Abstract
Background
Staphylococcus aureus is considered one of the major threats regarding food safety worldwide. Methicillin-resistant S. aureus (MRSA) strains in livestock, companion animals, and wild animals continue to be a potential risk to people working with them.
Aim
The current research aims to investigate the potential pathways of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) strains in the body after oral infection using the experimental mouse model.
Methods
Seven groups of SPF male mice were purchased and housed. On day 1, six groups of mice were infected orally by the sterile gastric probe using 100 μL/mice of LA-MRSA bacterial suspension (1 × 108 colony-forming units (CFU)/mL). The remaining group was kept as negative controls. Over 15 days, these animals have been monitored. Fresh fecal samples were screened for LA-MRSA at day 0, day 7 and day 14 following oral administration of MRSA strains. All animals were sacrificed at day 15, and internal organs (liver, lung, kidney, and intestine) were harvested aseptically and divided into two sections. The first part was histopathologically investigated, while the other half has been tested for LA-MRSA re-isolation.
Result
The oral challenge of mice by MRSA strains showed that MRSA was re-isolated from feces and intestines of all inoculated mice groups and from internal organs (liver, lung, kidney and intestine) of most mice. Results were confirmed by the detection of the bacteria in gram-stained tissue sections and changes in H&E-stained histopathological tissue sections from these organs.
Conclusion
Data from the present study indicate the possible colonization of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) in internal organs following oral infection and thus posing a risk for food-borne infection of MRSA. Infected animals could pass LA-MRSA through feces again, resulting in increased dispersion and environmental contamination.
Introduction
Food-borne diseases constitute a public health problem worldwide. To date, there have been more than 250 different foodborne diseases, most of which are infections caused by bacteria, viruses and parasites.
Staphylococcus aureus has a major threat regarding food safety and occupational health and is one of the most common agents incriminated in food poisoning outbreaks worldwide.Citation1 It is responsible for more than 10% of foodborne outbreaks associated with cheese, milk and other dairy products.Citation2
Methicillin-resistant S. aureus (MRSA) are of public health importance. MRSA infections are associated with a worse prognosis than methicillin-susceptible S. aureus infections.Citation3,Citation4 Emergence of these resistant strains is due to the acquisition of mecA gene encoding Penicillin-Binding Protein 2a (PBP2a), which belongs to the family of enzymes necessary for building the bacterial cell wall.Citation5 The presence of methicillin-resistant S. aureus strains (MRSA) in food-producing animals and its detection in retail meat samples raises the concern about the potential food-borne transmission of MRSA.Citation6
Before the 1990s, the majority of MRSA cases were hospital-associated (HA-MRSA); however, the community-associated MRSA (CA-MRSA) then found to cause infections outside the healthcare environment. The third major emergent type of MRSA has been reported in livestock animals [livestock-associated MRSA (LA-MRSA)].
This widespread of CA-MRSA and LA-MRSA has raised the question of whether MRSA is a potential foodborne pathogen or not. This prompted researches for determining the origin and pathways of LA-MRSA and its ability to cause zoonotic disease in human.Citation7 Furthermore, MRSA is in need to be studied closely in an attempt to control its spread.Citation8
Using animal models to study a particular disease whose features closely resemble those of disease in man are necessary in order to understand its pathogenesis and possible pathways. Numerous mouse models have been developed as substitutes for the study of infections with S. aureus occurring in humans. These include subcutaneous injection of staphylococci to generate skin and soft tissue infections,Citation9 intravenous challenge with staphylococci to induce sepsis,Citation10 or endocarditisCitation11 and intranasal instillation of staphylococci to induce pneumonia.Citation12 Our study used an oral-challenged mouse model to study the possible pathways of MRSA strains following oral infection and the understand the consequences of its sources and transmission.
Materials and Methods
Experimental design and protocols for laboratory animal housing and inoculations had been reviewed and approved by the Scientific Research Committee and Bioethics Board of Cairo University, Faculty of Veterinary Medicine, Giza, Egypt.
