Abstract
Purpose
Carbapenem-resistant Enterobacter cloacae complex has been reported worldwide and becomes a new challenge for clinical management. The present study was to characterize the IncX3 plasmid encoding blaNDM-1 and blaSHV-12 gene in E. hormaechei sequence.
Materials and Methods
EcHK001 was recovered from the sputum sample of a patient. Species identification and antimicrobial susceptibility testing were performed using the VITEK 2 system, while further classification was carried out by hsp60 typing. The presence of NDM-1 was detected by PCR and sequencing. Conjugation experiments and southern blotting were carried out to determine the transferability of the NDM-1-carrying plasmid. Whole-genome sequencing and analysis were conducted to better understand the molecular characteristics of the multi-drug resistant isolate.
Results
Strain EcHK001 was classified as E. hormaechei of new sequence type 1000. Multiple drug-resistant genes were detected. The blaNDM-1 and blaSHV-12 genes were located on a self-transferable IncX3 plasmid. Synonymous mutations were identified in the genes encoding TEM-1 and ACT-17. Phylogenetic analysis indicated that EcHK001 clustered into a different clade from domestic strains.
Conclusion
The rapid spread of the recurrent IncX3 plasmid highlights the need for continuous surveillance of the NDM-1 dissemination. The presence of mutations in existing carbapenem-resistant genes may generate potential new variants and raise serious challenges for clinical treatment.
Introduction
New Delhi metallo-β-lactamase-1 (NDM-1) is an enzyme with the ability to hydrolyze most β-lactams. Since first detected in 2009 from a Klebsiella pneumoniae strain,Citation1 21 NDM variants have been reported from a variety of Enterobacterales species.Citation2 The widespread of NDM is mainly due to diverse self-transferable plasmids. The co-existence of NDM and other resistance genes in a single plasmid is increasingly detected and confers higher resistance to carbapenems, raising great challenges for clinical management.Citation3,Citation4
As important opportunistic pathogens, Enterobacter cloacae complex is responsible for a variety of human infections in the respiratory tract, wound and urine.Citation5 Among the members of the Enterobacter cloacae complex, E. hormaechei was first reported in 1989Citation6 and has been increasingly detected worldwide with the acquisition of different beta-lactamase genes.Citation7,Citation8 In this study, we report a carbapenem-resistant E. hormaechei strain with an IncX3 plasmid coproducing NDM-1 and SHV-12. Susceptibility test, conjugation experiment and whole-genome sequencing were performed to investigate the molecular characteristics of the multiple drug-resistant strain.
Materials and Methods
Bacterial Strain Identification
The NDM-1-producing strain EcHK001 was isolated from the sputum sample of an 83-year-old male patient through routine surveillance in Haikou, China, in 2015. The E. cloacae strain was identified by the VITEK2 compact system (bioMérieux, France) and 16S rRNA gene sequencing.Citation9 The Enterobacter cloacae complex can be further classified by the heat shock protein 60-encoding (hsp60) gene,Citation10,Citation11 the sequence of which was obtained through amplification and sequencing using the primers of Hsp60-F (5ʹ-GGT AGA AGA AGG CGT GGT TGC-3ʹ) and Hsp60-R (5ʹ-ATG CAT TCG GTG GTG ATC ATC AG-3ʹ) as previously described.Citation12 Phylogenetic analysis of the hsp60 sequences from GenBank was performed to confirm the identification of E. hormaechei.
The presence of NDM, SHV, TEM, and ACT genes was detected by PCR and sequencing as previously described.Citation13–Citation16
Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) of E. hormaechei strain EcHK001 and the transconjugants were determined using the VITEK2 system with AST-GN09 card (bioMérieux) and interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI).Citation17 The following agents were tested: Ampicillin, Ampicillin/sulbactam, Piperacillin, Piperacillin/tazobactam, Cefazolin, Cefuroxime, Cefotetan, Ceftazidime, Ceftriaxone, Cefepime, Aztreonam, Imipenem, Meropenem, Amikacin, Gentamicin, Tobramycin, Ciprofloxacin, Levofloxacin, Nitrofurantoin, and Sulfamethoxazole/trimethoprim.
