Figure 2 Antimicrobial resistance of top ten isolated species from inpatients in this tertiary comprehensive hospital during 2014–2018. (A) Significant decreasing trends of antimicrobial resistance. (B) Significant increasing trends of antimicrobial resistance.
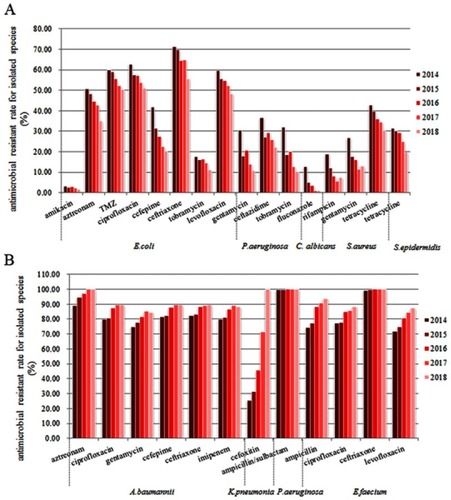
The isolated species which have significant increasing trends of antimicrobial resistance are as follows (). 1) For A. baumannii, the resistance rates to aztreonam (β=2.70), ciprofloxacin (β=2.79), gentamycin (β=2.76), cefepime (β=2.27), ceftriaxone (β=2.04), and imipenem (β=2.43) were significantly increased in 5 years. 2) For K. pneumonia, the resistance rate to cefoxitin was significantly increased year by year, from 25.34% in 2014 to 100.00% in 2018 (β=18.96). 3) For P. aeruginosa, the resistance rates to ampicillin/sulbactam were significantly increased (β=0.07). 4) For E. faecium, the resistance rates of ampicillin (β=5.25), ciprofloxacin (β=2.99), ceftriaxone (β=0.26), and levofloxacin (β=4.00) were significantly decreased.
However, the trend of isolated species including E. faecalis and C. glabrata found no statistical difference in various antibacterial resistance.
Correlation
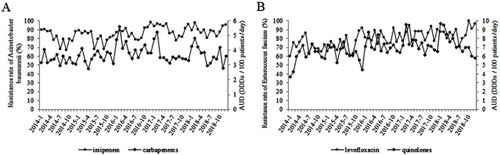
The association between antibacterial resistance and antibiotic usage from 2014 to 2018 is shown in Table S4. The analysis by the ARIMA model showed that the resistance rates of A. baumannii and E. faecium were significantly positively correlated with the AUD of antibiotics. The usage of carbapenems was positively correlated with the resistance rates of A. baumannii to imipenem (β=32.87, P<0.01) (). The β-value indicated that the resistance rate of A. baumannii to imipenem increased (or decreased) by 32.87% when carbapenem usage increased (or decreased) per AUD each month. The usage of quinolones was positively correlated with the resistance rates of E. faecium to levofloxacin (β=104.40, P<0.01) (). Nonetheless, we did not find any significant relationship between the usage of other antibacterial agents and resistance rates.
Figure 3 Correlation between antibiotics usage and resistance rates of A. baumannii and E. faecium in this tertiary comprehensive hospital during 2014–2018. (A) Correlation between usage of carbapenems and resistance rates of A. baumannii to imipenem. The resistance rate of A. baumannii to imipenem is represented as the AUD on the left y-axis and the consumption of carbapenems is shown on the right y-axis. (B) Correlation between usage of quinolones and resistance rates of E. faecium to levofloxacin. The resistance rate of E. faecium to levofloxacin is represented as the AUD on the left y-axis and the consumption of quinolones is shown on the right y-axis.
