Abstract
Purpose
Although immune dysfunction has been investigated in adult septic patients, early immune status remains unclear. In this study, our primary aim was to assess early immune status in adult patients with sepsis stratified by age and its relevance to hospital mortality.
Patients and Methods
A post hoc analysis of a multicenter, randomized controlled trial was conducted; 273 patients whose immune status was evaluated within 48 hours after onset of sepsis were enrolled. Early immune status was evaluated by the percentage of monocyte human leukocyte antigen-DR (mHLA-DR) in total monocytes within 48 hours after onset of sepsis and it was classified as immunoparalysis (mHLA-DR ≤30%) or non-immunoparalysis (>30%). Three logistic regression models were conducted to explore the associations between early immunoparalysis and hospital mortality. We also developed two sensitivity analyses to find out whether the definition of early immune status (24 hours vs 48 hours after onset of sepsis) and immunotherapy affect the primary outcome.
Results
Of the 181 elderly (≥60yrs) and 92 non-elderly (<60yrs) septic patients, 71 (39.2%) and 25 (27.2%) died in hospital, respectively. The percentage of early immunoparalysis in the elderly was twice of that in the non-elderly patients (32% vs 16%, p=0.006). For the elderly, hospital mortality was higher in the immunoparalysis ones than the non-immunoparalysis ones (53.4% vs 32.5%, p=0.009). But there was no significant difference in hospital mortality between immunoparalysis non-elderly patients and non-immunoparalysis non-elderly ones (33.5% vs 26.0%, p=0.541). By means of logistic regression models, we found that early immunoparalysis was independently associated with increased hospital mortality in elderly, but not in non-elderly patients. Sensitivity analysis further confirmed the definition of early immune status and immunotherapy did not affect the outcomes.
Conclusion
The elderly were more susceptible to early immunoparalysis after onset of sepsis. Early immunoparalysis was independently associated with poor prognosis in elderly, but not in non-elderly patients.
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.Citation1 After decades of effort, the mortality rate of sepsis has been decreasing; however, the absolute number of deaths is likely to continue to increase as the incidence of sepsis keeps rising.Citation2,Citation3 Although sepsis has been studied for decades, its pathogenesis remains unclear. A post-mortem study of septic patients demonstrated that patients who died of sepsis were associated with widespread severe immunosuppression.Citation4 Subsequently, numerous studies have demonstrated that severe immunosuppression is the main cause of high mortality in septic patients.Citation5,Citation6
Monocyte human leukocyte antigen-DR (mHLA-DR) is a key biomarker to assess immune status. Our previous study and other studies have revealed that both mHLA-DR and dynamic change of mHLA-DR were associated with poor prognosis in patients with sepsis.Citation7–Citation9 The percentage of mHLA-DR lower than or equal to 30% was widely accepted as immunoparalysis or severe immunosuppression.Citation9–Citation11 Furthermore, mHLA-DR has been applied to select severe immunosuppressed septic patients in the study of immunostimulant.Citation12 Therefore, the use of mHLA-DR as a biomarker to assess immune status in septic patients is supported by previous research.
It is well known that the immune function declines with age, so the elderly may be more susceptible to early immunoparalysis than younger patients.Citation13,Citation14 Elderly patients are more vulnerable to sepsis during hospitalization, and the mortality of elderly septic patients was higher than that of younger patients.Citation15–Citation17 Several studies found immunoparalysis in elderly patient increased the risks of death and secondary infection in the course of sepsis.Citation18,Citation19 Although immunoparalysis is associated with poor outcome in elderly septic patients, the timing of immunoparalysis remains unclear. Recently, Muszynski et al revealed that critically ill children with sepsis had immunoparalysis/severe immunosuppression from early stage (within 48 hours after onset of sepsis).Citation20 However, early immune status of elderly patients with sepsis has not been demonstrated yet.
In our current study, we aimed to assess early immune status in adult patients with sepsis stratified by age and determine their relevance to hospital mortality.
