Abstract
Background
In chronic hepatitis B virus (CHB) patients, both dendritic cells (DCs) and T cells are functionally impaired and consequently the HBV-specific cellular immune responses are downregulated. The present study aims to investigate whether monocyte-derived DC (MoDCs)-pulsed-HBV subviral particles (HBVsvp) can polarize Th1 cells to induce HBV-specific cytotoxic T-lymphocytes (CTL) responses in CHB patients.
Methods and Materials
To this end, the human hepatoma HepG2.2.15 cell line was used to produce HBVsvp as a culturing system, and HBVsvp were concentrated for highly virus titer using the polyethylene glycol protocol. Peripheral blood mononuclear cells (PBMCs), collected from CHB patients and healthy donors, were differentiated into MoDCs and T cells. PBMCs-derived MoDCs were first pulsed with HBVsvp and then cultured with PBMCs-derived T cells. MoDCs and/or T subsets cells were identified for phenotypic activation by FACS analysis. The cytokine secretion of IL-4, IL-12, and IFN-γ in the culture supernatants was detected.
Results
The MoDCs were restored for their activation upon pulsing with HBVsvp in vitro, as identified by significantly overexpression of both CD86 and HLA-DR, and overproduction of IL-4 and IL-12. Furthermore, MoDCs-pulsed-HBVsvp induced Th1 frequencies and activated HBV-specific CTL to produce significantly highest amount of IFN-γ. Enhanced HBV-specific CTL led to strong cytolytic capacity against HepG2.2.15.
Conclusion
Overall, our data suggest that in vitro activation of MoDCs with HBVsvp overcomes the functionally impaired DCs and T cells in CHB patients offering a promising tool for therapeutic or vaccine-based approaches against HBV.
Introduction
HBV remains a major global public health problem, an estimated more than 400 million people are infected with HBV and one million people die annually of HBV liver diseases.Citation1,Citation2 Up to recent, it is unknown why individual HBV-infected people develop a chronic carrier state.Citation3 The insufficient immune response of HVB patients probably has an important role in the chronicity of HBV infection.Citation3,Citation4 The immune system is specialized to recognize and eliminate foreign antigens. Therefore, if the immune response for any reason has become tolerant to HBV, the HBV-specific adaptive immune responses will be turned down to undetectable limits.Citation5–Citation7 Until now, it is not well determined how the virus escapes from immune protection leading to the chronic state characterized by absence of HBV-specific T-cell responses.Citation3,Citation5,Citation8 In acute infection, patients exhibit a significant multi-specific CD8 CTL and CD4 Th1 cell responses.Citation9,Citation10
Dendritic cells (DCs) are essential professionally antigen-presenting cells (APCs) and acting as key players initiating T cell activation against viral agentsCitation11,Citation12 by strongly expressing different costimulatory and/or adhesion molecules.Citation11 An increasing titer of viruses, such as human immunodeficiency virus (HIV),Citation13 hepatitis C virus (HCV),Citation14 herpes simplex virus,Citation15 measles virus,Citation16 vaccinia virus,Citation17 may induce functional paralysis of DC that suppresses antiviral immune responses. The basic role of DC in HBV infection is still unclarified.Citation12 However, the function of DC must be studied in relation to HBV infection to apply DC as an immunotherapeutic tool. There are many approaches that have been established for obtaining highly purified DCs from human and mice.Citation18–Citation22 DCs that generated from human peripheral blood progenitors in vitro are considered to be immature and possess the endocytosis capacity of soluble antigens (Ags) and present them to naïve T cells.Citation20 MoDCs isolated from CHB patients are functionally impaired.Citation23
The mature DCs can activate naïve T cells and determine their polarization into Th1 or Th2 cells. Th1 cells produce high amounts of type-1 cytokines (IFN-γ, TNF-α/β, and IL-2) which prime cell-mediated immunity against viruses.Citation24 Th2 cells produce significant levels of type-2 cytokines (IL-4, IL-5, IL-6, and IL-10) which induce humoral immunity. Only IL-12 or combined with both TNF-α/β and IFN- γ has been shown to be shared in the polarization of Th1 cells and the development of CTLs, T-memory cells, and humoral response.Citation25,Citation26 The HBV-specific CD4+ T cells are responsible for the induction and activation of HBV-specific CD8+ T cells. So, HBV particles-pulsed DC vaccine able to activate CD4+ T cells, B cells, and induce HBV-specific CD8+ T cells are considered important components of immunotherapy.Citation27
In our previous study, we demonstrated that DC can be activated in vitro specifically against HBV and initiate immune responses,Citation21,Citation22 and the tolerance of HBV transgenic mice was break-down by using DC-based immunization, which may also be applicable in vivo to treat CHB patients.Citation22 Also, we showed that HBVsvp can stimulate DC for production a large array of cytokines.Citation28 The aim of the present study was to investigate whether MoDCs-pulsed-HBVsvp can polarize Th1 cells and induce an HBV-specific cytotoxic T-lymphocytes response in CHB patients and healthy donors, in vitro activation of MoDC with HBVsvp can overcome the impaired DC and T-cell function in CHB, as well as vaccination with ex vivo activated MoDCs may be a promising tool for therapeutic or vaccine-based approaches against HBV.
