Abstract
Background and setting
Thailand is one of the highest tuberculosis (TB)-burdened countries. Chiang Rai, the northernmost province of Thailand has high tuberculosis and human immunodeficiency virus (HIV) prevalence and the laboratory workload for TB culture and drug susceptibility testing is increasing.
Objectives
To evaluate the simply modified microscopic-observation drug-susceptibility assay (MODS) in the setting of a developing country.
Methods
In this cross-sectional diagnostic study, a total of 202 sputum samples of clinically diagnosed TB patients were used to test the performance of MODS assay in reference to gold standard BACTEC™ MGIT™ 960 liquid culture system and Ogawa solid culture. Sputum samples were collected from clinically diagnosed TB patients. Culture growth rate and time to culture positivity were compared among three methods. Performance of modified MODS assay was evaluated for detection of mycobacterium drug resistance in reference to MGIT antimicrobial susceptibility test (AST).
Result
Median time to culture positivity by MODS, solid, and liquid culture were 12, 30, and 6 days respectively. Compared to the drug susceptibility test (DST) result of reference liquid culture, the sensitivity and specificity of MODS for detection of multidrug-resistant tuberculosis (MDR-TB) was 85.7% and 97.5% respectively. MODS assay has a positive predicative value of 80% and negative predictive value of 96.5% for isoniazid resistance, 70% and 100% for rifampicin resistance, and 66.7% and 99.1% for MDR-TB.
Conclusion
MODS is a highly effective screening test for detection of MDR-TB.
Introduction
Tuberculosis (TB) is a global disease with 9.5 million reported incident cases in 2009.Citation1,Citation2 By 2009, multidrug-resistant tuberculosis (MDR-TB) prevalence had increased and nearly half a million cases have been reported worldwide.Citation1 Thus, along with TB diagnosis, drug susceptibility testing (DST) has become an urgent clinical requirement for timely constitution of proper and effective TB treatment. However, TB diagnostic and laboratory capacity are still poor in many areas of highly TB-burdened countries.Citation2 It causes a crucial barrier for detection of human immunodeficiency virus (HIV)-associated and drug-resistant tuberculosis.Citation3
Thailand is one of the 22 most TB-burdened countries, having an estimated 130,000 TB cases (182/100,000 population) in 2010.Citation2 It has the highest HIV prevalence in the Southeast Asian region, which was reported to affect 1.4% of the general population in 2009.Citation3 It also has the highest MDR-TB prevalence in the Southeast Asian region, reported as 34.5% (28.2%–41.5%) MDR among previously treated cases.Citation4 The impact of TB-HIV dual epidemics has resulted in a high specimen workload for mycobacterial laboratories that exceeds human and technical resources.
The conventional solid culture method is slow and time consuming despite its high specificity. The highly accurate automated liquid culture method is rather costly for the developing world with a high TB burden. Therefore, a feasible, rapid, and sensitive TB culture and DST method applicable in a resource-challenged, high TB-burdened setting is an urgently needed priority.
Microscopic-observation drug-susceptibility assay (MODS), a noncommercial TB culture and DST method, is recommended by World Health Organization (WHO).Citation5,Citation6 It is cheaper and has shorter turn-around time than conventional gold standard methods.Citation7 It has been validated in Peru and evaluated in some developing countries.Citation6–Citation13 Drug resistance can be detected within 1–2 weeks even with low establishment cost and technical needs.Citation8 Therefore, it can be a reasonable solution for strengthening the TB diagnostic in developing countries.
In this study, we have made two simple modifications to MODS to satisfy completeness, safety, and less human resource needs, while conserving all the original culture technique.Citation14 The first modification was the addition of paranitrobenzoic acid (PNB) for identification of TB and non-TB mycobacteria. The second was modification of the reading process by using a digital microscope kept in a bio-safety cabinet. The objective of this prospective study was to compare the culture growth rate of modified MODS with conventional methods and validate its performance for detection of drug resistance in the practical setting of Chiang Rai, a TB–HIV-endemic area in northern Thailand on the border of Myanmar and Laos.
Patients, materials, and methods
Ethics
The protocol of this study has been approved by Chiang Rai Regional Hospital Ethics Committee Thailand. The study was approved by the Bureau of Tuberculosis, Ministry of Public Health, Thailand.