Patients and Methods
Study Design
The ETASS (Efficacy of Thymosin Alpha 1 for Severe Sepsis, ETASS) study was a multi-centre, randomized controlled study comparing the effect of thymosin alpha 1 (Tα 1) vs placebo in patients with severe sepsis.Citation21 A full description of the methods of the ETASS study, including the full study protocol, case report form, sample size, quality control, and main results can be found in the original paper.Citation21 In the ETASS study, severe sepsis was defined as the presence of a proven or suspected infection in at least one site, two or more signs of a systemic inflammatory reaction, and at least one acute sepsis-related organ dysfunction. Therefore, the term ‘severe sepsis’ in our previous study is approximately equal to the definition of sepsis in Sepsis 3.0.Citation1 Unless otherwise specified, ‘sepsis’ was used to replace “severe sepsis” in this study. Immunotherapy in the study was defined as patients have received at least one dose of thymosin alpha 1 in the ETASS study.
In the present study, the primary object was to assess early immune status in adult septic patients and its relevance to hospital mortality. All adult septic patients were divided into elderly and non-elderly group. According to China Country Assessment Report on Ageing and Health from the World Health Organization in 2015, elderly was defined as aged 60 years or over.Citation22 According to previous study, early immune status was defined as the immune status within 48 hours after onset of sepsis.Citation20 Immune status was measured by the expression of mHLA-DR because of its proven value in septic patients.Citation23 The detail method of mHLA-DR measurement had been described in Supplementary File 1. Therefore, only those patients with mHLA-DR measured within 48 hours after onset of sepsis were enrolled in this study (273/361). To assess different immune status, we divided early immune status into two categories: immunoparalysis (≤30%) and non-immunoparalysis (mHLA-DR > 30%).Citation10,Citation24 Changes in immune status were expressed by the value change in mHLA-DR on day 3 compared with the first measurement. According to our previous study, a change of mHLA-DR value of 4.8% on day 3 compared to initial measurement allowed discrimination between survivors and non-survivors.Citation8 Thus, the value change over 4.8% was defined as immune status improvement, and equal or less than 4.8% was defined as immune status non-improvement.
In addition, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score with or without the age component and the Sequential Organ Failure Assessment (SOFA) score were recorded during the first 24 hours after onset of sepsis. When using dichotomous variable for logistic analysis, high SOFA score was defined as the SOFA score more than 8 according to the previous study.Citation25 Other clinical or laboratory parameters were also recorded at the same time. For prognosis, we assessed hospital mortality, 28-day mortality, ICU mortality, length of ICU stay and mechanical ventilation (MV) support days.
Because we did a secondary selection of patients from the ETASS study, considering of the possible selection bias, the baseline clinical characteristics between included (273/361) and excluded (88/361) patients were compared.
Statistic Methods
Continuous variables with normal distribution were summarized as mean (standard deviation, SD) and compared by t-test; while non-normal distributed variables were described as median (interquartile range, IQR) and compared by the Wilcoxon rank sum test. Categorical data were presented as frequencies and percentages, and compared with Chi-squared tests. Logistic regression analysis was used to evaluate the association between early immunoparalysis and hospital mortality, stratified by age. In model 1, the crude odd ratios (ORs) and 95% confidence intervals (CIs) were calculated by entering only the variable for early immunoparalysis. In model 2, data were adjusted for sex, age, pre-existing condition, immunotherapy, and SOFA score. Then, we further adjusted for the dichotomous variable of immune status improvement in model 3. We also developed two sensitivity analyses to find out whether the definition of early immune status (24 hours vs 48 hours after onset of sepsis) and immunotherapy affect the primary outcome. A p-value <0.05 (two tailed) was considered statistically significant. All analyses were conducted using IBM SPSS software version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline Clinical Characteristics of Adult Patients with Sepsis
Of the 361 patients, 273 patients were enrolled in this study, including 181 elderly and 92 non-elderly (). There were no significant differences in most clinical variables between the included and excluded patients except for the prevalence of immunotherapy (54/88 vs 127/273, p=0.016). The excluded patients were more likely to receive immunotherapy than those included (Supplementary Table 1). Baseline clinical characteristic data of included patients are shown in . The mean APACHE II scores without the age component were 18.3 (7.4) in the elderly and 17.5 (6.9) in the non-elderly. The mean SOFA scores were 8.0 (3.9) in the elderly and 7.5 (3.6) in the non-elderly. There was no difference in the severity of sepsis between the elderly and non-elderly groups, but the elderly group had a higher percentage of pre-existing conditions than non-elderly (85.1% vs 70.7%, p=0.006).
