Abstract
Objective
The 16S rRNA methylase-mediated high-level resistance to aminoglycosides has become a great concern. The purpose of the study was to investigate the occurrence of 16S rRNA methyltransferase (RMTase) genes in carbapenem-resistant Klebsiella pneumoniae (CRKP) clinical isolates associated with bloodstream infections (BSIs) in China.
Methods
From July 2015 to December 2018, a total of 137 unique CRKP clinical isolates associated with BSIs were collected from 11 Chinese teaching hospitals. PCR and DNA sequencing were used to identify 16S RMTase genes. Whole-genome sequencing (WGS) was performed on all CRKP clinical isolates. Relevant information was extracted from WGS data (antibiotic resistance determinants, K-type and wzi allelic types). All 16S RMTase-producing CRKP clinical isolates were characterized by antimicrobial susceptibility testing, multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE).
Results
In this study, 137 CRKPs were found to harbor at least one carbapenemase gene. Among 137 CRKPs, 78 (56.9%, 78/137) were positive for 16S RMTase genes (5 for armA, 70 for rmtB, 3 for both armA and rmtB) and highly resistant to gentamicin and amikacin (MICs ≥256 mg/L). Seventy-five isolates harboring 16S RMTase genes also produced ESBLs. In this study, 5 sequence types (STs) and 6 capsule serotypes were found among 78 isolates positive for 16S RMTases genes, while 14 STs and 6 capsule serotypes were found among 59 isolates negative for 16S RMTases genes. Compared with the isolates negative for 16S RMTases genes, the STs and capsular serotypes of 16S RMTases-positive strains are more concentrated. Among 78 16S RMTases-positive strains, the most prevalent clone type is ST11-PFGE-B-KL64-wzi64 (62.8%, 49/78), which mainly carries the rmtB and blaKPC genes and is distributed in 7 provinces in China.
Conclusion
A high prevalence of 16S RMTase genes was found among CRKP clinical isolates associated with BSIs from Chinese teaching hospitals, which was attributed to the dissemination of the ST11-PFGE-B-KL64-wzi64 clone.
Introduction
Aminoglycosides are broad-spectrum and highly effective antibiotics, often used in combination with β-lactam antibiotics to treat bacterial infections. In the USA, the most commonly used prescription aminoglycosides for the treatment of serious infections include amikacin, gentamicin and tobramycin.Citation1 Although the nephrotoxicity and ototoxicity of aminoglycosides have been confirmed,Citation2,Citation3 they play a key role in antibacterial therapy, especially against multidrug resistance (MDR) Gram-negative bacilli.Citation4 However, increasing resistance to aminoglycosides is becoming an extremely serious clinical problem.Citation5–Citation7
Among the resistance mechanisms against aminoglycosides, the most common in Enterobacteriaceae are aminoglycoside modifying enzymes (AMEs).Citation8,Citation9 These enzymes include acetyltransferase (AAC), phosphotransferase (APH) and nucleotide transferase (ANT), which inactivate aminoglycosides by covalently modifying specific amino or hydroxyl groups.Citation9 In addition to AMEs, 16S RMTases have been shown to confer high-level resistance to all clinically relevant aminoglycosides (MICs≥256 mg/L).Citation10 Since the first 16S RMTases gene, armA, was identified in 2003.Citation10 Ten 16S RMTase-encoding genes, including armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA, have been found in clinical isolates of Gram-negative bacilli from multiple geographic locations.Citation11 Most of the genes encoding 16S RMTases are typically located on plasmids along with those for extended-spectrum beta-lactamases (ESBLs) and carbapenemases resistance determinants.Citation6,Citation10,Citation12 This may limit the choice of antimicrobials used to treat multi-drug resistant Gram-negative infections. The 16S RMTase-producing isolates have become a concern to clinical treatment in the world.Citation11 The reports about the prevalence of 16S RMTase genes among carbapenem-resistant Klebsiella pneumoniae (CRKP) clinical isolates associated with bloodstream infections (BSIs) in China are unknown. The aim of the present study was to investigate the occurrence of 16S RMTase genes in CRKP clinical isolates associated with BSIs in several Chinese teaching hospitals.