Study population and setting
The study included clinical specimens according to inclusion criteria and single specimen per case basic. Clinical specimens mean sputum specimens of diagnosed cases at TB clinics of study site hospitals. A total of 202 clinically diagnosed TB patients were recruited prospectively at TB clinics in 17 hospitals in Chiang Rai province from January 2010 to June 2010. All the MODS assay, automated liquid culture by BACTEC™ MGIT™ 960 (Becton, Dickinson and Company, Franklin Lakes, NJ) system and Ogawa solid culture were done at the mycobacterial laboratory of Chiang Rai Regional Hospital. A DST reference test by MGIT 960 was done at the National TB Reference Laboratory, a WHO Supranational Laboratory (SRL) in Bangkok serving four countries in Southeast Asia.
Sputum samples were collected by the time TB diagnosis was made clinically by physicians. Only the sputum samples meeting the following inclusion criteria were used for the study: (1) all pulmonary TB cases according to WHO criteria; (2) both acid-fast bacillus (AFB) smear positive and negative cases; and (3) both HIV-positive and -negative cases. Exclusion criteria were: (1) extrapulmonary TB; (2) age under 18 years; (3) sputum specimen collected more than 7 days after starting the anti-TB drugs; (4) sputum sample less than 2 mL; and (5) sputum sample transported to mycobacterial laboratory without proper packaging, such as released screw cap of sputum container inside the plastic bag with zip-top.
Storage of the sputum sample
Sputum samples were properly stored at 2°C–8°C in a household refrigerator after collection. Samples were cultured within 7 days of collection. The median duration of storage was 4 days.
MODS
All the MODS culture and drug susceptibility tests were carried out by the same technician who had more than 10 years of mycobacterial culture experience and a self-learned skill for MODS assay developed from MODS guidelines.Citation14
An inverted microscope modified with a digital camera connected to a personal computer (PC) was used to examine 24-well MODs culture plates. Six culture wells were used for each sample: two wells for culture, one well with PNB, one well with isoniazid (INH), and another well with rifampicin. The Middlebrook 7H9 Broth (Sigma-Aldrich, St Louis, MO) culture was used. All the steps of MODS assay developer’s guideline were followed except the amount of decontaminated sputum used to inoculate in each well of a culture plate was reduced.Citation14,Citation15 We used 500 μL instead of 750 μL. We have added two modifications as follows.
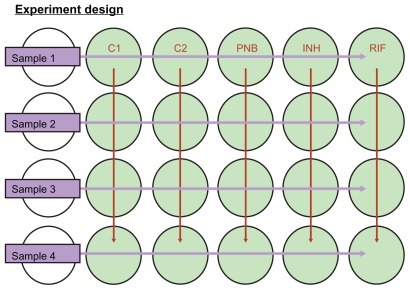
Modification 1: the addition of PNB test for identification of non-TB mycobacteria (NTM) PNB can differentiate Mycobacterium tuberculosis complex and NTM.Citation16 To obtain the culture, identification, and DST results from a one MOD package at a time, we tested the addition of a PNB culture well in each line for the identification of M. tuberculosis and NTM. All five wells in one line were used for one sputum sample (). One well in each line identified TB and NTB by using PNB. The PNB concentration in the well was 0.5 mg/mL.Citation16
Figure 1 Design of MODS culture plates containing one well for PNB.
Abbreviations: C1, C2 are culture wells 1 and 2; MODS, microscopic-observation drug-susceptibility assay; PNB, para-nitrobenzoic acid identification well; INH, isoniazid well for INH susceptibility test; RIF, rifampicin susceptibility test well.
Modification 2: Use of computer-assisted digital camera for reading phase to improve biosafety and to reduce human resource need for frequent reading. The modified microscope and culture plates were kept in a biosafety-level 2 A cabinet (SCV-1305EC2AB [class II B]; Hitachi, Tokyo, Japan) as it is smaller than the original microscope for MODS. The reading was not necessarily to perform by direct observation through the eyepiece of laboratory technician. The inverted microscope was modified by connecting to a digital microscope with 50× magnification on its viewer side. A Dino-Lite PLUS AM313T Digital Microscope (AnMo Electronics Corporation, New Taipei City, Taiwan) () was used for this modification. The digital microscope was connected to a computer. We used 50× magnifications to capture an image of one whole MODS culture well at a time. One whole plate could be read with 24 shots.