Table 1 Baseline Clinical Characteristics of Adult Patients with Sepsis
Outcomes of Adult Patients with Sepsis
In our study, 71 (39.2%) elderly and 25 (27.2%) non-elderly died in hospital (). The elderly patients received prolonged days of mechanical ventilation support than the non-elderly patients (6.2 vs 4.8; p=0.009), but there was no difference in the length of ICU stay (Days: 10.3 vs 9.0; p=0.103) between elderly and non-elderly patients ().
Table 2 Outcomes of Adult Patients with Sepsis
Immune Status in Adult Patients with Sepsis
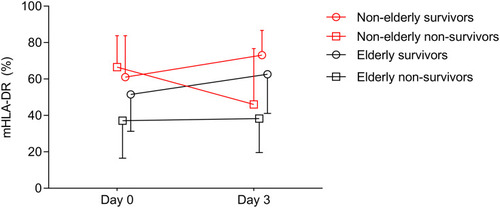
The percentage of mHLA-DR expression in elderly non-survivors was lower than that of elderly survivors on day 0 (Median: 37.1, interquartile range [IQR]: 16.5~71.4 vs 51.5, IQR: 31.3~77.1) and day 3 (38.3, IQR: 19.6~70.4 vs 62.6, IQR: 41.1~82.9) (). The mHLA-DR of non-elderly non-survivors was similar to that of non-elderly survivors on day 0 (66.5, IQR: 36.5~83.8 vs 61.0, IQR: 39.3~83.8). However, the mHLA-DR of non-elderly non-survivors decreased rapidly on day 3 (46.0, IQR: 20.5~76.7), while that of non-elderly survivors increased slightly (73.1, IQR: 49.4~86.7).
Figure 2 The changes of mHLA-DR in survivors and non-survivors in different age. The mHLA-DR in elderly non-survivors was lower than that of survivors on day 0 and day 3. However, the mHLA-DR of non-elderly non-survivors was similar to that of survivors on day 0, but mHLA-DR decreased rapidly in non-elderly non-survivors on day 3.
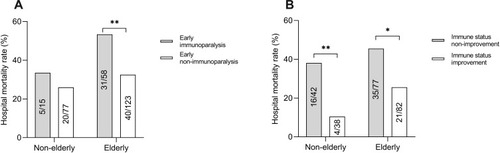
The percentage of early immunoparalysis (mHLA-DR ≤ 30%) in the elderly was twice of that in the non-elderly (32% vs 16%, p=0.006) patients (). The hospital mortality of immunoparalysis elderly patients were higher than that of non-immunoparalysis ones (53.4% vs 32.5%, p=0.009), but there was no significant difference in hospital mortality in the non-elderly between the immunoparalysis and non-immunoparalysis (33.5% vs 26.0%, p=0.541) (). To detect the change of immune status, 239 septic patients (80 non-elderly and 159 elderly) whose mHLA-DR was measured on day 3 were included. In these patients, about half of the elderly (82/159, 52%) and the non-elderly (38/80, 47%) patients had immune status improvement on day 3 (). We also found that patients with immune status improvement on day 3 had lower hospital mortality than those without improvement in both the elderly (21/82 vs 35/77) and the non-elderly (4/38 vs 16/42) groups ().
Figure 3 Early immune status and change of immune status in patients with sepsis. (A) The percentage of early immunoparalysis in elderly patients was twice of that of non-elderly patients (32% vs 16%, p=0.008). (B) About half of elderly (82/159, 52%) and non-elderly (38/80, 47%) patients had immune status improvement on day 3 (**p value <0.01).
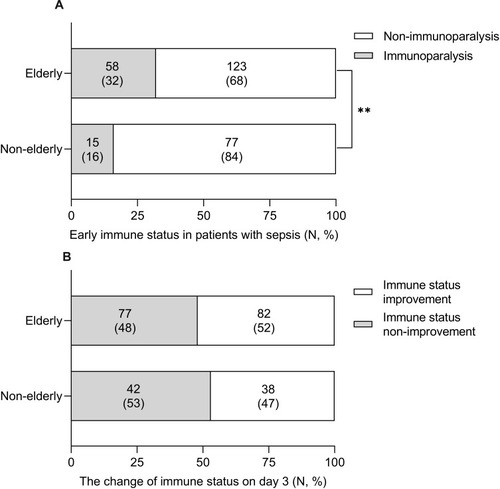
Figure 4 Immune status and hospital mortality. (A) The hospital mortality of immunoparalysis elderly patients were higher than that of non-immunoparalysis ones (31/58 vs 40/123), but there was no significant difference in hospital mortality in the non-elderly between immunoparalysis and non-immunoparalysis (5/15 vs 20/77). (B) Septic patients with immune status improvement on day 3 had lower hospital mortality than patients with non-improvement in both the elderly and the non-elderly groups (*p value <0.05; **p value <0.01).
