Abstract
Introduction
A serious problem affecting human society is the development of bacterial resistance. The purpose of the current study was to evaluate the antibiotic resistance of Gram-positive bacteria (GPB) and genotyping of common GPB causing hospital-acquired infections (HAIs) in patients who were referred to Children’s Medical Center during a 6-month period by random amplified polymorphic DNA (RAPD) and enterobacterial repetitive intergenic consensus sequence polymerase chain reaction (ERIC-PCR).
Methods
During the 6-month period, antimicrobial resistance profiles of GPB isolates recovered from patients in Children’s Medical Center were determined using the Kirby–Bauer disk diffusion and MIC. Typing of common GPB was performed and the results were analyzed by gel compare software.
Results
In this cross-sectional study, 6524 cultures were performed and 138 Ggram-positive bacteria were isolated (2%). Staphylococcus aureus strains showed the highest antibiotic penicillin resistance (96.3%). Twenty-six per cent of the strains were methicillin-resistant S. aureus (MRSA) and no resistance was found against vancomycin. All isolates of Enterococcus faecium were resistant to ciprofloxacin (100%). The resistance to vancomycin was very high (67%) and no resistance was observed to linezolid. The results of genotyping analysis of S. epidermidis strains showed the presence of two clones with a genetic relationship of over 80%. All of the S. aureus strains were in one cluster and half of the E. faecium strains were in a cluster with a genetic predilection of over 80%.
Conclusion
This study indicated frequent occurrence of antimicrobial resistance, especially in Enterococcus spp. isolates. Rapid spreads of MRSA and VREF from a clonal origin require implementing careful isolation and infection control measures.
Introduction
The administration and non-prescribed consumption of antibiotics has grown at a considerable rate, sometimes irrationally, in Iran. This process has become increasingly significant in recent years.Citation1,Citation2 According to several studies, the controlled consumption of antibiotics in patients suffering from bacterial infections leads to improved microorganism susceptibility, and the emergence of resistant strains are rarely observed.Citation3,Citation4 Among the Gram-positive bacteria, vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-resistant Enterococcus (VRE), methicillin-resistant S. aureus (MRSA) and beta-lactamase-resistant Streptococcus are considered the most important groups in terms of antibiotic resistance.Citation5,Citation6 Due to their importance, antibiotic consumption policies should be taken and implemented in the agenda of the infection control committees of hospitals.Citation7 Generally, studies covering the antibiotic profile of Gram-positive bacteria (GPB) applied a disk diffusion of a range of antibiotics according to the standard Clinical and Laboratory Standards Institute (CLSI) patterns for sensitivity.Citation8 Among these antibiotics, we can note penicillin, methicillin, ampicillin, trimethoprim-sulfamethoxazole, rifampin, clindamycin, ciprofloxacin, and gentamicin.Citation9 In addition, it is essential to understand pathogen distribution and relatedness to determine the epidemiology of hospital-acquired infections (HAIs) and aid to design the rational pathogen control methods.Citation10
Nowadays, researchers use various DNA-based, amplification-based and sequencing-based typing methods for genotyping of GPB.Citation11,Citation12 These techniques include RFLP (restriction fragment length polymorphism),Citation13 ERIC-PCR (enterobacterial repetitive intergenic consensus sequence polymerase chain reaction),Citation12 AP PCR (arbitrary primers-PCR),Citation14 ribotyping,Citation15 examination of plasmid profile,Citation16 RAPD (random amplified polymorphic DNA),Citation17 and PFGE (pulse field gel electrophoresis).Citation18 The purpose of the current study was to evaluate the antibiotic resistance of GPB and genotyping of common GPB causing HAI in the patients who were referred to Children's Medical Center during 6 months by RAPD and ERIC-PCR.
Materials And Methods
In the present study, the isolates were collected from the referral hospital of Tehran Children ‘s Medical Center for a 6-month period from July 2017 to January 2018. Isolation of bacteria was undertaken as part of the routine hospital laboratory procedure. The GPB strains isolated from clinical specimens of blood, wound and sterile fluids (pleural effusion, peritoneal and cerebrospinal fluid [CSF)] of patients hospitalized in this center were included in the study. All isolates were re-identified using conventional confirmatory tests, such as Gram stain, catalase and coagulase production, DNase, and mannitol fermentation. These specimens include S. aureus, coagulase-negative Staphylococcus, Streptococcus pneumoniae, Enterococcus spp. and Streptococcus viridans.