Southern Blotting and Conjugation Experiments
DNA fragments of strain EcHK001 were prepared in agarose plugs by electrophoresis and digested with S1 endonuclease (Takara, Dalian, China). Subsequent fragments were further separated by PFGE through a CHEF-DR Ⅲ system (Bio-Rad, Hercules, USA). A sheet of nylon membrane (Roche) was used to transfer the plasmid DNA to the membrane due to the negative charge of the DNA and positive charge of the membrane. Southern blotting specific to blaNDM-1 was performed using the Probe-NDM-F (5ʹ-GGC GGA ATG GCT CAT CAC GA-3ʹ) and Probe-NDM-R (5ʹ-CGC AAC ACA GCC TGA CTT TC-3ʹ) as previously described.Citation18
Conjugation experiment was carried out by broth and filter mating using strain EcHK001 and E. coli strain J53 Azr as the donor and recipient, respectively. The mixture was then incubated at 30°C for 18 hrs. Transconjugants were selected on MacConkey agar plates containing meropenem (4 μg/mL) plus sodium azide (100 μg/mL) at 37°C for 48 hrs. PCR amplification and sequencing were further performed to determine the presence of the blaNDM-1 and blaSHV-12 genes in the transconjugants.
Whole Genome Sequencing and Phylogenetic Analysis
Genomic DNA was extracted from the bacterial culture of E. hormaechei strain EcHK001 using the QIAamp DNA Mini Kit (Qiagen, Inc., Valencia, CA). Whole-genome sequencing was performed using Illumina HiSeq 2500 sequencer at Novogene Company (Beijing, China) with 109-fold coverage and the MinION sequencer with the nanopore library prepared using the Rapid Sequencing Kit SQK‐RAD004 (Oxford Nanopore Technologies, UK). Hybrid assembly of Illumina and Nanopore sequencing reads was carried out with Unicycler (v0.4.7).Citation19 The blaNDM-carried plasmid was designated as pNDM-EcHK001 here and annotated by RAST.Citation20 PlasmidFinderCitation21 was employed to determine the replicon type.
Strain typing was carried out by using the genomic sequence to query the seven house-keeping genes (pyrG, gyrB, rplB, leuS, dnaA, rpoB and fusA) of E. cloacae on the MLST web server.Citation22 All 7 strains of E. hormaechei from China and other 11 sequences of E. hormaechei were obtained from NCBI for phylogenetic tree construction using kSNP (v3.0).Citation23 The average nucleotide identities (ANIs) between strain EcHK001 and other isolates were calculated by JSpeciesWS web service.Citation24
Nucleotide Sequence Accession Numbers
Genomic and plasmid sequences of strain EcHK001 have been deposited into GenBank under accession NZ_PEIL01000000 and NZ_CM008823, respectively.
Results
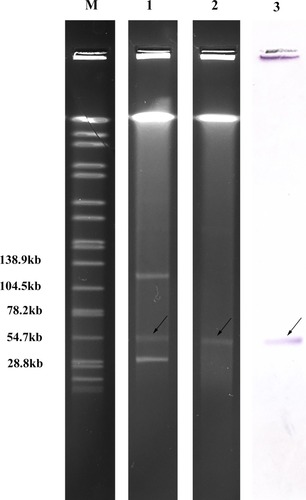
Microbiological Features of Strain EcHK001
Based on the hsp60 gene, the blaNDM-1 carrying strain EcHK001 was further classified into the E. hormaechei. EcHK001 exhibited resistance to most tested antimicrobial agents, but was still susceptible to amikacin, ciprofloxacin and levofloxacin (). S1-PFGE showed that EcHK001 contained three plasmids which sizes were ~33 kb, ~54kb and ~130 kb (). Electrophoresis and southern blotting revealed that the blaNDM-1 gene was located on the ~54kb plasmid. This plasmid, designated as pNDM-EcHK001, was successfully transferred via conjugation at a frequency of 2.94×10−2. Susceptibility testing showed the transconjugants acquired resistance to most tested β-lactams including ampicillin, ampicillin/sulbactam, piperacillin, cefazolin, cefuroxime, ceftazidime, ceftriaxone, aztreonam, imipenem and meropenem. In addition to blaNDM-1, multiple resistance genes including blaSHV-12, blaCTX-M-14, blaDHA-1, blaACT-17, blaTEM-1, qnrB4, brp, sul2, sul1, strA, strB, dfrA14 and aac(6ʹ)-IIc were identified through genome sequencing and analysis. Genome analysis indicated the blaDHA-1, blaCTX-M-14 and blaACT-17 genes were located on the chromosome, while other bla genes were on plasmids. The plasmid pNDM-EcHK001 had a length of 54,035 bp and carried both the blaNDM-1 and blaSHV-12 genes. The blaTEM-1 gene was located on a 124,544 bp plasmid which had an 11,452-bp resistance region containing ΔtnpA, tnpR, blaTEM-1, ISVsa3, strB, strA, sul2, IS4321, ΔtnpA and IS26, same as the region from plasmid p34998-210.894kb (GenBank accession no. CP012169.1).Citation10 Of note, synonymous mutations in the blaTEM-1 (T396G) and blaACT-17 (C96A, T276G, T675C, T690C, A708G, G777A and G807A) genes were detected and verified by PCR, indicating the potential to generate new variants of these β-lactamase genes. The variants of blaTEM-1 and blaACT-17 were deposited in GenBank under accession number MG818166 and MG865974.