Bacterial Strains
MRSA strains previously obtained from milk of Mastitic animals (Cattle, buffalo and goat) were used in this study. The used strains were related to Dorgham et al.Citation13
Bacterial strains were inoculated onto trypticase soy agar with 5% sheep blood and incubated for 18 to 24 hrs at 35°C.
Bacterial suspension was prepared by mixing the obtained colonies in sterile 0.9% NaCl.
MRSA cells were suspended at a concentration of 1 × 108 colony-forming units (CFU)/mL in saline using McFarland standard.Citation14
In vivo Infectivity Assays Mice
Four weeks old, SPF male mice weighing 25 to 33 g were purchased. Mice were maintained under standard ethical conditions recommended by the Committee for the Care and Use of Laboratory Animals. Upon arrival, mice were placed and divided into 7 groups (five animals each). Experimental animal groups were individually housed in separate cages and were managed and kept at the same environmental and nutritional conditions. All animals received a common laboratory diet and water and all efforts were made to minimize the suffering of animals throughout the experiment. Fecal samples were collected from all mice and tested for the presence of MRSA strains before beginning of the experiment.
On day 1, six groups of mice were infected orally with 100 μL of the bacterial suspension/mice using a sterile gastric probeCitation14 while the remaining group was kept as negative controls.
Infected animals were monitored for morbidity or mortality over a period of 15 days.
Re-Isolation and Identification of MRSA Strains from the Inoculated Mice
Following oral administration of MRSA strains, fresh fecal samples were aseptically collected from mice at day 0, day 7 and day 14 post inoculations by gentle pressure on their abdomens. At day 15, all animals were sacrificed and internal organs (liver, lung, kidney and intestine) were aseptically collected from mice and divided into two parts. The first part was fixed in 10% neutral buffered formalin while the other was plated onto Columbia Agar base with 5% defibrinated sheep blood. All samples were transferred directly to the laboratory for further processing. Fresh fecal and tissue samples were plated onto Columbia Agar base with 5% defibrinated sheep blood (Oxoid, Germany). Test plates were incubated for 24–48 hrs at 37°C±1°C. All isolates were identified based on the colony morphology, Gram staining, coagulase plasma test, catalase test and occurrence of hemolysis. Additionally, an API-Staph Kit (bioMerieux, Durham, N.C.) was also used for identification of S. aureus.
Molecular Confirmation of the Re-Isolated MRSA Strains
A total of 5~10 S. aureus colonies were suspended in 200 µL TE buffer. The suspension was incubated for 10 min at 56°C, and then for 10 min at 95°C before being spun at 16000 × g for 2 min. After centrifugation, 5 μL of the supernatant were used as template in a 50 μL PCR reaction.
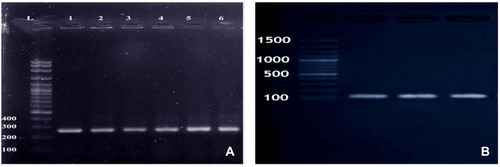
Molecular confirmation was done by amplification of the S. aureus-specific nuc gene and mecA gene. The primer pairs sequence used in the PCR assays are listed in .
Table 1 Primer Sequences Used for Amplification of (Nuc) and (mecA) Genes and the Suspected Product Size
PCR assay for the detection of nuc gene (encoding for the S. aureus specific thermonuclease) was performed as previously mentioned.Citation15 The extracted DNA was amplified for 35 cycles consisted of 30 s at 94°C for denaturation, 30 s at 55°C for annealing and 60 s at 72°C for primer extension and a final extension for 10 min at 72°C. For amplification of the mecA gene (encoding for the methicillin-resistant S. aureus), PCR conditions included a 4 min initial denaturation at 94°C, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min and a final extension for 10 min at 72°C.Citation16 Twenty microliters of the obtained PCR product were then visualized and photographed after being electrophoresed on 1.5% agarose gel.