Materials and Methods
Collection and Identification of CRKP Isolates
From July 2015 to December 2018, a total of 137 unique (one isolate per patient) CRKP isolates were collected from 11 tertiary teaching hospitals in eight provinces of China, including Jiangxi (n = 40), Shandong (n = 19), Shanghai (n = 18), Henan (n = 15), Zhejiang (n = 18), Hubei (n = 11), Fujian (n = 9), and Hunan (n = 7). All isolates were isolated from patients with BSIs. All isolates were identified as K. pneumoniae by Gram staining and a VITEK-2 automated platform (bioMérieux, Marcy l’Etoile, France) according to the manufacturer’s protocol. Escherichia coli ATCC25,922, Pseudomonas aeruginosa ATCC27853 and Staphylococcus aureus ATCC25923 were used as control strains for the species identification. In this study, carbapenem resistance was defined as resistance to meropenem or imipenem based on the 2018 Clinical and Laboratory Standards Institute (CLSI) guidelines.Citation13 Clinical data were extracted from medical records and oral informed consent was obtained from the relevant patients.
Detection of 16S RMTase Genes
The 16S RMTase genes, including armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and nmpA, were detected using polymerase chain reaction (PCR) as described previously.Citation6,Citation14,Citation15 After PCR, PCR products were sequenced by Sanger sequencing. The obtained DNA sequences were compared with the DNA sequence in GenBank using the BLAST program.
Phenotype Confirmation of Carbapenemase
A modified carbapenem inactivation test (mCIM) for 137 CRKP isolates to detect carbapenemases according to CLSI 2018 standards.Citation13 All tested CRKP isolates were incubated with meropenem disk (BIO-KONT, Wenzhou, China) in 2 mL TSB at 37°C for 4 hours. The E. coli ATCC25922 was used as an Indicator bacteria. A suspension of E. coli ATCC 25922 was adjusted to 0.5 McFarland standard with sterile physiological saline solution. The E. coli ATCC25922 suspension was evenly spread on MH agar plates. After drying for 3–10 minutes, the meropenem disk (10 μg) was placed on the surface of the agar plate. All treated plates were incubated at 37°C for 18–24 hours. Following incubation, measure the zones of inhibition.
Whole-Genome Sequencing (WGS) and Analysis
DNA extraction was carried out using the Ezup Column Bacteria Genomic DNA Purification Kits (Sangon Biotech, shanghai, China) according to the manufacturer’s instructions. Genomic sequencing libraries were prepared using the Nextera XT kit and sequenced using the Illumina Miseq platform (paired end reads of 150 bp) according to the manufacturer’s protocol. Trimmed reads were de novo assembled using SPAdes 3.9.1.Citation16 Resistance genes carried by all isolates were evaluated using Resfinder program provided by the Center for Genome Epidemiology (https://cge.cbs.dtu.dk/services/).Citation17 Multi-locus Sequence Typing (MLST) was also determined from the assembled genome. The serotypes (K-types and wzi allelic types) of all K. pneumoniae isolates were analyzed using the Klebsiella WGS data online platform tool Kaptive-web (http://kaptive.holtlab.net/).
Antimicrobial Susceptibility Testing
The minimum inhibitory concentration (MIC) of various antibacterial drugs for CRKP isolates tested was determined by the broth micro-dilution method and interpreted according to CLSI criteria as described previously.Citation13,Citation18 The MIC was defined as the lowest concentration of antibiotic completely inhibiting visible growth. A total of 17 antimicrobials including carbapenems (imipenem and meropenem), aminoglycosides (amikacin and gentamicin), β-lactams/β-lactamase inhibitor complexes (ceftazidime-avibactam and piperacillin-tazobactam), cephalosporin (ceftazidime, cefepime, cefotaxime and cefoxitin), fluoroquinolones (ciprofloxacin), folate metabolic pathway inhibitors (sulfamethoxazole), polymyxin B, tetracyclines (tigecycline, minocycline and tetracycline) and monocyclic β-lactam (aztreonam) were tested. The E. coli ATCC25922 was used as control strain for determining the antimicrobial susceptibility for K. pneumoniae clinical isolates.
Multilocus Sequence Typing (MLST)
Multilocus sequence typing (MLST) was used to investigate the genetic relationship of the isolates positive for 16S RMTase genes. Amplification of seven standard housekeeping loci by PCR, including gapA, infB, mdh, pgi, phoE, rpoB and tonB, followed by Sanger sequencing as described previously.Citation19 The alignment analysis was performed using an online database on the Pasteur Institute MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html) to obtain the assigned sequence type (ST) of the isolates.