Table 1 Antibiotic Susceptibilities of Strain EcHK001 and the E. coli J53 Transconjugants
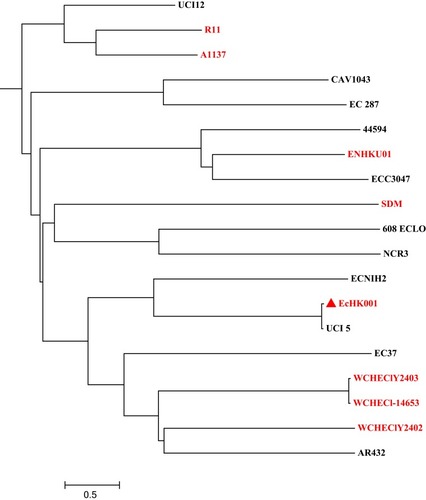
Strain Typing and Phylogenetic Analysis
In silico MLST analysis identified a new allele of the house-keeping genes pyrG designated as pyrG-281, and strain EcHK001 was assigned a new sequence type 1000. Whole-genome phylogenetic analysis revealed EcHK001 formed a clade with strains isolated in USA and China (). Sequence alignments revealed the ANI values between EcHK001 and three isolates from Chengdu, China ranged from 99.22% to 99.23%, while the ANI between EcHK001 and the strain UCI_5 from Irvine, California, USA was 99.30%. However, the ANI between EcHK001 and the other Chinese isolates ranged from 86.5 to 87.2. These results indicated that EcHK001 had a close relationship with overseas strains and may have originated from an earlier ancestor than domestic strains.
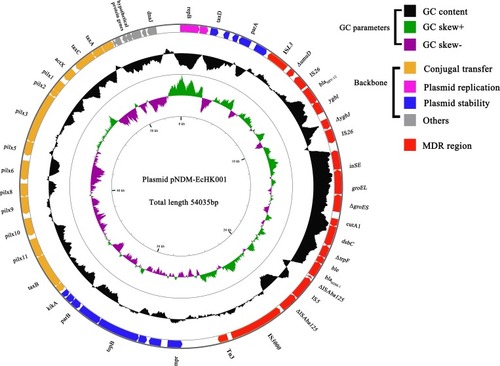
Characterization of Plasmid pNDM-EcHK001
Plasmid pNDM-EcHK001 had a length of 54,035 bp and 52 ORFs (). The plasmid, assigned to replicon type IncX3, consists of a 39.6 kb backbone and a 19.2 kb multiple drug resistance (MDR) region. The backbone carries genes responsible for plasmid replication (repB), conjugal transfer (taxA, taxB, taxC and pilx1-pilx11, etc.) and plasmid stability (parA, parB, etc.). The MDR region is composed of Tn3, IS3000, the transposon Tn125 encoding blaNDM-1, a mobile element containing blaSHV-12 and ISL3.
Figure 3 The genetic map of plasmid pNDM-EcHK001. GC content, GC skew+ and GC skew- are, respectively, indicated in black, green and purple. The MDR regions are indicated in red. The modules of conjugal transfer, plasmid replication and plasmid stability are, respectively, indicated in orange, pink and blue. The other elements are indicated in grey.