Histopathological Examination
Tissue samples from liver, kidneys, lungs and intestine collected from the control and inoculated mice groups were routinely processed for histopathological examination to obtain 5 μm sections. The sections were stained with hematoxylin and eosin and Gram’s stain and examined under the microscope.Citation17
Statistical Analysis
Data were analysed with the Chi-square (X2) test using PASW Statistics, Version 18.0 software (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Results
Mortality Rate
The mortality rate in all mouse groups was reported throughout the experiment. None of the mice were found dead from the beginning to the end of the experiment.
Isolation, Identification and Molecular Confirmation of Re-Isolated MRSA Strains from Fecal Samples of Mice
Fecal samples from all mice under experiment were free from MRSA before the oral challenge. Following oral administration, MRSA strains were re-isolated through the study period from the collected fecal samples of mice under experiment and completely identified by morphological, biochemical characterization and by molecular amplification of nuc and mecA genes .
Detection of MRSA in Internal Organs (Liver, Lung, Kidney and Intestine) and in Histopathological Sections
MRSA strains were re-isolated from intestine of all mouse groups after oral administration. The results of re-isolation and identification of MRSA strains from liver, lung, kidney and intestine are showed in . The proportion of LA-MRSA positive samples between different organs (liver, kidney, lung and intestine) showed no significant difference.
Table 2 Re-Isolated MRSA Strains from Internal Organs Following Oral Challenge of Mice
Histopathological Changes
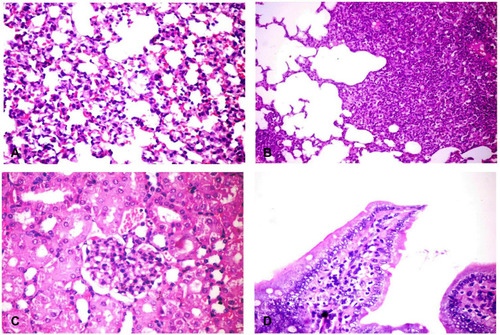
No pathological changes had been detected in internal organs of the control group. However, liver of infected mice showed multiple minute focal areas to large patchy areas of hepatocellular necrosis infiltrated with neutrophils and mononuclear cells (). Sinusoidal dilation and kupffer cells activation were also observed. The portal area revealed portal congestion, bile duct hyperplasia and leukocytic aggregations mostly, with neutrophils, macrophages and lymphocytes (). Lung tissues revealed thickening of the alveolar wall with dilated perialveolar blood capillaries, inflammatory cells mainly neutrophils and mononuclear cells (). In two cases, severe fibrinopurulent lobar pneumonia was observed (). Massive aggregation of neutrophils, macrophages and necrotic cell debris together with fibrinopurulent exudates were noticed in the alveolar lumen. Some bronchi and bronchioles showed hyperplasia of its epithelial cells with peribronchial and peribronchiolar inflammatory cell aggregation. Kidneys from infected animals showed glomerulonephritis, characterized by mesangial hypercellularity (), the intertubular blood vessels were dilated and the renal tubular epithelium was vacuolated. Intestine showed sloughing and desquamation of individual enterocytes with increase lamina proprial macrophages, lymphocytes and neutrophils (). Some enterocytes were apoptotic showing shrinkage with pyknotic nuclei.
Figure 2 Histopathological changes in liver of challenged mice. (A) Liver showing patchy area of hepatic cellular necrosis mixed with polymorph nuclear and mononuclear inflammatory cells (arrow), portal congestion, bile duct hyperplasia and leukocytic aggregations mostly, with neutrophils, macrophages and lymphocytes (H&E X400). (B) Liver showing multiple focal areas of hepatocellular necrosis with neutrophils (arrows) and few mononuclear cell aggregation, sinusoidal dilation and kupffer cells activation (H&E X400). (C) Liver showing focal area of hepatic cellular necrosis (rectangle) mixed with polymorph nuclear, mononuclear inflammatory cells and fragmented nuclei (H&E X400). (D) Liver showing intense periportal inflammatory cell aggregation (arrow) (H&E X400). H&E, hematoxylin and eosin stain.