Reading and recording
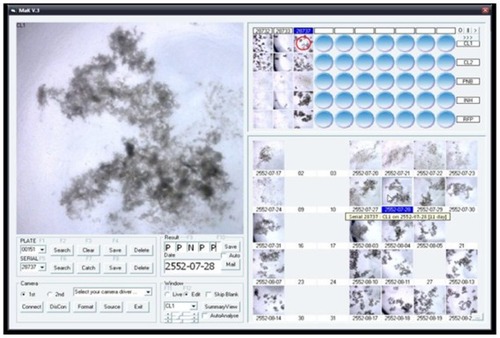
It was compulsory to read the control plate before reading each batch of MODS culture sample plates. All the samples of a culture batch were discarded if the control plate result was not valid. A positive result was decided by seeing growth in digital images on successive days (). A positive result was decided by observation of the appearance of new growth compared with a clear culture well in the digital images taken previously. New appearance was defined as any pattern of growth. Negative culture was made sure by absence of growth after 35 days of reading.
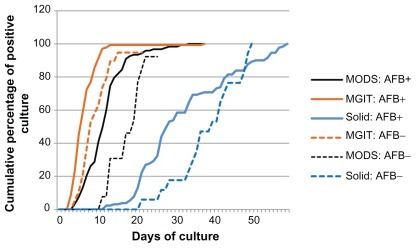
Figure 4 Cumulative culture positive rates of MODS assay, automated liquid culture by BACTEC™ MGIT™ 960 (Becton, Dickinson and Co, NJ, NY) and solid culture by Ogawa media.
Abbreviations: AFB+, acid fast bacilli positive sputum samples; AFB−, acid fast bacilli negative sputum samples; MODS, microscopic -observation drug susceptibility assay.
Only after the growth had been seen in C1 and C2 wells, PNB-, isoniazid-, and rifampicin-containing wells were checked compulsorily (see ). Each culture plate was read by keeping an inverted light microscope inside the biosafety-level 2A cabinet. Results were read every day except during holidays and recorded in the logbook and computer software developed by the project. Positivity was judged by the same technician looking at pictures captured by the system and shown on the laptop computer monitor. Each specimen was determined by two technologists. The consensus between two opinions was checked by a statistician and unmatched results were repeated and concurred between two technologists. MODS culture results were judged independently, and MGIT results were blinded for individual specimens.
Drug susceptibility tests
For the purposes of quality control, a line of negative control wells was used in every plate. A positive control was prepared with a separate plate as a daily routine of MODS procedure. A negative control column of four wells on each plate was made to be sure that cross-contamination was not occurring (see ). The positive controls were run on a separate plate at the end of every day to check that (a) the media supported growth normally, and (b) the concentrations of rifampicin (1 μg/mL) and isoniazid (0.1 μg/mL) were correct. Citation17 INH concentration 0.1 μg/mL and rifampicin 1 μg/mL solution were used in the drug susceptibility test.
Solid and liquid culture
MGIT 960 automated liquid culture method
This is the gold standard and reference method in this study. The manufacturer’s instruction was strictly followed for the BACTECTMMGIT 960TM automated TB liquid culture system. The culture inoculation was done at the mycobacterial laboratory of Chiang Rai Regional Hospital. The MGIT AST tests were performed at National TB Reference Laboratory of Thailand in Bangkok.
Ogawa solid culture
This is the routine culture method currently used in Chiang Rai. Solid cultures were inoculated in parallel with MODS and MGIT at the mycobacterial laboratory, Chiang Rai Regional Hospital. The routine protocol of the Chiang Rai Laboratory was followed for solid culture in Ogawa media.
Statistical analysis
Stata software (version 11; StataCorp LP, College Station, TX) was used for statistical data analysis. The Mann–Whitney U test was used for comparing median time to positive culture growth between MODS versus each two methods. DST performance of MODS assay was validated in reference to the gold standard MGIT 960 and parameters were computed by Stata software.
Results
Altogether there were 202 sputum samples in this study, which comprised 72.77% of the smear AFB-positive sputum samples. shows the characteristics of TB patients in the study population. Most of the cases were new cases (88.12%). Moreover, 16.34% of the studied participants were HIV-coinfected.
Table 1 Characteristic of the pulmonary TB patients at the study entry
Time to culture positivity
Median time to culture positivity was 12 days by MODS, 6 days by MGIT 960 automated liquid culture, and 30 days by Ogawa solid culture. MODS assay has a shorter median time to culture positivity than solid culture, but longer than the automated liquid culture (). The contamination rate of MODS is higher than that of liquid and solid culture. The contamination rates of the MODS, MGIT, and Ogawa methods were 15.8% (32 samples), 11.9% (24 samples), and10.9% (22 samples), respectively. Contamination rate in smear positive samples were 3.4% in MGIT, 6.12% in solid culture and 10.2% in MODS whereas contamination rate in smear negative sample were 23.64% in solid culture, 30.91% in MODS and 34.55% in MGIT. Moreover, culture growth rates were 71.14% in HIV-positive patients and 82.35% in HIV-negative patients.