Pulsed-Field Gel Electrophoresis (PFGE)
PFGE analysis was performed on 78 clinical isolates of CRKP positive for 16S RMTase genes. The K. pneumoniae cells were embedded in agar plates and digested with XbaI for 3.5 hours at 37°C to prepare genomic DNA. Electrophoresis was carried out for 19 hours using the Bio-Rad CHEF III system with parameters set to 14°C, 120° angle, 6 V/cm, and switching times of 6 and 36 seconds. The Salmonella enterica serotype Braenderup strain H9812 was chosen as the control standard strain and universal molecular weight marker. Electrophoresis results are interpreted according to the criteria proposed by Tenover et al.Citation20 Analysis of PFGE results using Bioices software (Applied Maths, Sint-Martens-Latem, Belgium) with the Dice similarity coefficients. Clinical isolates with more than 80% homology are defined as the same PFGE cluster.
Statistical Analysis
SPSS statistical software (version 23, IBM SPSS Statistics) was used for statistical analysis. The χ2 or Fisher’s exact tests were used for categorical variables. P <0.05 was considered statistically significant.
Results
Detection of 16S RMTase Genes
Among 137 tested CRKP isolates, 78 (78/137, 56.9%) were positive for 16S RMTase genes determined by PCR and Sanger sequencing. Of 78 isolates with 16S RMTase genes, 70 were positive for rmtB gene and were distributed in 8 provinces, including Jiangxi (n = 22), Shandong (n = 11), Hubei (n = 9), Henan (n = 8), Shanghai (n = 7), Zhejiang (n = 5), Fujian (n = 5) and Hunan (n = 3); 5 were positive for armA gene (3 in Shanghai and 2 in Henan); 3 were found to carry both rmtB and armA (2 in Shanghai and 1 in Zhejiang). Each of the 137 CRKP isolates carries at least one carbapenem-resistant gene, which contains five different carbapenemase genes, including blaKPC-2 (n = 110), blaNDM-5 (n = 18), blaNDM-1 (n = 16), blaIMP-4 (n = 1) and blaIMP-30 (n=2). Among them, 6 isolates possessed both blaNDM-1 and blaKPC-2, 3 isolates harbored blaNDM-5 and blaKPC-2, and 1 isolate carried blaIMP-4 and blaKPC-2. Seventy-five (75/78, 96.2%) of 78 isolates harboring 16S rRNA methylase genes were extended-spectrum β-lactamase (ESBL) producers. Seventy and Thirty-six isolates with 16S rRNA methylase genes were positive for blaCTX-M and blaSHV genes, respectively. Among the 78 isolates, it was confirmed that 3 strains carried blaCTX-M-3, 1 strain carried blaCTX-M-14, 3 strains carried blaCTX-M-15, 1 strain carried blaCTX-M-27, 66 strains carried blaCTX-M-65 and 36 strains carried blaSHV-12. In this study, compared to 16S RMTases-negative strains, 16S RMTases-positive strains harbored fewer blaNDM genes, but carried more blaKPC genes (). Carbapenemase genes and ESBL genes were extracted from WGS data.
Table 1 Antibiotic Resistance Gene Profiles in 16S RMTases-Positive and -Negative Strains
Aminoglycoside Modification Enzyme (AME) Genes
Besides the 16S RMTase genes, other aminoglycoside resistance genes, including AME genes and streptomycin-related resistance genes, were identified by analyzing the WGS data of 78 CRKP isolates. Various aminoglycoside modifying enzyme genes were identified, including aac3-IId (23/78, 29.5%), aph3-Ia (5/78, 6.4%), aadA2 (65/78, 83.3%), aadA1-pm (2/78, 2.6%), aadA16 (3/78, 3.8%) and aadB (6/78, 7.7%). In addition, 24 isolates were also found to carry streptomycin-related resistance genes strA and strB.
Phenotypical Identification of All CRKP Isolates
All 137 CRKP isolates were found to produce carbapenemases determined by the mCIM assay.