The antibiotic susceptibility of the isolates was evaluated according to the guidelines published in 2016 by CLSI.Citation19 The measured antibiotics include penicillin (10 U), gentamicin (10 μg), ciprofloxacin (5 μg), cefoxitin (30 μg), trimethoprim/sulfamethoxazole (1.25/23.75 μg), erythromycin (15 μg), clindamycin (2 μg), ampicillin (10 μg), linezolid (30 μg), cefazolin (30 μg), and ampicillin-sulbactam (10/10 μg). All disks were purchased from Mast Co., UK. S. aureus ATCC 25923 was used for quality control of the test. The MICs of vancomycin were determined by E-test methods.
Following the assessment of antimicrobial resistance in each organism, the genotyping of the common hospital -cquired GPB (S. aureus, coagulase-negative Staphylococcusand E. faecium) was accomplished. To achieve this, bacterial genomic DNA extraction was performed using a DNA extraction kit (Bioneer, South Korea) according to manufacturer’s guidelines. RAPD-PCR was accomplished for E. faecium genotyping, whereas ERIC-PCR was done to assess the genotyping of S. aureus and S. epidermidis. Briefly, DNA amplification was performed on a thermo cycler (Bio Rad, USA) in a final volume of 25 mL. The amount of PCR materials for both sets was mentioned in .
Table 1 The PCR Components And Procedures Used For Each Species
The PCR products were loaded on a 1% (w/v) agarose gel containing 1/10,000 gel red (Biotium, USA), and were analyzed by gel electrophoresis and banding patterns were visualized and photographed in Gel-Documentation system (Uvitec, UK). A 100-bp Plus DNA ladder (Thermos Scientific, USA) was used as a molecular size standard. Comparison of PCR fingerprinting profile was performed using GelCompar II software, version 6.5 (Applied Maths, Sint-Martens-Latem, Belgium). Each gel photograph was inverted as TIFF images and then normalized using the reference marker. Similarity analysis of results was calculated using the Dice coefficient/unweighted pair-group method with arithmetic mean (UPGMA). The criterion for related clones was taken as profiles with 80% or more similar bands.
Statistical Analysis
Statistical analysis of the results was performed by the statistical package SPSS 13.0 (SPSS Inc. Chicago, IL, USA).
Results
In the current cross-sectional study, among a total of 6524 collected isolates (5243 blood culture, 889 CSF, 139 dialysis fluid, 48 pleural fluid, 60 ascites, 44 joint fluid, 66 wound and 35 CV line) over the course of the 6 months, 138 (2%) GPB were separated among 88 males patients (36.2%). Ninety-two children (66.7%) were under the age of one year, and only 20 patients (14.49%) were above 5 years of age. A little over half (55.1%) had a history of previous hospitalization. Among 121 GPB, which were assessed for nosocomial infection, 64 isolates (52.9%) had the HAI criteria. In addition, the HAI criteria were met by all patients who utilized a CV line, and 89% of patients who used a catheter. There was also one child who used a ventilator, due to having the criteria of nosocomial infection.
The prevalence of Gram-positive organisms isolated was: 76 (55.1%) S. epidermidis, 27 (19.5%) S. aureus, 12 (8.7%) E. faecium, 9 (6.5%) S. viridans, 7 (5.1%) S. haemolyticus, 4 (2.9%) S. pneumoniae, 2 (1.4%) Streptococcus group B, and 1 (0.7%) diphtheroid. The frequency of HAI among isolated bacteria is shown in . Obviously, S. epidermidis was reported as the most frequent bacteria having nosocomial infection (64.2%), followed by S. aureus (17.9%).
Table 2 Frequency Of Nosocomial Infections Among Isolated Bacteria
Most of the GPB were isolated from the Pediatric Intensive Care Unit, which was responsible for 20.3% of isolates, followed by the emergency unit (18.1%). The most isolated bacteria separated from these units were S. epidermidis (75% and 68%, respectively).