The genome sequence of pNDM-EcHK001 shared >99% similarity with plasmid pNDM-HN380 (GenBank accession no. JX104760) in K. pneumoniae, pNDM-HF727 (GenBank accession no. KF976405) in E. cloacae,Citation25 pYE315203 (GenBank accession no. JX254913) in Citrobacter freundii and pKPN5047 (GenBank accession no. KC311431) (), which are differed by only 14 SNPs in the IS5 element upstream of the blaNDM-1 gene. Plasmids pEC55-NDM4 (GenBank accession no. KX470734) and pZHDC33 (GenBank accession no. KX094555) also shared >99% identity with pNDM-EcHK001 but containing different blaNDM alleles (). The blaNDM-4 (T354G) and blaNDM-13 genes (C531T) differ from blaNDM-1 and blaNDM-4 by only a single nucleotide, respectively, suggesting a close relationship among these three plasmids. On these above plasmids, the blaNDM-1 gene was located in a Tn125 variant while the blaSHV-12 gene in a composite transposon. The transposon Tn125 served as a mobile element for the transfer of blaNDM-1 and comprised ΔISAba125, blaNDM-1 (locus tag, CS291_RS00145), the bleomycin resistance gene ble (locus tag, CS291_RS00140), ΔtrpF (locus tag, CS291_RS00135), tat (locus tag, CS291_RS00130), dct (locus tag, CS291_RS00125), groES (locus tag, CS291_RS00120), groEL (locus tag, CS291_RS00115) and insE (locus tag, CS291_RS00115) as previously described.Citation25 Compared with the prototype Tn125, the upstream ΔISAba125 is disrupted by IS5 while the downstream copy is absent. The ΔISAba125 and IS5 were each surrounded by two inverted repeats (IRs) (15 bp and 12 bp), respectively. Two direct repeats (DRs) (21 bp and 11 bp) were located at the end of groEL and insE.
Table 2 pNDM-EcHK001-Like Plasmids Harboring blaNDM Among Enterobacterales
Figure 4 The genetic structure features of pNDM-EcHK001-like plasmids. The blaNDM-1 and blaSHV-12 gene are indicated in red and yellow. The blaNDM variants and the blaSHV-11 gene are indicated in purple and dark orange. The insertion sequences ΔISAba125, IS5 and IS26 are indicated in black. Other genes in ΔTn125 and blaSHV-12-carrying transposon are indicated in light gray and dark gray, respectively.
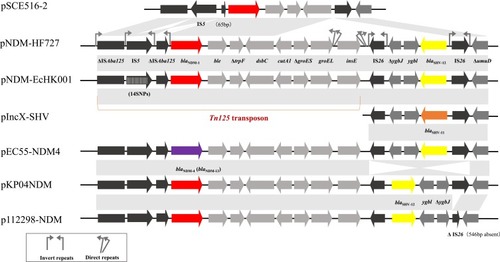
The blaSHV-12-carrying transposon is sequentially organized as ΔygbJ (locus tag, CS291_RS00085), ygbI (locus tag, CS291_RS00080) and blaSHV-12 (locus tag, CS291_RS00075), which were located within two opposite IS26 elements. And each IS26 was surrounded by 14 bp IRs. In comparison with pNDM-EcHK001, the inversion of the blaSHV-12–carrying transposon happened in plasmids pKP04NDM (GenBank accession no. KU314941)Citation26 and p112298-NDM (GenBank accession no. KP987216).Citation27 In addition, p112298-NDM had a 546-bp-deletion in the IS26 gene. Plasmid pIncX-SHVCitation28 had a similar backbone as pNDM-EcHK001 but with the deletion of Tn125 and a different blaSHV allele, while pSCE516-2 (GenBank accession no. JN247852) had only the NDM-1-carrying Tn125 with an inversion of IS5 and a shorter ΔISAba125 region with only 65 bp remaining, suggesting the composite transposon may serve as the major vehicle for SHV-12 (GenBank accession no. KX023261) (). The information of all the above plasmids is shown in .
Discussion
Previous studies have shown that the blaNDM-1-positive isolates in diverse species of Enterobacterales often exhibited high resistance to beta-lactam antibiotics and posed a significant threat to public health.Citation3,Citation29 We provided a detailed description of a multidrug-resistant E. hormaechei strain EcHK001, which had an IncX plasmid pNDM-EcHK001 co-harboring blaNDM-1 and blaSHV-12. The IncX plasmids have been repeatedly reported in Enterobacterales, carrying different resistance genes.Citation30 The plasmid pNDM-EcHK001 had a relatively high conjugation frequency compared with other IncX3 plasmids,Citation31 which may facilitate rapid dissemination of the blaNDM-1 gene.