Antimicrobial Resistance Among 16S RMTases-Positive and -Negative CRKP Isolates
Among tested 137 CRKP isolates, 94 (68.6%) and 78 (56.9%) isolates were resistant to gentamicin and amikacin. All 78 amikacin-resistant isolates were concomitantly resistant to gentamicin and positive for 16S rRNA methylase genes. Relative to 16S RMTases-negative isolates, 16S RMTases-positive isolates were more sensitive to ceftazidime/avibactam, tetracycline, minocycline and sulfamethoxazole, but had higher resistance rates to aztreonam, gentamicin, amikacin and ciprofloxacin. All 78 isolates positive for 16S RMTases genes were resistant to clinically often used antimicrobial agents, including imipenem, meropenem, cefoxitin, cefotaxime, cefepime, ceftazidime, aztreonam, gentamicin and amikacin, but more sensitive to ceftazidime/avibactam, polymyxin B and tigecycline. All 16S rRNA methylase gene-positive isolates had high levels of resistance to gentamicin and amikacin (MICs ≥256 μg/mL) ().
Table 2 The Antimicrobial Resistance Profiling of 16S RMTases-Positive and -Negative Strains
Capsular Serotyping of 16S RMTases-Positive and -Negative CRKP Isolates
Of 78 16S RMTases-positive isolates, 6 capsular serotypes were identified, including KL64-wzi64 (70.5%, 55/78), KL47-wzi209 (20.5%, 16/78), KL19-wzi19 (5.1%, 4/78), KL3-wzi59 (1.3%, 1/78), KL27-wzi27 (1.3%, 1/78), and KL24-wzi101 (1.3%, 1/78). A total of 11 capsular serotypes were found in 59 16S RMTases-negative strains, including KL64-wzi64 (35.6%, 21/59), KL24-wzi101 (11.9%, 7/59), and KL21-wzi21 (10.2%, 6/59), KL47-wzi209 (10.2%, 6/59), KL48-wzi151 (5.1%, 3/59), KL102-wzi173 (5.1%, 3/59), KL19-wzi19 (3.4%, 2/59), KL67-wzi198 (3.4%, 2/59), KL2-wzi72 (1.7%, 1/59), KL37-wzi37 (1.7%, 1/59), and KL74-wzi174 (1.7%, 1/59). Six 16S RMTases-negative isolates (10.2,6/59) were not typed successfully. The results showed that compared with 16S RMTases-negative strains, 16S RMTases-positive strains had fewer types of capsular serotypes and more concentrated distribution. In all 16S RMTases-positive and -negative CRKP strains, the major capsular serotype were KL64-wzi64. However, more 16S RMTases-positive isolates (70.5%) belonged to KL64-wzi64 relative to 16S RMTases-negative isolates (35.6%).
Molecular Characteristics for 16S RMTases-Positive and -Negative CRKP Isolates
Multi-locus sequencing typing (MLST) was performed on all 16S RMTases-positive and -negative CRKP isolates. Among 78 16S RMTases-positive isolates tested, 5 STs were identified. ST11 was the most prevalent ST (92.3%, 72/78), followed by ST15 (2.6%, 2/78) and ST2237 (2.6%, 2/78). ST45 and ST395 were found among only one isolate. Among 59 16S RMTases-negative strains, a total of 14 sequence types (STs) were identified, including ST11 (45.8%, 27/59), ST45 (11.9%, 7/59), ST290 (11.9%, 7/59), and ST15. (8.5%, 5/59), ST438 (3.4%, 2/59), ST1319 (3.4%, 2/59), ST307 (3.4%, 2/59), ST1692 (1.7% 1/59), ST375 (1.7% 1/59), ST35 (1.7% 1/59), ST107 (1.7% 1/59), ST485 (1.7% 1/59), ST2390 (1.7% 1/59), and ST2236 (1.7% 1/59). The results showed that 16S RMTases-positive strains had fewer types of STs and more concentrated distribution relative to the isolates negative for RMTases genes. In all 16S RMTases-positive and -negative CRKP strains, the predominant ST was ST11. However, more 16S RMTases-positive isolates (92.3%) belonged to ST11 clonal strains relative to 16S RMTases-negative isolates (45.8%).