As shown in , S. aureus and S. epidermidis were determined as the most resistant to penicillin (96.3% and 98.6%, respectively), and all were sensitive to vancomycin. Among S. aureus strains, 26% MRSA strains were reported. The highest resistance rate among S. viridans strains was observed to be against erythromycin, while none was resistant to vancomycin, clindamycin, and trimethoprim/sulfamethoxazole. The whole Enterococcusspp. isolates were resistant to ciprofloxacin, and along with that, sensitive to linezolid.
Table 3 Antibiotic Resistance Rates Of Evaluated Gram-Positive Bacteria
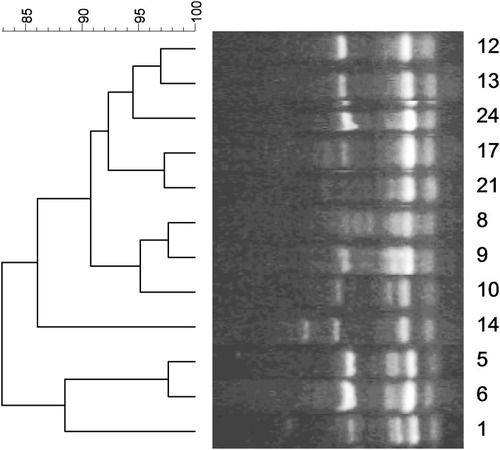
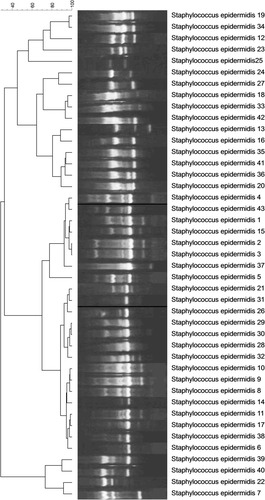
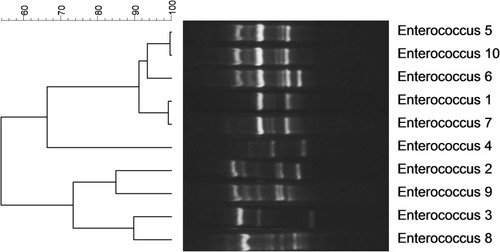
The genotyping of S. aureus causing hospital-acquired infection showed the presence of all strains in one cluster with more than 80% genetic similarity (). In addition, the dendrogram, based on analysis of S. epidermidis strains indicated the presence of two clones with 80% genetic similarity. Twenty-six strains were located in one cluster and 17 other strains were in another (). Genotyping of Enterococcusspp. strains demonstrated the presence of three clusters, of which 50% of strains was placed in one of them ().
Discussion
In the current investigation, we assessed the antimicrobial resistance and genotyping of GPB isolated from patients who were referred to Children’s Medical Center over a period of 6 months from July 2017 to January 2018.
In our current study, the most frequent Gram-positive organisms were S. epidermidis (n= 76, 55.1%) and S. aureus (n= 27, 19.5%). In the earlier study by Mamishi et al.,Citation20 coagulase-negative Staphylococcus(48.4%) and S. aureus (16.7%) were reported as the most frequent bacteria. In addition to the mentioned organisms, Enterococcusspp. were also recognized as the most frequent GPB in the study performed by Bagherzadeh et al.Citation21
In S. aureus strains, the highest antibiotic resistance rate was identified in penicillin (96%), while the highest sensitivity rate was observed in vancomycin (100%) and trimethoprim/sulfamethoxazole (96%). Also, 26% of isolates were MRSA, which was compatible with the two studies performed in Kuwait, and in Saudi Arabia, Brazil and Iran;Citation22–Citation25 however, they were far lower than the figures reported by the previous studies in the Children’s Medical Center.Citation20,Citation26–Citation28
Enterococcus spp. strains indicated significantly high resistance to several used drugs (vancomycin, 67%; ciprofloxacin, 100%; gentamycin, 90%; ampicillin, 83%; ampicillin-sulbactam, 87.5%). In the study of Sattari-Maraji et al., E. faecium was found to have high resistance against ampicillin (92.5%), ciprofloxacin (96%), and vancomycin (70%).Citation29 In our study, all isolates were sensitive to linezolid, which is consistent with previous studies.Citation20,Citation30–Citation33
Although the occurrence of VRE varies in different countries, a high frequency was described in Iran, Ethiopia and Turkey.Citation29,Citation34–Citation36