Methods and Materials
Generation of HBVsvp
HBVsvp were prepared as described previously.Citation21,Citation22 In brief, the cell line human hepatoma HepG2.2.15 was kindly provided by Dr. Stefan Urban (Heidelberg University, Germany), where the cell lines have not been purchased from an accredited commercial source and was approved by the ethics committee at the CCHE. The cells were cultured in Williams’ E medium (Sigma-Aldrich), supplemented with 100 U of penicillin/mL, 100 µg of streptomycin/mL, 2 mL glutamine, and 10% (v/v) FCS (Invitrogen), 5µg/mL insulin and 5µg/mL of hydrocortisone (Sigma-Aldrich). The HepG2.2.15 cells were cultured at 37°C in a CO2 incubator, which resulted in a strong production of HBsAg in the supernatant as shown in our previous results.Citation28 The HBVsvp was concentrated from the medium as described previouslyCitation21,Citation22 using polyethylene glycol (PEG) protocol. Briefly, HBVsvp containing supernatant was incubated with PEG (40%) overnight at 4°C and then centrifuged at 8000 rpm (4°C) for one hour. The pellets were resuspended in FCS/PBS (25%) followed by incubation at 4°C for overnight. The HBVsvp were harvested by centrifugation at 4000 rpm (4°C) for 20 min. Finally, the HBVsvp were quantified for HBsAg by ELISA technique and stored at −80°C.
Samples Collection
Peripheral heparinized blood samples were collected from 20 CHB patients and 20 healthy donors as control (). CHB patients who had received antiviral therapy within the past 6 months, or concurrent with HCV, HDV, or HIV infection or any immunological disorders were excluded.
Table 1 Shows the Patients and Healthy Controls Clinical Characteristics
For CHB patients, the median serum HBV DNA load was 65 × 105 geq/mL (range, 16 × 103–4 × 107) with mean 10.6 × 105 geq/mL, the median of alanine aminotransferase level (ALT) was 64 U/L (range, 17–156) with mean 67.35 U/L, the median of aspartate aminotransferase level (AST) was 67.5 U/L (range, 12–130) with mean 64.75 U/L, and the median of total bilirubin level was 1.25 (mg/dL) (range, 0.2–3.5) with mean 1.4 mg/dL (). The 20 patients were HBV surface antigen (HBsAg)-positive, and 18 patients were HBV envelope antigen (HBeAg)-positive. For controls, participants were negative to HBV, HCV, and HIV and free from any liver diseases. Control and CHB subjects were age/sex-matched.
All participants were consented to use their samples and clinical data (CHB patients) for this study. The consent form and the research proposal were approved by the ethical committee at the CCHE and the liver institute(s) that the CHB patients were recruited. After being consented, 30 mL of peripheral blood were collected from each participant using EDTA vacutainer.
Generation of Monocyte-Derived DCs (MoDCs)
PBMCs were prepared using a blood cell separator, then MoDCs were generated. Briefly, PBMCs (5×106 cells/mL) were suspended in 6-well plates in serum-free RPMI medium (Sigma-Aldrich) and incubated for 2 hours. Then, the non-adherent cells were gently collected and cryopreserved and used for autologous T cells. On the other hand, the adherent cells were incubated in complete RPMI medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) (20ng/mL) and IL-4 (20ng/mL) (Sigma-Aldrich) at 37°C in CO2 (5%) incubator. Fresh medium containing IL-4 and GM-CSF were replenished every 2 days. On day 7, the MoDC was harvested for phenotypic analysis by FACS analysis.