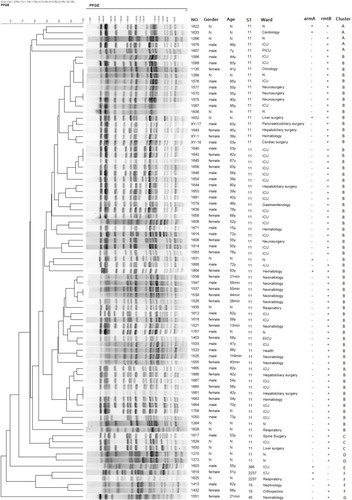
PFGE results revealed seven distinct clusters (cluster A to cluster G). Among them, 72 ST11 isolates were divided into four different PFGE clusters, including A clusters (5/78, 6.4%), B clusters (62/78, 79.5%), C clusters (3/78, 3.8%) and D clusters (2/78, 2.6%). Two ST2237 and two ST15 isolates belong to the same cluster (cluster F). Each of the remaining two isolates formed a singleton (cluster E and cluster G). ()
Figure 1 PFGE results for 78 CRKP isolates harboring 16S rRNA methyltransferase genes. The adjacent information shown on the right represents number of strains, gender, age, ST type, ward, armA and rmtB presence, and cluster, respectively.
Abbreviations: PFGE, pulsed-field gel electrophoresis; CRKP, carbapenem-resistant Klebsiella pneumoniae.
Characteristics of Different Clonetypes in 16S RMTases-Positive Strains
Among 78 16S RMTases-positive strains, 11 different clonal strains were identified, including ST11-PFGE-A-KL64-wzi64 (3/78, 3.8%), ST11-PFGE-A-KL47-wzi209 (2/78, 2.6%), ST11-PFGE-B-KL64-wzi64 (49/78, 62.8%), ST11-PFGE-B-KL47-wzi209 (12/78, 15.4%), ST11-PFGE-B-KL27-wzi27 (1/78, 1.3%), ST11-PFGE- C-KL64-wzi64 (3/78, 3.8%), ST11-PFGE-D-KL47-wzi209 (2/78, 2.6%), ST15-PFGE-F-KL19-wzi19 (2/78, 2.6%), ST45-PFGE-G-KL24-wzi101 (1/78, 1.3%), ST395-PFGE-E-KL3-wzi59 (1/78, 1.3%), and ST2237-PFGE-F -KL19-wzi19 (2/78, 2.6%). ST11-PFGE-B-KL64-wzi64 (62.8%) is the most prevalent clone strains, distributed in 7 provinces in China, including Jiangxi (n = 18), Zhejiang (n = 2), Fujian (n = 2), and Shandong (n = 10), Hubei (n = 7), Henan (n = 4), and Shanghai (n = 6). The main prevalent clonal strains of ST11-PFGE-B-KL64-wzi64, mainly carrying the rmtB (48/49, 98%) gene.()
Table 3 Characteristics of Different Clonotypes in 16S RMTases-Positive Strains
Discussion
The emergence of CRKPs poses an urgent threat to public health worldwide.Citation21,Citation22 BSI caused by CRKP is a more serious problem due to ineffective antibiotics and high mortality.Citation23–Citation26 Clinically, therapeutic options against CRKP infections are limited. Aminoglycosides are still important companion antibiotics in combination therapy.Citation27 However, 16S RMTases have been found to mediate high levels of resistance to all clinically relevant aminoglycosides, such as amikacin, tobramycin and gentamicin.Citation10 To the best of our knowledge, this is the first report of describing the prevalence of 16S RMTase genes in CRKP clinical isolates associated with BSIs in several Chinese teaching hospitals.