Early Immunoparalysis Was Associated with Increased Mortality in Elderly Patients
In univariate logistic regression analysis (model 1), early immunoparalysis was associated with increased hospital mortality in elderly (ORs: 2.382; 95% CIs: 1.257~4.514; p=0.008), but not in non-elderly (ORs: 1.425; 95% CI 0.434–4.676; p=0.559) patients (). After variables of age, sex, pre-existing conditions, immunotherapy and SOFA score being adjusted in model 2, early immunoparalysis was independently associated with increased hospital mortality in elderly (ORs: 2.257; 95% CI 1.130–4.506; p=0.021), but not in non-elderly (ORs: 1.074; 95% CI 0.242–4.763; p=0.925) patients (). Then, we conducted another model (model 3) to add the immune status improvement on day 3 into model 2. In model 3, early immunoparalysis was also only associated with increased hospital mortality in elderly (ORs: 2.684; 95% CIs: 1.224~5.883; p=0.014), but not in non-elderly (ORs: 1.604; 95% CI 0.351–7.331; p=0.542) patients (). In addition, we also found that immune status improvement on day 3 was associated with reduced hospital mortality in both elderly (ORs: 0.335; 95% CI 0.159–0.706; p=0.004) and non-elderly (ORs: 0.131; 95% CI 0.029–0.584; p=0.008) patients.
Table 3 Early Immunoparalysis Was Associated with Increased Hospital Mortality in Elderly Patients
The definition of early immune status is a controversial issue, and it was defined as immune status within 48 hours after onset of sepsis in our study. To evaluate whether the definition of early immune status was driving our results, we performed a sensitivity analysis in which early immune status was measured within 24 hours after onset of sepsis. Consistently, early immunoparalysis was independently associated with increased hospital mortality in elderly patients (ORs: 5.507; 95% CIs: 1.497~20.258; p=0.010), but not in non-elderly (ORs: 1.536; 95% CI 0.236–10.023; p=0.654) ones (Supplementary Figure 1A). Then, we also performed another sensitivity analysis to further confirm whether immunotherapy affected our results. We selected patients with sepsis without immunotherapy, and the results were consistent with above that early immunoparalysis was independently associated with increased hospital mortality in elderly patients (ORs: 3.638; 95% CIs: 1.116~11.852; p=0.032), but not in the non-elderly (ORs: 7.002; 95% CI 0.678–72.325; p=0.102) ones (Supplementary Figure 1B).
Discussion
The present study is, to the best of our knowledge, the first to evaluate early immune status in elderly septic patients. Our study has a few important findings. First, our data indicated the elderly had greater risk of developing immunoparalysis within 48 hours after onset of sepsis, and the rate was twice of that of the non-elderly. Second, early immunoparalysis in the elderly was associated with poor prognosis, but not in non-elderly patients. Third, immune status improvement was associated with reduced mortality in both the elderly and the non-elderly.
Immunoparalysis/severe immunosuppression was associated with increased mortality and secondary infection, prolonged length of ICU stay and aggravated organ dysfunction in adult and children with sepsis.Citation19,Citation20,Citation26,Citation27 However, the initiation of immunoparalysis remains controversial, so it is difficult to determine when to start immune monitoring and administer immunotherapy. A recent study demonstrated that septic paediatric patients were severe immunosuppressed within the first 48 hours after sepsis, and such early immunoparalysis/severe immunosuppression was significantly associated with prolonged organ dysfunction time.Citation20 In our study, about one-third of the elderly (58/181) had early immunoparalysis within 48 hours, and more than half of the immunoparalysis patients (31/58) died in hospital. All these results displayed that the elderly were more vulnerable to immune dysfunction early after onset of sepsis. Therefore, the immune status of elderly patients needs to be monitored from the early stage of sepsis.