In vitro Activation of MoDCs with HBVsvp
On day 7, MoDCs (2×106 cells/mL) were placed in a 12-well culture plate and checked for their immaturity by FACS analyses. One day later, the MoDCs were activated by either HBVsvp, E. coli lipopolysaccharide (LPS) (1 μg/mL), HBVsvp + LPS or adding nothing as a negative control. On day 9, MoDCs were harvested, extensively washed, and measured for expression of activating markers by FACS analyses.
Flow Cytometric Analysis for MoDCs
MoDCs cells (2×105) were resuspended in FACS buffer (0.5% BSA-PBS) and stained with either anti-CD11c, anti-CD86, or anti-HLA-DR (BD Bioscience) on for 30 min on ice in dark. Cells were centrifuged at 2000 rpm for 3 min and washed twice with FACS buffer. Unstained cells were used for determination of the fluorescence baseline. All analyses were performed on a BD FACS Calibur™ flow cytometer and analyzed using Cell Quest Pro software (Beckton Dickinson, USA).
Th1/Tc Polarization Assessment
Among the non-adherent PBMCs, autologous T cells were determined by FACS analysis using anti-CD4 or anti-CD8 (BD Bioscience). The T cell polarization capacity of the MoDCs was determined using autologous T cells as responder T cells, which were co-cultured with MoDCs. Briefly, MoDCs pulsed with HBVsvp were co-cultured with autologous T cells at a MoDCs: T cells ratio of 1:5 in 24-well plates in complete RPMI1640 medium and incubated for 3 days. T cells were cultured alone as a control. On day 4, T cells from each well were detected T cells subsets.
Detection of HBV-Specific-CTL Cytotoxicity
HBV-specific CTL obtained from CHB patients and healthy donors were used to evaluate cytotoxicity by FACS analysis. Cells were counted and checked for viability by trypan blue exclusion. HBV-specific CTL was used as effector cells. Since the cell line HepG2.2.15 already has an HBV genome integrated into its chromosome was used as target cells. HepG2.2.15 was labelled with HEA125 FITC (5 µL), as a specific antibody, for 30 min on ice and incubated in the dark. Target cells were washed twice in FACS buffer. Approximately 2x105 target cells and 1×106 or 2×106 effectors cells (1:5 or 1:10 ratio) were resuspended in complete RPMI medium. Cells were centrifuged for 1 min at 800 rpm, incubated at 37°C in a CO2 incubator for 5–6 hours, and then were washed twice with FACS buffer. Cells were resuspended in 200 µL FACS buffer with 1.3 µg/mL propidium iodide (PI) to stain dead cells. For instrument settings and compensations, individual FITC-labelled target cells and HBV-specific CTL were used. Analysis gates were set on the target cells and the percentage of FITC+/PI+ cells were determined using Cell Quest Pro software.
Detection of Cytokines Activity by ELISA
The cytokines (IL-4, IL-12, and IFN-γ) were measured in the culture supernatants by ELISA kits according to the manufacturer’s instructions. The actual cytokine concentrations (pg/mL) were determined using standard reagents provided by the manufacturer.
Statistical Analysis
All data were analyzed by SPSS software (version 23) and summarized as the mean ± SD. ANOVA analysis was used to test for differences in marker values between normal control and chronic patient groups at different treatments. T-test was used to determine statistical differences (P<0.05 is significant).
Results
MoDCs are Strongly Activated by HBVsvp and Secreted High Levels of Cytokines in vitro
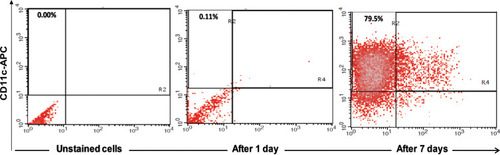
After incubation for 7 days, approximately 79.5% of the cultured PBMCs were positive for CD11c, indicating the differentiation of monocytes towards MoDCs (). showed strong activation of MoDCs by HBVsvp and the co-stimulatory factor LPS in vitro, expressing higher levels of CD86 and HLA-DR than negative control. The MoDCs activation was significant (p < 0.001) increased in both CHB patients and healthy donors compared to the negative control.