To date, three plasmid-encoded 16S RMTase, including ArmA, RmtB and RmtC, have been found in clinical isolates of Gram-negative bacilli in China.Citation28,Citation29 The overall prevalence (56.9%) of 16S RMTase genes in CRKP Clinical isolates in the present study is much higher than what was found in Greece.Citation30 Surprisingly, the prevalence of 16S RMTase genes in CRKP isolates has reached 75% reported by a teaching hospital in northeast China in 2019.Citation31 The difference in the prevalence of 16S RMTase genes may be due to the type of specimens collected. This study shows that rmtB is the most prevalent gene among the 16S RMTase genes identified, which is consistent with previous studies.Citation31,Citation32 In addition, among 78 isolates positive for 16S RMTase genes, 72 (72/78, 92.3%) also harbored one or more AME genes. Due to the coexistence of these genes, this will make it difficult to predict the aminoglycoside resistance profile based on the effects of a single gene. Most (96.2%; 75/78) of the 16S RMTase-producing isolates in our collection were extended-spectrum β-lactamase (ESBL) producers and each isolate harbored at least one carbapenemase gene. These results indicate that a strong association between these resistance mechanisms. Therefore, the treatment of 16S RMTase-producing CRKP isolates with aminoglycoside and beta-lactam antibiotic combinations may have lost its clinical significance. Among contemporary CRKP clinical isolates from China, where KPC-producing K. pneumoniae remain predominant, followed by NDM-producing K. pneumoniae. The results are similar to those reported in previous studies of CRKP clinical isolates from different geographic regions in China.Citation33–Citation35 In this study, 78 16S RMTases positive strains were identified with 5 STs and 6 capsule serotypes. Compared with 16S RMTases-negative strains, the STs and capsular serotypes of 16S RMTases-positive strains are more concentrated, which may be caused by the dissemination of clone strains. In all 16S RMTases-positive and -negative CRKP strains, the major clonotype was ST11-KL64-wzi64, which is in accordance with previous study. A tertiary hospital in China reported the dominant clone ST11 CRKPs has undergone subclonal shift, of which the previously prevalent capsule locus (KL) 47 has been replaced by KL64.Citation36 PFGE results showed that most (62/78, 79.5%) 16S RMTase-producing CRKP isolates belong to cluster B, indicating that horizontal gene transfer and clonal spread were responsible for the dissemination of armA and rmtB genes. Among 78 16S RMTases-positive strains, the most prevalent clone type is ST11-PFGE-B-KL64-wzi64 (62.8%, 49/78), which mainly carries the rmtB and blaKPC genes and is distributed in 7 provinces in China. These results indicate that the ST11-PFGE-B-KL64-wzi64 CRKP clone strains may be spread in China, resulting in multiple resistance to aminoglycosides and other antibiotics.
In conclusion, a high prevalence of 16S RMTase genes was found among CRKP clinical isolates associated with BSIs in Chinese teaching hospitals. Dissemination of 16S RMTase genes among multidrug resistance (MDR) isolates is an unwelcome event. Therefore, intense molecular surveillance is thus imperative to inform strategies for containment.
Ethics Statement
Because the Klebsiella pneumoniae isolates in this study were part of the routine hospital laboratory procedure, the Ethics Committee of the first affiliated hospital of Wenzhou Medical University exempted this research for review.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors specially thank Professors Liang Chen and Mingliang Chen for their outstanding technical support.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
- Gonzalez LS 3rd, Spencer JP. Aminoglycosides: a practical review. Am Family Phy. 1998;58(8):1811–1820.
- Jiang M, Karasawa T, Steyger PS. Aminoglycoside induced cochleotoxicity: a review. Front Cell Neurosci. 2017;11:308.29062271
- O’Sullivan ME, Perez A, Lin R, Sajjadi A, Ricci AJ, Cheng AG. Towards the prevention of aminoglycoside related hearing loss. Front Cell Neurosci. 2017;11:325. doi:10.3389/fncel.2017.0032529093664
- Iovleva A, Doi Y. Carbapenem resistant enterobacteriaceae. Clin Lab Med. 2017;37(2):303–315. doi:10.1016/j.cll.2017.01.00528457352
- Yamane K, Wachino J, Doi Y, Kurokawa H, Arakawa Y. Global spread of multiple aminoglycoside resistance genes. Emerging Infect Dis. 2005;11(6):951–953. doi:10.3201/eid1106.04092415963295
- Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45(1):88–94. doi:10.1086/51860517554708
- Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother. 2000;44(12):3249–3256. doi:10.1128/AAC.44.12.3249-3256.200011083623
- Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(2):479–487. doi:10.1128/AAC.49.2.479-487.200515673721
- Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Res Updates. 2010;13(6):151–171. doi:10.1016/j.drup.2010.08.003
- Galimand M, Courvalin P, Lambert T. Plasmid-mediated high-level resistance to aminoglycosides in enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother. 2003;47(8):2565–2571. doi:10.1128/AAC.47.8.2565-2571.200312878520
- Doi Y, Wachino JI, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infectious Dis Clin North Am. 2016;30(2):523–537. doi:10.1016/j.idc.2016.02.01127208771
- Bercot B, Poirel L, Nordmann P. Updated multiplex polymerase chain reaction for detection of 16S rRNA methylases: high prevalence among NDM-1 producers. Diagn Microbiol Infect Dis. 2011;71(4):442–445. doi:10.1016/j.diagmicrobio.2011.08.01622000158
- CLSI. 2018 CLSI performance standards for antimicrobial susceptibility testing. 28th ed.CLSI supplement M100.Wayne. PA. 2018;2018:320.