The pNDM-EcHK001-like plasmids, which share the same backbone and have both blaNDM-1 and blaSHV-12, have a broad host range and the ability of transfer among Enterobacterales.Citation32 However, it is noteworthy that there were no previous reports of such plasmids in E. hormaechei. The co-occurrence of blaNDM-1 and other beta-lactamase genes mediated resistance to broad-spectrum antibiotics such as carbapenems and cephalosporins, raising challenges in rapidly tailoring clinical therapy.
These plasmids were first detected and repeatedly reported from different geographical locations mainly in China. The isolates carrying these plasmids were all recovered from samples of human patients, which had no history of foreign travel or epidemiological relationship. These evidences indicated a natural reservoir of these plasmids may exist in China. The slight variations among the pNDM-EcHK001-like plasmids suggesting they may be descended from a common ancestor. Whole-genome phylogenetic analysis showed that EcHK001 had a close relationship with foreign isolates. More data are needed to further understand the mechanisms underlying the dissemination of these pNDM-EcHK001-like plasmids.
Conclusions
In summary, our study characterized a novel E. hormaechei ST1000 strain EcHK001, which had a different origination with domestic isolates through phylogenetic analysis. The strain carried multiple bla genes and conferred increased resistance to carbapenems. The coexistence of the blaNDM-1 and blaSHV-12 genes is repeatedly reported on the pNDM-EcHK001-like plasmids, which had a high transferability and may serve as a common vehicle for rapid dissemination of carbapenemase-encoding genes. Our findings further highlight the spread of NDM-1 in EnterobacteralesEnterobacteriaceae. More attention should be devoted to the surveillance of the rapid dissemination of the NDM-1 gene.
Ethics Approval
The authors state that all experimental protocols were approved by the institutional ethics committees of Academy of Military Medical Sciences.
Data Sharing Statement
Genomic and plasmid sequences of strain EcHK001 were deposited in GenBank under accession NZ_PEIL01000000 and NZ_CM008823. The variants of blaTEM-1 and blaACT-17 were deposited in GenBank under accession number MG818166 and MG865974.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by a grant from the National Science and Technology Major Project (no. 2018ZX10712001-002-002), the Beijing Natural Science Foundation (no. 5172029) and the Beijing Noval Program (no. Z181100006218110).
Disclosure
The authors report no conflicts of interest in this work.
References
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054. doi:10.1128/AAC.00774-0919770275
- Liu L, Feng Y, McNally A, Zong Z. blaNDM-21, a new variant of blaNDM in an escherichia coli clinical isolate carrying blaCTX-M-55 and rmtB. J Antimicrob Chemother. 2018;73(9):2336–2339. doi:10.1093/jac/dky22629912337
- Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi:10.1016/S1473-3099(10)70143-220705517
- Naas T, Cuzon G, Bogaerts P, Glupczynski Y, Nordmann P. Evaluation of a DNA microarray (check-MDR CT102) for rapid detection of TEM, SHV, and CTX-M extended-spectrum β-lactamases and of KPC, OXA-48, VIM, IMP, and NDM-1 carbapenemases. J Clin Microbiol. 2011;49(4):1608–1613. doi:10.1128/JCM.02607-1021325547
- Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 2012;7(7):887–902. doi:10.2217/fmb.12.6122827309
- O’Hara CM, Steigerwalt AG, Hill BC, Farmer JJ 3rd, Fanning GR, Brenner DJ. Enterobacter hormaechei, a new species of the family enterobacteriaceae formerly known as enteric group 75. J Clin Microbiol. 1989;27(9):2046–2049.2778068
- Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing enterobacteriaceae: report from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2008;52(2):570–573. doi:10.1128/AAC.01114-0718070960
- Sampaio JL, Ribeiro VB, Campos JC, et al. Detection of OXA-370, an OXA-48-related class D beta-lactamase, in enterobacter hormaechei from Brazil. Antimicrob Agents Chemother. 2014;58(6):3566–3567. doi:10.1128/AAC.02510-1324709259
- Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74(8):2461–2470. doi:10.1128/AEM.02272-0718296538