Consistent with previous studies, we found elderly patients with sepsis have higher mortality than the non-elderly.Citation2,Citation28 However, it appears that it is not the age per se but rather the associated factors, such as severity of illness or immune status, contribute to the increased mortality rate.Citation29 In our study, the severity of sepsis (SOFA score and APACHE-Ⅱ score without an age component) was similar in the elderly and non-elderly, but the percentage of early immunoparalysis in the elderly was twice of that in the non-elderly patients. We also found that more than half of the immunoparalysis elderly (31/58, 53%) died in hospital, but only one-third of the non-elderly (5/15, 33%) died during hospitalization. Furthermore, immunoparalysis was associated with increased hospital mortality in the elderly, but not in non-elderly patients. That is to say, immunoparalysis may be responsible for the increased mortality in elderly patients with sepsis.
Numerous studies revealed that mHLA-DR can be used to predict prognosis and to select septic patients who needed immunostimulant.Citation8,Citation9,Citation30 Monneret et al previously found that mHLA-DR decreased in septic patients on days 1–2 and days 3–4 after onset of sepsis, but only low mHLA-DR (≤30%) on days 3–4 was independently associated with increased 28-day mortality in patients with sepsis.Citation10 However, Perry et al reported a different result that mHLA-DR on days 1–3 cannot help to predict outcome in sepsis.Citation31 In Perry’s research, the median age of septic patients was about 56 with a range from 20 to 84, while in Monneret’s study, the median age was 64 (IQR: 48~75). We speculated that the age differences in septic patients may be the cause of the opposite outcomes in the two studies. In current study, our results indicated that early low mHLA-DR expression was an independent risk factor for poor outcome in elderly, but not in non-elderly septic patients. Our previous study demonstrated that dynamic change of mHLA-DR was a reliable predictor for mortality in septic patients.Citation8 Then, we combined early immune status with changes of immune status to evaluate hospital mortality. In this study, early immunoparalysis was associated with increased hospital mortality in the elderly, and immune status improvement on day 3 was associated with reduced hospital mortality in both elderly and non-elderly patients. Therefore, monitoring of early immune status should be carried out in elderly patients, and it may be beneficial to monitor the dynamic changes of immune status in both elderly and non-elderly patients.
Several limitations should be noted in our study. Firstly, our data came from a clinical study of immunotherapy for patients with sepsis. Considering the interference of immunotherapy in our study, we took immunotherapy as a fixed covariate in multivariate logistic regression analysis for mortality and found immunotherapy did not affect the prognosis of elderly patients. Secondly, only 15 non-elderly septic patients with early immunoparalysis were included in our study, so a larger study is needed to further verify the results that early immunoparalysis was not associated with poor prognosis in non-elderly patients.
Conclusions
In adult patients with sepsis, the elderly were more susceptible to early immunoparalysis after onset of sepsis. Early immunoparalysis was associated with poor prognosis in elderly patients, but not in non-elderly patients. In addition, immune status improvement was associated with reduced mortality in both elderly and non-elderly patients.
Abbreviations
APACHE II, Acute Physiology and Chronic Health Evaluation II; CIs, confidence intervals; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ETASS, Efficacy of Thymosin Alpha 1 for Severe Sepsis; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; mHLA-DR, monocyte human leukocyte antigen-DR; MV, mechanical ventilation; ORs, odds ratios; RRT, renal replacement therapy; SBP, systolic blood pressure; SD, standard deviation; SOFA, sequential organ failure assessment; SSC, surviving sepsis campaign; Tα 1, thymosin alpha 1.
Data Sharing Statement
The datasets in this study are available from the corresponding author on reasonable request.
Ethics Approval
The original study was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University, the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, the Ethics Committee of Sixth Affiliated Hospital, Sun Yat-sen University, the Ethics Committee of Sun Yat-sen University Cancer Center, Sun Yat-sen University, the Ethics Committee of Foshan First Municipal People’s Hospital and the Ethics Committee of Guangzhou First Municipal People’s Hospital. Written informed consents were obtained from patients or next of kin for patients unable to consent. The clinical trial registry number is NCT0711620. We make sure to conduct this trial in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank all of the doctors, nurses, technicians, and patients involved at the six participating centres for their dedication to the ETASS study.
Disclosure
All authors declare that they have no competing interests.