Table 2 Median time to culture growth in three different TB culture methods: Ogawa solid culture, BACTEC™ MGIT™ 960 liquid culture system and MODS assay in sputum samples of clinically diagnosed TB patients, Chiang Rai, Thailand 2010
Performance of MODS for detection of drug resistance in clinical sample
The performance of the MODS assay in clinical sample was compared with the reference MGIT 960 result for detection of mycobacterial drug resistance (). Of the 129 cases tested for rifampicin resistance, the MODS assay was in agreement with the MGIT 960 system in 126 cases. The positive predicative value (PPV) was 70% (95% confidence interval [CI]: 34.8–93.3) and the negative predictive value (NPV) was 100% (95% CI: 96.9–100). Of the 130 cases tested for INH resistance, MODS assay was in agreement with the MGIT 960 AST result in only 123 cases. The PPV of MODS for INH resistance was 80% (95% CI: 51.9–95.7) and the NPV was 96.5% (95% CI: 91.3–99%). Of the 126 specimens tested for MDR, MODS assay was in agreement with the MGIT 960 AST result in 122 cases (). The PPV for detection of MDR-TB was 66.7% (95% CI: 29.9–92.5) and NPV was 99.1% (95% CI: 95.3–100) ().
Table 3 DST performance result of MODS in reference to BACTEC™ MGIT™ 960 automated liquid culture for detection of drug-resistant M. tuberculosis in sputum
Table 4 Performance characteristic MODS assay compared to reference BACTEC™ MGIT™ 960 automated liquid culture for detection of drug-resistant M. tuberculosis in sputum, Chiang Rai, Thailand, 2010
Negative predicative values in detection of INH resistance, rifampicin resistance, and MDR-TB were consistently high. Positive likelihood ratio for detection of rifampicin resistance (40.7), INH resistance (28.5), and MDR-TB (34) indicated that MODS assay would be an effective DST for ruling out the mycobacterial drug resistance ().
Identification of mycobacterium species by PNB
In this study, MODS assay could identify only one case of NTM infection. Reference method PNB on L-J media identified six NTM cases out of 202. MODS results agreed with reference method in 98.28% (114/116) of samples tested for NTM idetification, but there was no positive result agreed between two methods.
Discussion
A TB culture method which has fast turnaround time and a rapid DST result with a reasonable price is an operational need in the TB control program in Chiang Rai and many areas with similar tuberculosis burdens across the world. MODS assay is a reasonable candidate by existing evidences.
Two meta-analyses have reported that MODS can outperform other non-commercial methods of DST.Citation5,Citation18 However, Banerjee et alCitation19 point out the weakness of meta-analyses as lack of studies in developing countries. In the current study, we have tested the performance of MODS in a study population entirely composed of clinically diagnosed TB patients’ sputum samples. This study was a prospective study conducted in a real clinical setting in a highly TB-burdened area with limited resources. All processes involved with sputum sample collection, sputum specimen transport, storage, and processing were conducted through a routine TB control program service at 16 district hospitals and a regional hospital. Therefore, our study finding is expected to provide existing and growing evidence for TB diagnostic pipe lines following research from these routine processes.
The culture growth rate and time to culture positivity were compared to reference liquid culture and Ogawa solid culture. We have seen faster median time to culture growth of MODS assay than in Ogawa solid culture. However, MODS assay is longer than automated liquid culture. A community based study in Peru has demonstrated that the growth rate of MODS is faster than that of the MGIT liquid culture at 7 days and 13 days, respectively.Citation7 Clinical studies from Ethiopia reported that the median turn-around time of smear AFB-positive sputum samples by MODS culture was 9 days and the growth rate was 96.9% (n = 262).Citation8 Our finding about the culture growth rate and time to culture positivity of MODS is rather different from those previous findings. In the current study of simply modified MODS assay, the culture-growth rate of sputum smear AFB-positive samples was 82.99% and median time to detect culture growth was 12 days (see ).