- Wu Q, Zhang Y, Han L, Sun J, Ni Y. Plasmid-mediated 16S rRNA methylases in aminoglycoside-resistant enterobacteriaceae isolates in Shanghai, China. Antimicrob Agents Chemother. 2009;53(1):271–272. doi:10.1128/AAC.00748-0818955532
- Taylor E, Sriskandan S, Woodford N, Hopkins KL. High prevalence of 16S rRNA methyltransferases among carbapenemase-producing enterobacteriaceae in the UK and Ireland. Int J Antimicrob Agents. 2018;52(2):278–282. doi:10.1016/j.ijantimicag.2018.03.01629596903
- Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Computational Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
- Zankari E, Hasman H, Cosentino S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi:10.1093/jac/dks26122782487
- Yu T, He T, Yao H, et al. Prevalence of 16S rRNA methylase gene rmtB among escherichia coli isolated from bovine mastitis in Ningxia, China. Foodborne Pathogens Dis. 2015;12(9):770–777. doi:10.1089/fpd.2015.1983
- Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–4182. doi:10.1128/JCM.43.8.4178-4182.200516081970
- Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233–2239. doi:10.1128/JCM.33.9.2233-2239.19957494007
- Nordmann P. Carbapenemase-producing enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. 2014;44(2):51–56. doi:10.1016/j.medmal.2013.11.00724360201
- Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895.27379038
- Endimiani A, Depasquale JM, Forero S, et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrobial Chemother. 2009;64(5):1102–1110. doi:10.1093/jac/dkp327
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69(4):357–362. doi:10.1016/j.diagmicrobio.2010.10.01321396529
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infection Control Hospital Epidemiol. 2008;29(12):1099–1106. doi:10.1086/592412
- Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62:2.
- Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health. 2019;7:151. doi:10.3389/fpubh.2019.0015131245348
- Yu FY, Yao D, Pan JY, et al. High prevalence of plasmid-mediated 16S rRNA methylase gene rmtB among Escherichia coli clinical isolates from a Chinese teaching hospital. BMC Infect Dis. 2010;10:184. doi:10.1186/1471-2334-10-18420573216
- Huang J, Deng S, Ren J, Tu J, Ye M, Wang M. Characterization of a blaNDM1harboring plasmid from a Salmonella enterica clinical isolate in China. Mol Med Rep. 2017;16(2):1087–1092. doi:10.3892/mmr.2017.673328627648
- Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Souli M. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect Dis. 2019;19(1):167. doi:10.1186/s12879-019-3801-130770727
- Lin L, Xiao X, Wang X, Xia M, Liu S. In vitro antimicrobial susceptibility differences between carbapenem-resistant KPC-2-producing and NDM-1-producing Klebsiella pneumoniae in a teaching hospital in Northeast China. Microbial Drug Resist. 2019.
- Yu F, Wang L, Pan J, et al. Prevalence of 16S rRNA methylase genes in Klebsiella pneumoniae isolates from a Chinese teaching hospital: coexistence of rmtB and armA genes in the same isolate. Diagn Microbiol Infect Dis. 2009;64(1):57–63. doi:10.1016/j.diagmicrobio.2009.01.02019232867
- Yu X, Zhang W, Zhao Z, et al. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae isolates with focus on antimicrobial resistance. BMC Genomics. 2019;20(1):822. doi:10.1186/s12864-019-6225-931699025
- Zhang Y, Jin L, Ouyang P, et al. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrobial Chemother. 2019.
- Zeng L, Deng Q, Zeng T, Liu Y, Zhang J, Cao X. Prevalence of carbapenem-resistant klebsiella pneumoniae infection in southern China: clinical characteristics, antimicrobial resistance, virulence, and geographic distribution. Microb Drug Resist. 2019.
- Zhou K, Xiao T, David S, et al. Novel subclone of carbapenem-resistant Klebsiella pneumoniae sequence type 11 with enhanced virulence and transmissibility, China. Emerging Infect Dis. 2020;26(2):289–297. doi:10.3201/eid2602.19059431961299