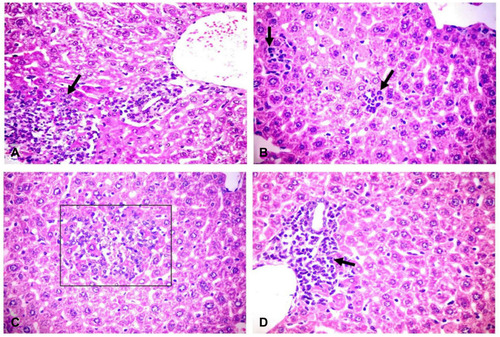
Figure 3 Histopathological changes in different organs of challenged mice. (A) Lung showing thickening of the alveolar wall by dilated perialveolar blood capillaries, inflammatory cells mainly neutrophils and mononuclear cells (H&E X400). (B) Lung showing severe fibrinopurulent pneumonia with giant alveoli (H&E X400). (C) Kidney showing glomerular hypertrophy with mesangial hypercellularity (H&E X400). (D) Intestine showing sloughing and desquamation of individual enterocytes with increased lamina propria macrophages, lymphocytes and neutrophils (H&E X200). H&E, hematoxylin and eosin stain.
Discussion
S. aureus is frequently colonizing most animal species worldwide; however, the emergence of MRSA strains in several food producing animals, including pigs, cattle, chicken and other animals has a serious impact regarding food safety.Citation18 Contact with animals is recognized as a risk factor for MRSA carriage. Available data in previous literature proved that MRSA are found colonizing pigs, pig farmers and their families who are in contact with pigs in the Netherlands.Citation19 Also, people in occupational contact with livestock, eg, farmers, veterinarians and abattoirs workers are frequently exposed and found colonized with LA-MRSA.Citation20 Furthermore, MRSA strains have been detected in different foods for human consumption including bovine milk, cheese, meat products and raw chicken meat.Citation21,Citation22
It is important to understand the LA-MRSA source and dynamics of transmission in order to monitor and prevent the contamination of the LA-MRSA in domestic animals and retail meat.
In the present study, LA-MRSA was re-isolated from feces of all experimentally infected mice, and this may give rise to more environmental contamination. In this regard, in a recent study, LA-MRSA has been recovered from the paws of control mink groups neighboring to other minks infected with LA-MRSA spiked feed within 24 hrs following exposure to contaminated feed due to environmental dispersion.Citation23
In addition, the presented data from our experiment support the evidence of MRSA colonization in internal organs following oral administration.
MRSA was found colonizing the intestinal mucosa. This may be due to the presence of surface proteins “microbial surface components recognizing adhesive matrix molecules” (MSCRAMMs), which appear to play a key role in initiation of endovascular infections.Citation24,Citation25 After bacterial adherence, it will be able to grow by forming biofilm that enable it to evade the host defense mechanism.Citation26,Citation27 Deregulated barrier function of the intestinal surface epithelial lining and the ability of MRSA to invade and survive inside the epithelial and endothelial cells believed to be a key factor for mucosal bacterial invasion.Citation28 During infection, S. aureus produces numerous enzymes, such as proteases, lipases, and elastases that enable it to invade and destroy host tissues and metastasize to other sites.Citation29 When the bacteria gain access to the bloodstream through invasions of intestinal capillaries, it drains into the portal vein then to general circulation with dissemination of MRSA to different organs as liver, kidney and lung. This hypothesis was confirmed in our experiment by re-isolation of MRSA and histopathological examination of liver, kidney and lung tissues. This bacterial colonization in the lung and kidney may give rise to shedding of bacteria in respiratory discharge or urinary tract.
LA-MRSA could also be re-isolated at the end of this study from internal organs (), and all examined feces, this denoted that LA-MRSA would persist in mice for 15 days after oral administration.
In a similar study in Denmark, LA-MRSA was re-isolated from paws and pharynx of minks (Neovison vison) after giving them LA-MRSA spiked feed. However, the infected animals were being able to get rid of being carriers after stopping of MRSA administration (Fertner et al 2019b).Citation30 On the other hand, data from our study were denied by the conclusion of Wendlandt et alCitation7 who stated that although all types of MRSA may be present in/on human food, it could not be considered as a food-borne pathogen.