Discussion
China is one of the countries with serious problems in terms of antibiotic misuse and antibiotic resistance.Citation23–Citation25 The government has closely followed the issue of antibiotic resistance in China and has developed a series of strategies at the national level. This study used the standardized ATC/DDD index to study the utilization and changing patterns of antimicrobials. In this study, the use of penicillins, monobactams, aminoglycosides, macrolides and lincosamides was significantly reduced, while the use of second-generation cephalosporins, quinolones, and tetracyclines was significantly increased in China’s tertiary comprehensive hospitals from 2014 to 2018. The AUD of antibiotics was reduced from 50.66 in 2014 to 44.28 in 2018. Compared to previous reports, the antibiotic consumption of other studies done in China was similar to our study. In the study of tertiary comprehensive hospital, the use of monobactams, aminoglycosides, macrolides and lincosamides was decreased from 2011 to 2017.Citation26 The studies of 65 public general hospitals and 89 tertiary general hospitals showed AUD of antibiotics was decreased in China from 2010 to 2014 and from 2011 to 2015.Citation22,Citation27 However, a previous study showed stable consumptions of several antibiotics, such as second-generation cephalosporins and quinolones, whose consumptions were increased in our study.Citation26 In addition, according to the requirements for Chinese Antimicrobials Special Rectification Activity issued by the National Health Commission, the AUD of antibiotic should be limited to less than 40,Citation28 suggesting that Chinese hospitals still need strong management standards for antibiotic use, especially for the AUD increased types, to promote the selection, dosage, and course of antibiotics.Citation29
In the analysis of antibiotic consumption and resistance, the detection ratios of gram-negative bacteria and gram-positive bacteria in our study were basically consistent with the overall situation in China.Citation10 Additionally, in accordance with previous studies, the imipenem-resistant A. baumannii was associated with usage of carbapenem in our study. Carbapenem-resistant A. baumannii (CRAB) is known worldwide as an extremely important hospital pathogen. It mainly affects weak patients, causes pneumonia and blood infections, and has a high mortality rate.Citation30,Citation31 CRAB infection has become a serious clinical challenge due to its very limited therapeutic options.Citation32 The mechanisms of resistance mainly include overexpression of intrinsic and/or acquired β-lactamases and overexpression of the efflux pump which expels antibiotics and from alterations in outer membrane porins.Citation33,Citation34 Some clinical studies and literature reviews have indicated that exposure to carbapenems significantly increases the risk of acquiring CRAB.Citation35,Citation36 Similar to the results of Wickman et al, the resistance rate of E. faecium to levofloxacin was strongly correlated with quinolones usage in our study.Citation37 Infections caused by E. faecium have been increasing and currently account for around 40% of all enterococcal infections.Citation38 Moreover, outbreaks of hospital-acquired vancomycin-resistant enterococci infections are increasingly reported worldwide, causing nosocomial bacteremia, infective endocarditis, and intra-abdominal and urinary tract infections that have limited treatment options.Citation39,Citation40 If quinolones must be used to treat serious E. faecium infections, it is recommended to select drugs such as DX-619 that have the activity and propensity to select susceptible mutants.
There are some limitations to this study. First, the scope of research is only one tertiary hospital. Although comprehensive monitoring networks for bacterial resistance have been established in China, the data of antibiotic use for participating hospitals were not fully included; therefore, the next step is to collect data exploring the relationship between antibiotic resistance and consumption in a future multicenter study. Second, the relationship between antibiotic resistance and consumption may be nonlinear, and further optimization of statistical methods can be attempted.Citation41 Finally, the use of AUD for antibiotic consumption may not be well correlated with subsequent antibiotic resistance development.Citation20
In conclusion, the consumption of antibiotics and antibiotic resistance has been significantly improved in China. After clarifying the relationship between antibiotics and drug resistance, the next step may be to explore the critical threshold of antibiotic selection pressures, beyond which resistant genes and pathogens gain a survival advantage.Citation41 These findings may help clinicians take appropriate precautions to reduce mortality in such patients.Citation42
Abbreviations
E. coli, Escherichia coli; A. baumannii, Acinetobacter baumannii; K. pneumonia, Klebsiella pneumonia; P. aeruginosa, Pseudomonas aeruginosa; C. albicans, Candida albicans; S. aureus, Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; E. faecium, Enterococcus faecium; E. faecalis, Enterococcus faecalis; C. glabrata, Candida glabrata; TMZ, trimethoprim/sulfamethoxazole; ATC, Anatomical Therapeutic Chemical; AUD, antimicrobial use density; CRAB, carbapenem-resistant A. baumannii.
Ethical approval
This study was reviewed and approved by the Ethical Committee of Xiangya Hospital of Central South University (Approval No. 2018121128).
Informed consent
According to the “Human Biomedical Research Ethical Review Procedures” approved by the National Health and Family Planning Committee of China (No. 11, Section 39), informed consent was waived because of the retrospective nature of the study. After the following circumstances have been reviewed and approved by the ethics committee, the informed consent form can be waived if research is conducted using human body materials or data that can identify information, and the subjects cannot be found, and the research project does not involve personal privacy and commercial interests.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was funded by the Hospital Management Research Foundation of Xiangya Hospital (No. 2016GL21), the Clinical Big Data System Construction Project of Central South University (No. 46), and the Finance Department Project of Hunan Province, People's Republic of China (No. (2016) 129).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- O’Neill J[Website on the Internet]. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; 2016 Available from: http://amr-review.org/Publications.html. Accessed April 24, 2019.