The dendrogram of genotyping of S. epidermidis strains depicted the presence of two clones with more than 80% genetic similarity, suggesting the existence of an outbreak in the hospital. In addition, all S. aureus isolates belonged to one cluster with more than 80% genetic similarity. The analysis of genotyping of Enterococcus spp. demonstrated the presence of 50% of strains in one cluster with more than 80% genetic similarity. In the study of Banerjee et al. in India, RAPD typing of a multidrug-resistant E. faecium urinary isolate showed two major clusters, one of which had 10 strains of 100% similarity and were isolated from a common source.Citation37 According to their high resistance rate to vancomycin in the current study and the presence of half of the strains in one cluster, it might be considered as an alarm for transmission of these strains among different units and patients. In the study of Pourakbari et al.,Citation34 the results of RAPD-typing of Enterococcus strains demonstrated the presence of four distinct clusters, of which 100% of VREF isolates belonged to one cluster, indicating a nosocomial infection among units.
Vancomycin-resistant enterococci are thought to be spread mostly through cross-colonization.Citation38 To minimize this high pressure, health care workers (HCWs) hand washing and using gloves are crucial actions.Citation38 Besides, reducing the movement of HCWs between colonized and non-colonized patients may be attained by creating cohorts of either patients or nursing staff, and also by restricting the number of physicians entering patients' rooms during rounds.Citation38
In conclusion, this study indicated frequent occurrence of antimicrobial resistance, especially in Enterococcusspp. isolates. Rapid spreads of MRSA and VREF from a clonal origin require implementing careful isolation and infection control measures. Therefore, environmental control by routine disinfection of patient area, in addition to screening high-risk patients and isolation of colonized patients, should be imposed to diminish the risk of acquiring nosocomial VRE.
Acknowledgments
This study was taken from Dr. Maryam Mohammadian’s postgraduate thesis and was supported by a grant (number: 96-03-88-35761) from Tehran University of Medical Sciences to Dr. Setareh Mamishi.
Disclosure
The authors report no conflicts of interest in this work.
References
- Mahmoudi S, Mamishi S, Mohammadi M, et al. Phenotypic and genotypic determinants of mupirocin resistance among Staphylococcus aureus isolates recovered from clinical samples of children: an Iranian hospital-based study. Infect Drug Resist. 2019;12:137–143. doi:10.2147/IDR.S18561030655680
- Valian SK, Mahmoudi S, Pourakbari B, Banar M, Ashtiani MTH, Mamishi S. The causative organisms of bacterial meningitis and their antimicrobial resistance profiles in Iranian children in 2011–2016. Infect Disord Drug Targets. 2018. doi:10.2174/1871526519666181123130101
- Tunger O, Karakaya Y, Cetin CB, Dinc G, Borand H. Rational antibiotic use. J Infect Dev Ctries. 2009;3(2):088–093. doi:10.3855/jidc.54
- Sani R, Garba S, Oyewole O, Ibrahim A. Antibiotic resistance profile of gram positive bacteria isolated from wound infections in Minna, Bida, Kontagora and Suleja area of Niger State. J Health Sci. 2012;2(3):19–22.
- Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59:S4–S16. doi:10.1016/S0163-4453(09)60003-719766888
- Pourakbari B, Mahmoudi S, Moradzadeh M, et al. Antimicrobial resistance patterns of the gram-positive bacteria isolated from children with bloodstream infection in an Iranian referral hospital: a 6-year study. Infect Disord Drug Targets. 2018;18(2):136–144. doi:10.2174/187152651766617082116434328828970
- Vessal G, Afhami S, Gholami K, Shafaghi B, Hekmat Yazdi S. Evaluation of antimicrobial resistance among gram-negative isolates collected from intensive care units and reliability of routine disc susceptibility tests at a teaching hospital in tehran. Iran J Pharm Res. 2010;89–100.24363712
- Karimzadeh I, Mirzaee M, Sadeghimanesh N, Sagheb MM. Antimicrobial resistance pattern of Gram-positive bacteria during three consecutive years at the nephrology ward of a tertiary referral hospital in Shiraz, Southwest Iran. J Res Pharm Pract. 2016;5(4):238. doi:10.4103/2279-042X.19246027843959
- Kassim A, Omuse G, Premji Z, Revathi G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: a cross-sectional study. Ann Clin Microbiol Antimicrob. 2016;15:21. doi:10.1186/s12941-016-0135-327068515
- Nanvazadeh F, Khosravi AD, Zolfaghari MR, Parhizgari N. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39(7):1409–1413. doi:10.1016/j.burns.2013.03.00823773789
- Tenover FC, Arbeit RD, Goering RV. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18(6):426–439. doi:10.2307/301412529181401
- Ranjbar R, Karami A, Farshad S, Giammanco G, Mammina C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014;37(1):1–15.24531166
- Okhravi N, Adamson P, Matheson MM, Towler HM. Lightman S. PCR-RFLP–mediated detection and speciation of bacterial species causing endophthalmitis. Invest Ophthalmol Vis Sci. 2000;41(6):1438–1447.10798660
- Delboni MG, Gomes BP, Francisco PA, Teixeira FB, Drake D. Diversity of Enterococcus faecalis genotypes from multiple oral sites associated with endodontic failure using repetitive sequence-based polymerase chain reaction and arbitrarily primed polymerase chain reaction. J Endod. 2017;43(3):377–382. doi:10.1016/j.joen.2016.10.04228131414
- Schumann P, Pukall R. The discriminatory power of ribotyping as automatable technique for differentiation of bacteria. Syst Appl Microbiol. 2013;36(6):369–375. doi:10.1016/j.syapm.2013.05.00323827045
- El-Sayed ZM, Al-Ghamdi AK, Azhar EI, Khalifa NA, Ashshi AM, Faidaha HS. Multidrug resistant bacterial strains and their associated plasmid profile. Life Sci J. 2015;12:1.
- Sadeghifard N, Ranjbar R, Zaeimi J, et al. Antimicrobial susceptibility, plasmid profiles, and RAPD-PCR typing of Acinetobacter bacteria. Asian Biomed. 2010;4(6):901–911. doi:10.2478/abm-2010-0118
- Salipante SJ, SenGupta DJ, Cummings LA, Land TA, Hoogestraat DR, Cookson BT. Application of whole genome sequencing for bacterial strain typing in molecular epidemiology. J Clin Microbiol. 2015;03385–03314.
- CLSI C. Performance standards for antimicrobial susceptibility testing. Clin Lab Stand Inst. 2016.
- Mamishi S, Pourakbari B, Ashtiani MH, Hashemi FB. Frequency of isolation and antimicrobial susceptibility of bacteria isolated from bloodstream infections at Children’s Medical Center, Tehran, Iran, 1996–2000. Int J Antimicrob Agents. 2005;26(5):373–379. doi:10.1016/j.ijantimicag.2005.08.00416213124
- Bagherzadeh Yazdchi S, Pourmand M, Hajiabdolbaghi M, Hoseini M, Mardani N. Molecular characterization of hypervariable region (HVR) and antibiotic susceptibility patterns of staphylococcus aureus strains isolates collected from Tehran University of Medical Sciences hospitals. J School Publ Health Inst Publ Health Res. 2008;6(2):39–47.