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.028726903338
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi:10.1097/CCM.0b013e31827c09f823442987
- Suarez De La Rica A, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Ann Transl Med. 2016;4(17):325. doi:10.21037/atm.2016.08.5927713883
- Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi:10.1001/jama.2011.182922187279
- Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi:10.1097/CCM.000000000000207427632674
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi:10.1038/nri355224232462
- Gouel-Cheron A, Allaouchiche B, Floccard B, Rimmele T, Monneret G. Early daily mHLA-DR monitoring predicts forthcoming sepsis in severe trauma patients. Intensive Care Med. 2015;41(12):2229–2230. doi:10.1007/s00134-015-4045-126359166
- Wu JF, Ma J, Chen J, et al. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit Care. 2011;15(5):R220. doi:10.1186/cc1045721933399
- Landelle C, Lepape A, Voirin N, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36(11):1859–1866. doi:10.1007/s00134-010-1962-x20652682
- Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32(8):1175–1183. doi:10.1007/s00134-006-0204-816741700
- Lin HY, Guo XS, Yao YM, et al. Clinical trial to verify the value of the CD14(+) monocyte human leukocyte antigen DR as a marker in evaluating immunosuppression in patients with severe sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15(3):135–138.12831612
- Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–648. doi:10.1164/rccm.200903-0363OC19590022
- Caruso C, Buffa S, Candore G, et al. Mechanisms of immunosenescence. Immun Ageing. 2009;6(1):10. doi:10.1186/1742-4933-6-1019624841
- Martin S, Perez A, Aldecoa C. Sepsis and immunosenescence in the elderly patient: a review. Front Med (Lausanne). 2017;4:20. doi:10.3389/fmed.2017.0012928293557
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi:10.1097/01.CCM.0000261890.41311.E917414736
- Baykara N, Akalin H, Arslantas MK, et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care. 2018;22(1):93. doi:10.1186/s13054-018-2013-129656714
- Lee SH, Hsu TC, Lee MG, et al. Nationwide trend of sepsis: a comparison among octogenarians, elderly, and young adults. Crit Care Med. 2018;46(6):926–934. doi:10.1097/CCM.000000000000308529533311
- Inoue S, Suzuki-Utsunomiya K, Okada Y, et al. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med. 2013;41(3):810–819. doi:10.1097/CCM.0b013e318274645f23328259
- Suzuki K, Inoue S, Kametani Y, et al. Reduced immunocompetent B cells and increased secondary infection in elderly patients with severe sepsis. Shock. 2016;46(3):270–278. doi:10.1097/SHK.000000000000061927172158
- Muszynski JA, Nofziger R, Moore-Clingenpeel M, et al. Early immune function and duration of organ dysfunction in critically iii children with sepsis. Am J Respir Crit Care Med. 2018;198(3):361–369. doi:10.1164/rccm.201710-2006OC29470918
- Wu J, Zhou L, Liu J, et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17(1):R8. doi:10.1186/cc1193223327199
- World Health Organization. China country assessment report on ageing and health. 2015 Available from: http://www.who.int/ageing/publications/china-country-assessment/en/. Accessed 1013, 2019..
- Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: utility of innate immune response markers. Intensive Care Med Exp. 2017;5(1):49. doi:10.1186/s40635-017-0163-029063386
- Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation–rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl 4):S474–S481. doi:10.1007/BF17437278923092
- Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi:10.1001/jama.286.14.175411594901
- Remy S, Kolev-Descamps K, Gossez M, et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study. Ann Intensive Care. 2018;8(1):36. doi:10.1186/s13613-018-0382-x29536210
- Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi:10.1016/S1473-3099(13)70001-X23427891
- Biston P, Aldecoa C, Devriendt J, et al. Outcome of elderly patients with circulatory failure. Intensive Care Med. 2014;40(1):50–56. doi:10.1007/s00134-013-3121-724132383
- Flaatten H, de Lange DW, Artigas A, et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 2017;43(9):1319–1328. doi:10.1007/s00134-017-4718-z28238055
- Lukaszewicz AC, Grienay M, Resche-Rigon M, et al. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37(10):2746–2752. doi:10.1097/CCM.0b013e3181ab858a19707128
- Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intensive Care Med. 2003;29(8):1245–1252. doi:10.1007/s00134-003-1686-212774155