- Roope LSJ, Smith RD, Pouwels KB, et al. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019;364:6435. doi:10.1126/science.aav6390
- Lishman H, Costelloe C, Hopkins S, et al. Exploring the relationship between primary care antibiotic prescribing for urinary tract infections, Escherichia coli bacteraemia incidence and antimicrobial resistance: an ecological study. Int J Antimicrob Agents. 2018;52(6):790–798. doi:10.1016/j.ijantimicag.2018.08.01330145249
- Gajdacs M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8:2. doi:10.3390/antibiotics8020052
- Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49(8):1175–1184. doi:10.1086/60563019739972
- Gajdacs M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24:5. doi:10.3390/molecules24050892
- Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol. 2015;49(11):6772–6782. doi:10.1021/acs.est.5b0072925961663
- European Centre for Disease Prevention and Control (ECDC) [Website on the Internet]. Europe. Directory of Online Resources for the Prevention and Control of Antimicrobial Resistance (AMR) and Healthcare-associated Infections (HAI); 2014. Available from: http://www.ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/guidance-infection-prevention-control/Pages/guidance-prevention-control-infections-caused-by-multidrug-resistant-bacteria-and-healthcare-associated-infections.aspx. Accessed April 24, 2019.
- Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol. 2016;22(Suppl 1):S9–S14. doi:10.1016/j.cmi.2016.01.001
- Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–S134. doi:10.1093/cid/ciy65730423045
- National Health Commission [Website on the Internet]. China. National Action Plan for Containing Bacterial Resistance; 2016 Available from: http://www.nhc.gov.cn/xxgk/pages/viewdocument.jsp?dispatchDate=&staticUrl=/yzygj/s3593/201608/f1ed26a0c8774e1c8fc89dd481ec84d7.shtml. Accessed April 24, 2019.
- Centers for Disease Control and Prevention, US Department of Health and Human Services [Website on the Internet]. US. Antibiotic Resistance Threats in the United States; 2013 Available from: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed April 24, 2019.
- Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi:10.1016/S0140-6736(15)00474-226603918
- Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nature Rev Microbiol. 2010;8(4):260–271. doi:10.1038/nrmicro231920208551
- Callens B, Cargnel M, Sarrazin S, et al. Associations between a decreased veterinary antimicrobial use and resistance in commensal Escherichia coli from Belgian livestock species (2011–2015). Prev Vet Med. 2018;157:50–58. doi:10.1016/j.prevetmed.2017.10.01330086849
- Asencio Egea MA, Huertas Vaquero M, Carranza Gonzalez R, Herraez Carrera O, Redondo Gonzalez O, Arias Arias A. Trend and seasonality of community-acquired Escherichia coli antimicrobial resistance and its dynamic relationship with antimicrobial use assessed by ARIMA models. Enferm Infecc Microbiol Clin. 2018;36(8):502–506. doi:10.1016/j.eimc.2017.10.01329217096
- Thaulow CM, Berild D, Eriksen BH, Myklebust TA, Blix HS. Potential for more rational use of antibiotics in hospitalized children in a country with low resistance: data from eight point prevalence surveys. Pediatr Infect Dis J. 2019;38(4):384–389. doi:10.1097/INF.000000000000210630882728
- Cui D, Liu X, Hawkey P, et al. Use of and microbial resistance to antibiotics in China: a path to reducing antimicrobial resistance. J Int Med Res. 2017;45(6):1768–1778. doi:10.1177/030006051668623029239248
- Kim B, Kim Y, Hwang H, et al. Trends and correlation between antibiotic usage and resistance pattern among hospitalized patients at university hospitals in Korea, 2004 to 2012: a nationwide multicenter study. Medicine. 2018;97(51):e13719. doi:10.1097/MD.000000000001371930572507
- Zou YM, Ma Y, Liu JH, et al. Trends and correlation of antibacterial usage and bacterial resistance: time series analysis for antibacterial stewardship in a Chinese teaching hospital (2009–2013). Eur J Clin Microbiol Infect Dis. 2015;34(4):795–803. doi:10.1007/s10096-014-2293-625487131
- WHO Collaborating Centre For Drug Statistic Methodology [homepage on the Internet]. Norway. Anatomical Therapeutic Chemical Code; 2013 Available from: (www.whocc.no. Accessed April 24, 2019.