- Chavda KD, Chen L, Fouts DE, et al. Comprehensive genome analysis of carbapenemase-producing enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. MBio. 2016;7(6):e02093–16. doi:10.1128/mBio.02093-1627965456
- Hoffmann H, Roggenkamp A. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol. 2003;69(9):5306–5318. doi:10.1128/AEM.69.9.5306-5318.200312957918
- Morand PC, Billoet A, Rottman M, et al. Specific distribution within the enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol. 2009;47(8):2489–2495. doi:10.1128/JCM.00290-0919515837
- Aminov RI, Chee-Sanford JC, Garrigues N, et al. Development, validation, and application of PCR primers for detection of tetracycline efflux genes of gram-negative bacteria. Appl Environ Microbiol. 2002;68(4):1786–1793. doi:10.1128/AEM.68.4.1786-1793.200211916697
- Coque TM, Novais Â, Carattoli A, et al. Dissemination of clonally related escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg Infect Dis. 2008;14(2):195. doi:10.3201/eid1402.07035018258110
- Tijet N, Alexander DC, Richardson D, et al. New delhi metallo-β-lactamase, Ontario, Canada. Emerg Infect Dis. 2011;17(2):306. doi:10.3201/eid1702.10156121291614
- Yan -J-J, Wu S-M, Tsai S-H, Wu -J-J, Su I-J. Prevalence of SHV-12 among clinical isolates of klebsiella pneumoniae producing extended-spectrum β-lactamases and identification of a novel AmpC enzyme (CMY-8) in Southern Taiwan. Antimicrob Agents Chemother. 2000;44(6):1438–1442. doi:10.1128/AAC.44.6.1438-1442.200010817689
- CLSI C. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. M100-S24 January. 2014.
- Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii acinetobacter spp. in China. J Antimicrob Chemother. 2012;67(9):2114–2122. doi:10.1093/jac/dks19222604448
- Wick RR, Judd LM, Gorrie CL, Holt KE, Phillippy AM. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi:10.1371/journal.pcbi.100559528594827
- Aziz RK, Bartels D, Best AA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9(1):75. doi:10.1186/1471-2164-9-7518261238
- Carattoli A, Zankari E, García-Fernández A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi:10.1128/AAC.02412-1424777092
- Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50(4):1355–1361. doi:10.1128/JCM.06094-1122238442
- Gardner SN, Slezak T, Hall BG. kSNP3. 0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics. 2015;31(17):2877–2878. doi:10.1093/bioinformatics/btv27125913206
- Richter M, Rossello-Mora R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106(45):19126–19131. doi:10.1073/pnas.090641210619855009
- Ho PL, Li Z, Lo WU, et al. Identification and characterization of a novel incompatibility group X3 plasmid carrying bla NDM-1 in enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect. 2012;1(11):e39. doi:10.1038/emi.2012.3726038408
- Lai CC, Chen CC, Lu YC, et al. Simultaneous three enterobacteriaceae with different bla NDM-1-encoding plasmids in a patient transferred from mainland China to Taiwan. Infect Drug Resist. 2018;11:2555–2560. doi:10.2147/IDR.S17902430573984
- Feng J, Qiu Y, Yin Z, et al. Coexistence of a novel KPC-2-encoding MDR plasmid and an NDM-1-encoding pNDM-HN380-like plasmid in a clinical isolate of citrobacter freundii. J Antimicrob Chemother. 2015;70(11):2987–2991. doi:10.1093/jac/dkv23226260129
- Garcia-Fernandez A, Villa L, Carta C, et al. Klebsiella pneumoniae ST258 producing KPC-3 identified in italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob Agents Chemother. 2012;56(4):2143–2145. doi:10.1128/AAC.05308-1122252815
- Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi metallo-beta lactamase (NDM-1) in enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58(3):147–149.20848811
- Kopotsa K, Osei Sekyere J, Mbelle NM. Plasmid evolution in carbapenemase-producing enterobacteriaceae: a review. Ann N Y Acad Sci. 2019. doi:10.1111/nyas.14223
- Wang Y, Tong MK, Chow KH, et al. Occurrence of highly conjugative IncX3 epidemic plasmid carrying bla NDM in enterobacteriaceae isolates in geographically widespread areas. Front Microbiol. 2018;9:2272. doi:10.3389/fmicb.2018.0227230294321
- Tardif G, Grant RB. Transfer of plasmids from escherichia coli to pseudomonas aeruginosa: characterization of a pseudomonas aeruginosa mutant with enhanced recipient ability for enterobacterial plasmids. Antimicrob Agents Chemother. 1983;24(2):201–208. doi:10.1128/AAC.24.2.2016416159