- Udo E, Al-Sweih N, Dhar R, et al. Surveillance of antibacterial resistance in Staphylococcus aureus isolated in Kuwaiti hospitals. Med Princ Pract. 2008;17(1):71–75. doi:10.1159/00010959418059105
- Madani TA, Al-Abdullah NA, Al-Sanousi AA, Ghabrah TM, Afandi SZ, Bajunid HA. Methicillin-resistant Staphylococcus aureus in two tertiary-care centers in Jeddah, Saudi Arabia. Infect Control Hosp Epidemiol. 2001;22(4):211–216. doi:10.1086/50189111379711
- Neves FPG, Marlow MA, Rezende-Pereira G, et al. Differences in gram-positive bacterial colonization and antimicrobial resistance among children in a high income inequality setting. BMC Infect Dis. 2019;19(1):478. doi:10.1186/s12879-019-4104-231142269
- Mamishi S, Mahmoudi S, Bahador A, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus in an Iranian referral paediatric hospital. Br J Biomed Sci. 2015;72(2):47–51. doi:10.1080/09674845.2015.1166679526126318
- Mamishi S, Mahmoudi S, Sadeghi R, Movahedi Z, Hadipour R, Pourakbar B. Genotyping of Staphylococcus aureus strains among healthcare workers and patients in the tertiary referral Children’s Medical Hospital in Tehran, Iran. Br J Biomed Sci. 2012;69(4):173. doi:10.1080/09674845.2012.1206914823304794
- Sabouni F, Mahmoudi S, Bahador A, et al. Virulence factors of Staphylococcus aureus isolates in an Iranian referral children’s hospital. Osong Public Health Res Perspect. 2014;5(2):96–100. doi:10.1016/j.phrp.2014.03.00224955319
- Sabouni F, Ranjbari R, Pourakbari B, et al. Staphylococcus aureus infections in children in an Iranian referral pediatric Hospital. J Prev Med Hyg. 2013;54(4):205.24779281
- Sattari-Maraji A, Jabalameli F, Node Farahani N, Beigverdi R, Emaneini M. Antimicrobial resistance pattern, virulence determinants and molecular analysis of Enterococcus faecium isolated from children infections in Iran. BMC Microbiol. 2019;19(1):156. doi:10.1186/s12866-019-1539-y31286887
- Zhanel GG, DeCorby M, Adam H, et al. Prevalence of antimicrobial-resistant pathogens in Canadian hospitals: results of the Canadian Ward Surveillance Study (CANWARD 2008). Antimicrob Agents Chemother. 2010;54(11):4684–4693. doi:10.1128/AAC.00469-1020805395
- Bhat R, Lewis LES, Vandana K. Bacterial isolates of early-onset neonatal sepsis and their antibiotic susceptibility pattern between 1998 and 2004: an audit from a center in India. Ital J Pediatr. 2011;37(1):32. doi:10.1186/1824-7288-37-3221745376
- Mahmoudi S, Mahzari M, Banar M, et al. Antimicrobial resistance patterns of Gram-negative bacteria isolated from bloodstream infections in an Iranian referral paediatric hospital: a 5.5-year study. J Glob Antimicrob Resist. 2017;11:17–22. doi:10.1016/j.jgar.2017.04.01328729206
- Sabouni F, Movahedi Z, Mahmoudi S, Pourakbari B, Keshavarz Valian S, Mamishi S. High frequency of vancomycin resistant Enterococcus faecalis in children: an alarming concern. J Prev Med Hyg. 2016;57(4):E201–E204.28167857
- Pourakbari B, Mahmoudi S, AGHDAM MK, et al. Clonal spread of vancomycin resistance Enterococcus faecalis in an Iranian referral pediatrics center. J Prev Med Hyg. 2013;54(2):87.24396988
- Yilema A, Moges F, Tadele S, et al. Isolation of enterococci, their antimicrobial susceptibility patterns and associated factors among patients attending at the University of Gondar Teaching Hospital. BMC Infect Dis. 2017;17(1):276. doi:10.1186/s12879-017-2363-328412932
- Saba Copur S, Sahin F, Gocmen JS. Determination of virulence and multidrug resistance genes with polymerase chain reaction method in vancomycin-sensitive and -resistant enterococci isolated from clinical samples. Turk J Med Sci. 2016;46(3):877–891. doi:10.3906/sag-1412-8627513269
- Banerjee T. Random amplified polymorphic DNA (RAPD) typing of multidrug resistant enterococcus faecium urinary isolates from a Tertiary Care Centre, Northern India. J Clin Diagn Res. 2013;7(12):2721–2723. doi:10.7860/JCDR/2013/6541.374224551622
- Bonten MJ, Slaughter S, Ambergen AW, et al. The role of colonization pressure in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998;158(10):1127–1132. doi:10.1001/archinte.158.10.11279605785