These culture performance results may be due to the use of a relatively smaller amount of decontaminated sputum (500 μL) for inoculating in Middlebrook 7H9 Broth or, due to the modification of using digital microscope and computerized recording. Because the lens resolution is not very high, the colony growth may not be obviously seen in the early days of bacterial growth. The modification has improved safety, whereas in the earlier days of MODS there were biohazard concerns.Citation20 Moreover, the tedious daily workload of checking the culture plates was also a major weak point.Citation20
We have modified MODS to solve these issues. The reading process of MODS was modified by using a computer assisted digital camera. The potential advantages are: (1) all the culture material and microscope can be kept in a bio-safety cabinet, minimizing the chance of biohazard to laboratory workers, (2) the morphological changes in MODS culture wells can be checked more frequently with less human effort, (3) the morphology of culture wells can be compared day by day, (4) the culture results can be stored in digital copy, enabling sharing so that technical experts can review the results. In this study we used daily recording of the morphological changes in culture wells.
During the current study, similar kinds of reading system were reported by Zimic et al.Citation21 However, their adjustable magnification was different from ours. Zimic et al used an artesenal inverted microscope with 60–100× amplifier for direct visualization of MODS cultures which aimed to capture a cording image. We used 50× magnifications to capture an image of one whole well at a time. It made the reading process faster. Identification by using PNB well was tested but did not work.
The diagnostic performance parameters of the MODS indicated that it could be a highly valuable screening tool for identifying MDR-TB. Negative predictive values of the test were consistently high for INH resistance, rifampicin resistance, and MDR-TB. In our study, a higher concentration of INH 0.1 μg/mL was used. Positive and negative control wells were carefully checked for quality of DST. However, area under the relative operating characteristic (ROC) curve for INH resistance was rather small at 0.862 compared to other previous studies.Citation5 Likewise, the meta-analysis by Bwanga et alCitation5 has reported the good performance of MODS despite variable pooled sensitivity and specificity in detection of INH resistance. In the current study we found that modified MODS had lower sensitivity in detection of INH resistance than the pooled sensitivity reported by that meta-analysis.Citation5 However, sensitivity is high for detection of rifampicin resistance which is a good proxy indicator of MDR-TB. Therefore, it would be a better option to use the MODS assay for screening drug-resistant cases and confirm the positive results by using a more specific test. MODS is a nonpatented, low cost and low technology culture method. With our modification of reading process it would be a low workload culture method and therefore usable in resource-challenged settings.
Limitation of the study
We could not compare the time to result, total time taken from taking sample to giving culture and DST result back to clinician, in each method. The reference methods of DST were carried out at the National Reference Laboratory in Bangkok. Median time to result is 70 days for Chiang Rai province. In this study MODS assay has culture turnaround time of 12 days but we did not deliver the result to clinicians. So, we did not compare the time to result between MODS and reference method.
In our sample, 42% of the study population were non-Thai patients. The distance of hill tribe villages from the TB laboratory, the language barrier, and health illiteracy among such patients were also limitations in practice to get the sputum samples which met the eligibility criteria of the study.
We have calculated the sample size necessary for power of 80% with 95% CI based on prevalence of INH resistance in Chiang Rai and found it was 250 samples. After application of eligibility criteria, only 202 samples remained for analysis. To keep the internal validity of the study, we sacrificed some samples.
Conclusion
In a highly TB-burdened area like Chiang Rai, Thailand, a rapid, sensitive, and affordable culture method is operationally necessary. After applying a modification for better safety and averting human resource need by use of computer-assisted reading, MODS would be a safer and effective culture and DST method for screening multidrug- resistant tuberculosis.
Authors' contribution
Norio Yamada, Myo Nyein Aung, Saiyud Moolphate and Boonchai Chaiyasirinroje designed the study. Myo Nyein Aung developed the research proposal which was edited by Norio Yamada, Satoshi Mitarai, Somsak Rienthong, and Dhanida Rienthong in October 2009. Myo Nyein Aung and Boonchai Chaiyasirinroje developed the standard operating procedures for the study in November 2009. Boonchai Chiyasirinrote carried out all the TB cultures and MODS procedures with the help of Oranuch Nampaisan. Supalert Nedsuwan, Wiravoot Sangchun, and Narin Suriyon contributed to the research process and collection of data. Saiyud Moolphate and Myo Nyein Aung analyzed the data and interpreted the results. Myo Nyein Aung wrote the manuscript. Norio Yamada, Somsak Rienthong, Yuthichai Kasetjaroen, Dhanida Rienthong, Satoshi Mitarai and Boonchai Chaiyasirinroje critically reviewed, revised, and amended the manuscript. All the authors contributed their comments which have been amalgamated into the final manuscript by Myo Nyein Aung.