The findings presented in this study may partly help to answer the controversial question posing a potential health risk; does isolation of MRSA from poultry, beef, and meat products linked to contamination from food handlers and poor hygiene during processing, or it comes from the animal itself.
Hence, effective control measures to prevent dispersion of MRSA begins from good hygienic practices, good manufacturing practices and hazard analysis critical control point for products from animal origin throughout the food chain production system from animal feeding and rearing in the farms to retail facilities.Citation31
Generally, limiting the irresponsible use of antimicrobials in veterinary medicine in treatment and their use as growth promotors is very valuable to prevent dispersion of antimicrobial resistance.Citation32,Citation33
Conclusion
The present study revealed that oral administration of MRSA strains in experimental mice model resulted in their colonization into the internal organs and the infected animals could pass the resistant bacteria through feces again giving rise to more dispersion and environmental contamination. Good hygienic and manufacturing practices throughout all stages of food chain from animal husbandry to consumption of animal products are very valuable in elimination of bacterial contamination from animal feed and subsequently prevent their infection and dispersion of antimicrobial-resistant bacteria.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hennekinne JA, De Buyser ML, Dragacci S. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev. 2012;36(4):815–836. doi:10.1111/j.1574-6976.2011.00311.x22091892
- Sasidharan S, Prema B, Latha LY. Antimicrobial drug resistance of Staphylococcus aureus in dairy products. Asian Pac J Trop Biomed. 2011;1(2):130–132. doi:10.1016/S2221-1691(11)60010-523569742
- Chen C, Fan H, Huang Y, et al. Recombinant lysostaphin protects mice from methicillin-resistant Staphylococcus aureus pneumonia. Biomed Res Int. 2014;2014.
- Hesari MR, Salehzadeh A, Darsanaki RK. Prevalence and molecular typing of methicillin-resistant Staphylococcus aureus carrying Panton–Valentine leukocidin gene. Acta Microbiol Immunol Hung. 2018;65(1):93–106. doi:10.1556/030.64.2017.03228859499
- Gajdács M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8(2):52. doi:10.3390/antibiotics8020052
- Chon J, Sung K, Khan S. Methicillin-resistant Staphylococcus aureus (MRSA) in food-producing and companion animals and food products. Frontiers in Staphylococcus Aureus. 2017;8:47. doi.10.5772/66645 Available from: https://www.intechopen.com/books/frontiers-in-i-staphylococcus-aureus-i-/methicillin-resistant-staphylococcus-aureus-mrsa-in-food-producing-and-companion-animals-and-food-pr
- Wendlandt S, Schwarz S, Silley P. Methicillin-resistant Staphylococcus aureus: a food-borne pathogen? Annu Rev Food Sci Technol. 2013;28(4):117–139. doi:10.1146/annurev-food-030212-182653
- Gajdács M, Zsoldiné Urbán E. Epidemiology and resistance trends of Staphylococcus aureus isolated from vaginal samples: a 10-year retrospective study in Hungary. Acta Dermatovenerol Alp Pannonica Adriat. 2019;28(4):143–147. doi:10.15570/actaapa.2019.3531855266
- Voyich JM, Otto M, Mathema B, et al. Is Panton‐Valentine leukocidin the major virulence determinant in community‐associated methicillin‐resistant Staphylococcus aureus disease? J Infect Dis. 2006;194(12):1761–1770. doi:10.1086/50950617109350
- Kim HK, DeDent A, Cheng AG, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 2010;28(38):6382–6392. doi:10.1016/j.vaccine.2010.02.09720226248
- Panizzi P, Nahrendorf M, Figueiredo JL, et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat Med. 2011;17(9):1142–1146. doi:10.1038/nm.242321857652
- Wardenburg JB, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75(2):1040–1044. doi:10.1128/IAI.01313-0617101657
- Dorgham SM, Hamza DA, Khairy EA, Hedia RH. Methicillin-resistant staphylococci in mastitic animals in Egypt. Glob Vet. 2013;11(6):714–720.