- Chen J, Min R, Wang H, Zhao S, Li H, Fang P. Trends and drivers of inpatient antibiotic consumption among 89 China Tertiary General Hospitals from 2011Q1 to 2015Q4. Biomed Res Int. 2018;2018:5968653. doi:10.1155/2018/596865330519582
- Tang Q, Song P, Li J, Kong F, Sun L, Xu L. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends. 2016;10(1):1–6. doi:10.5582/bst.2016.0103426961210
- Meng X, Liu S, Duan J, et al. Risk factors and medical costs for healthcare-associated carbapenem-resistant Escherichia coli infection among hospitalized patients in a Chinese teaching hospital. BMC Infect Dis. 2017;17(1):82. doi:10.1186/s12879-017-2757-228095785
- Liu T, Zhang Y, Wan Q. Pseudomonas aeruginosa bacteremia among liver transplant recipients. Infect Drug Resist. 2018;11:2345–2356. doi:10.2147/IDR.S18028330532566
- Zhang D, Hu S, Sun J, et al. Antibiotic consumption versus the prevalence of carbapenem-resistant Gram-negative bacteria at a tertiary hospital in China from 2011 to 2017. J Infect Public Health. 2019;12(2):195–199. doi:10.1016/j.jiph.2018.10.00330385238
- Bao L, Peng R, Wang Y, et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010–2014. PLoS One. 2015;10(3):e0118868. doi:10.1371/journal.pone.011886825767891
- National Health Commission [Website on the Internet]. China. Notice on Further Carrying Out Antimicrobials Special Rectification Activity on Clinical Rational Use; 2013 Available from: http://www.nhc.gov.cn/yzygj/s3585u/201305/823b9d131ff4416ab7b41b2c4e1f0e83.shtml. Accessed April 24, 2019.
- Yusuf E, Versporten A, Goossens H. Is there any difference in quality of prescribing between antibacterials and antifungals? Results from the first global point prevalence study (Global PPS) of antimicrobial consumption and resistance from 53 countries. J Antimicrob Chemother. 2017;72(10):2906–2909. doi:10.1093/jac/dkx23629091210
- Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol. 2019. doi:10.1016/j.cmi.2019.03.014
- Huang ZY, Li J, Shui J, Wang HC, Hu YM, Zou MX. Co-existence of blaOXA-23 and blaVIM in carbapenem-resistant Acinetobacter baumannii isolates belonging to global complex 2 in a chinese teaching hospital. Chin Med J. 2019. doi:10.1097/CM9.0000000000000193
- Nie XM, Huang PH, Ye QF, Wan QQ. The distribution, drug resistance, and clinical characteristics of Acinetobacter baumannii infections in solid organ transplant recipients. Transplant Proc. 2015;47(10):2860–2864. doi:10.1016/j.transproceed.2015.09.03726707303
- Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. 2019. doi:10.1016/j.ajic.2019.03.003
- Chen F, Wang L, Wang M, et al. Genetic characterization and in vitro activity of antimicrobial combinations of multidrug-resistant Acinetobacter baumannii from a general hospital in China. Oncol Lett. 2018;15(2):2305–2315. doi:10.3892/ol.2017.760029434938
- Abdallah M, Badawi M, Alzaagi I, Issa KN, Rasheed A, Alharthy A. Effect of short-term carbapenem restriction on the incidence of non-pseudomonal multi-drug resistant Gram-negative bacilli in an intensive care unit. J Chemother. 2019;1–6. doi:10.1080/1120009X.2019.1601802
- Munoz-Price LS, Rosa R, Castro JG, et al. Evaluating the impact of antibiotic exposures as time-dependent variables on the acquisition of carbapenem-resistant Acinetobacter baumannii. Crit Care Med. 2016;44(10):e949–e956. doi:10.1097/CCM.000000000000184827167999
- Wickman PA, Black JA, Smith Moland E, Thomson KS, Hanson ND. In vitro development of resistance to DX-619 and other quinolones in enterococci. J Antimicrob Chemother. 2006;58(6):1268–1273. doi:10.1093/jac/dkl42117062613
- Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. 2018;41:76–82. doi:10.1016/j.mib.2017.11.03029227922
- Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10(5):436–440. doi:10.1016/j.mib.2007.07.00417765004
- Guo Y, Tomich AD, McElheny CL, et al. High-level fosfomycin resistance in vancomycin-resistant Enterococcus faecium. Emerg Infect Dis. 2017;23(11):1902–1904. doi:10.3201/eid2311.17113029048285
- Lopez-Lozano JM, Lawes T, Nebot C, et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nature Microbiol. 2019;4(7):1160–1172. doi:10.1038/s41564-019-0410-0
- Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev. 2017;30(1):1–22. doi:10.1128/CMR.00042-1627795305