Acknowledgments
This work was sponsored partly by Research Institute of Tuberculosis, Japan. David AJ Moore is acknowledged for his technical advice. Hideki Yani is acknowledged for his suggestions and research protocol comments. Nurses, medical technologists and TB clinic staff of 17 hospitals in Chiang Rai province are acknowledged for their cooperative assistance.
Disclosure
The authors declare no conflict of interest in this work.
References
- World Health Organization (WHO)Global tuberculosis control2010 Available from: http://www.who.int/tb/publications/global_report/2010/en/index.htmlAccessed August 8, 2011
- World Health Organization (WHO)Global tuberculosis control2011 Available from: http://www.who.int/tb/publications/global_report/en/Accessed October 24, 2011
- World Health Organization (WHO)TB diagnostics and laboratory strengthening2011 Available from: http://www.who.int/tb/laboratory/en/Accessed August 8, 2011
- World Health Organization (WHO)Regional Report 2011: Tuberculosis control in Southeast Asain Region2011 Available from: http://www.searo.who.int/LinkFiles/TB_Day_Kit_TB_Annual_Report_2011pdfAccessed June 8, 2011
- BwangaFHoffnerSHaileMJolobaMDirect susceptibility testing for multi drug resistant tuberculosis: a meta-analysisBMC Infect Dis200996719457256
- MooreDAMendozaDGilmanRHMicroscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settingsJ Clin Microbiol200442104432443715472289
- MooreDAEvansCAGilmanRHMicroscopic-observation drug-susceptibility assay for the diagnosis of TBN Engl J Med2006355151539155017035648
- ShiferawGWoldeamanuelYGebeyehuMGirmachewFDemessieDLemmaEEvaluation of microscopic observation drug susceptibility assay for detection of multidrug-resistant Mycobacterium tuberculosisJ Clin Microbiol20074541093109717251409
- MichaelJSDaleyPArmstrongLProspective evaluation of microscopic observation drug-susceptibility (MODS) assay for the diagnosis of active tuberculosis (TB) in India – preliminary analysisInt J Infect Dis200812Suppl 1e324
- MelloFCQAriasMSRosalesSClinical evaluation of the microscopic observation drug susceptibility assay for detection of Mycobacterium tuberculosis resistance to isoniazid or rifampinJ Clin Microbiol200745103387338917699652
- EjiguGSWoldeamanuelYShahNSGebyehuMSelassieALemmaEMicroscopic-observation drug susceptibility assay provides rapid and reliable identification of MDR-TBInt J Tuberc Lung Dis200812333233718284841
- AriasMMelloFCPavónAClinical evaluation of the microscopic observation drug-susceptibility assay for detection of tuberculosisClin Infect Dis200744567468017278058
- LimayeKKanadeSNatarajGMehtaPUtility of microscopic observation of drug susceptibility (MODS) assay for Mycobacterium tuberculosis in resource constrained settingsIndian J Tuberc201057420721221141339
- CoronelJRoperMCaviedesLMooreDMODS. A user guideMicroscopic-observation drug-susceptibility assay2008 Available from: http://www.modsperu.org/MODS_user_guide.pdfAccessed October 12, 2009
- MooreDAJMODS laboratory accreditation2008 http://www.modsperu.org/MODS_laboratory_validation.pdfAccessed October 12, 2009
- GiampagliaCMSMartinsMCChimaraEDifferentiation of Mycobacterium tuberculosis from other mycobacteria with rho-nitrobenzoic acid using MGIT960Int J Tuberc Lung Dis200711780380717609058
- MooreDAJMODS QC question from TB HIV research consortium ThailandAungMN2009
- MinionJLeungEMenziesDPaiMMicroscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysisLancet Infect Dis2010101068869820813587
- BanerjeeYTaranikantiVBayoumiRAssays for drug resistant tuberculosis in high burden countriesLancet Infect Dis201111316116221371651
- PalominoJCMartinAPortaelsFMODS Assay for the Diagnosis of TBN Engl J Med2007356218818917215539
- ZimicMVelazcoACominaGDevelopment of low-cost inverted microscope to detect early growth of Mycobacterium tuberculosis in MODS culturePLoS ONE201053e957720351778