- Lkhagvadorj E, Nagata S, Wada M, et al. Anti‐infectious activity of synbiotics in a novel mouse model of methicillin‐resistant Staphylococcus aureus infection. Microbiol Immunol. 2010;54(5):265–275. doi:10.1111/j.1348-0421.2010.00224.x20536723
- Al-Amery K, Elhariri M, Elsayed A, et al. Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrob Resist Infect Control. 2019;8(1):129. doi:10.1186/s13756-019-0585-431404199
- Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–5033. doi:10.1128/JCM.43.10.5026-5033.200516207957
- Morgan A, Galal MK, Ogaly HA, et al. Tiron ameliorates oxidative stress and inflammation in titanium dioxide nanoparticles induced nephrotoxicity of male rats. Biomed Pharmacother. 2017;93:779–787. doi:10.1016/j.biopha.2017.07.00628709131
- De Neeling AJ, Van den Broek MJ, Spalburg EC, et al. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet Microbiol. 2007;122(3–4):366–372. doi:10.1016/j.vetmic.2007.01.02717367960
- Van Duijkeren E, Ikawaty R, Broekhuizen-Stins MJ, et al. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet Microbiol. 2008;126(4):383–389. doi:10.1016/j.vetmic.2007.07.02117765409
- European Food Safety Authority. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin‐resistant Staphylococcus aureus in food‐producing animals and food. EFSA J. 2012;10(10):2897. doi:10.2903/j.efsa.2012.2897
- Normanno G, Corrente M, La Salandra G, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int J Food Microbiol. 2007;117(2):219–222. doi:10.1016/j.ijfoodmicro.2007.04.00617533002
- Dai J, Wu S, Huang J, et al. Prevalence and characterization of Staphylococcus aureus isolated from pasteurized milk in China. Front Microbiol. 2019;10:641. doi:10.3389/fmicb.2019.0064131001225
- Fertner M, Pedersen K, Jensen VF, et al. Within-farm prevalence and environmental distribution of livestock-associated methicillin-resistant Staphylococcus aureus in farmed mink (Neovison vison). Vet Microbiol. 2019;231:80–86. doi:10.1016/j.vetmic.2019.02.03230955829
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–958. doi:10.1038/nrmicro128916322743
- Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:S350–9. doi:10.1086/53359118462090
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi:10.1128/CMR.15.2.167-193.200211932229
- Salehzadeh A, Zamani H, Langeroudi MK, Mirzaie A. Molecular typing of nosocomial Staphylococcus aureus strains associated to biofilm based on the coagulase and protein A gene polymorphisms. Iran J Basic Med Sci. 2016;19(12):1325.28096965
- Bettenworth D, Nowacki TM, Friedrich A, Becker K, Wessling J, Heidemann J. Crohn’s disease complicated by intestinal infection with methicillin-resistant Staphylococcus aureus. World J Gastroenterol. 2013;19(27):4418. doi:10.3748/wjg.v19.i27.441823885156
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi:10.1056/NEJM1998082033908069709046
- Fertner M, Pedersen K, Chriél M. Experimental exposure of farmed mink (Neovison vison) to livestock-associated methicillin-resistant Staphylococcus aureus contaminated feed. Vet Microbiol. 2019;231:45–47. doi:10.1016/j.vetmic.2019.02.03330955822
- European Food Safety Authority. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008‐Part A: MRSA prevalence estimates. EFSA J. 2009;7(11):1376. doi:10.2903/j.efsa.2009.1376
- Sergelidis D, Angelidis AS. Methicillin‐resistant Staphylococcus aureus: a controversial food‐borne pathogen. Lett Appl Microbiol. 2017;64(6):409–418. doi:10.1111/lam.1273528304109
- Gajdács M, Paulik E, Szabó A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: a cross-sectional survey in Hungary (KAPPhA-HU). Antibiotics. 2020;9(2):41. doi:10.3390/antibiotics9020041