Table 2 Shows Strong Activation of MoDCs by HBVsvp in vitro, Which Expressed High Levels of CD86 and HLA-DR Than Negative Control. The MoDCs Activation Was Significantly (p < 0.001) Increased in Healthy Donors and Chronic Patients Comparing with the Negative Control for the Expression Levels of CD86 and HLA-DR Activation Markers
Figure 1 Showed FACS analysis of MoDCs, identified by expression of CD11c after 7 days of maturation, approximately 79.5% of the cultured cells were positive for CD11c, indicating the differentiation of monocytes towards MoDCs.
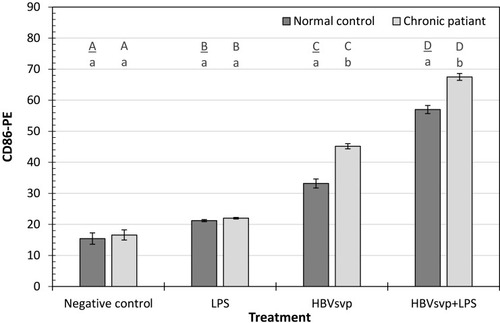
Our results indicated that after 24 hours incubation of MoDCs of healthy donors or CHB patients with HBVsvp and LPS, CD86 activating marker of MoDCs was significantly (p < 0.001) increased than the negative control. Regarding the incubation with HBVsvp alone, CD86 activating marker of MoDCs was also increased significantly (p < 0.001) comparing with negative control ().
Figure 2 Showed CD86-PE activating marker of MoDCs detected in healthy donors and chronic patient groups at different treatment. Small letters represent the significance between normal control and chronic patient within each treatment. Capital letters represent the significance of normal control or chronic patient at different treatments. Error bars represent standard error (SE).
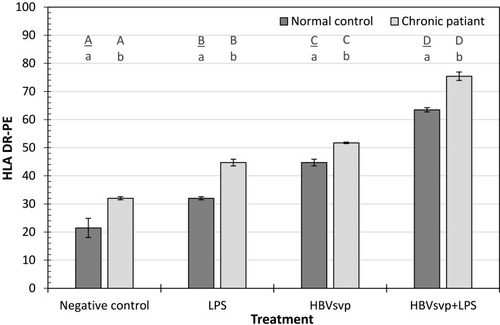
For the HLA-DR activating marker, our results showed after 24 hours incubation of MoDCs of healthy donors or chronic patients with HBVsvp plus LPS, the HLA-DR activating marker of MoDCs was significantly (p < 0.001) increased than the negative control. Regarding the incubation with HBVsvp alone, HLA-DR activating marker of MoDCs was also increased significantly (p < 0.001) comparing with the negative control (). Our results demonstrated that pulsing of MoDCs with HBVsvp alone or coupled with LPS upregulated expression of costimulatory molecules on MoDCs.
Figure 3 Showed HLA DR-PE activating marker of MoDCs detected in healthy donors and chronic patient groups at different treatment. Small letters represent the significance between normal control and chronic patient within each treatment. Capital letters represent the significance of normal control or chronic patient at different treatments. Error bars represent standard error (SE).
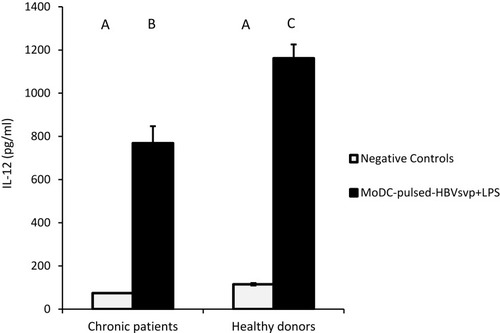
For cytokines secretions, our data showed that MoDCs pulsed with HBVsvp plus LPS produced significantly highest levels of IL-4 in both chronic HBV patients (p < 0.05) and healthy donors (p < 0.001), in vitro (). Regarding IL-12, our results showed that MoDCs co-cultured with HBVsvp plus LPS produced significantly elevated amounts of IL-12 in chronic HBV patients (p < 0.05) and healthy donors (p < 0.001), in vitro (). Our above-mentioned data indicated that pulsing of MoDCs with HBVsvp plus LPS upregulated expression of costimulatory molecules and enhanced the cytokine production ability of MoDCs.
Figure 4 Showed MoDCs from healthy donors and chronic patients were secreted significantly larger amounts of the effector cytokine IL-4 into the culture medium when co-cultured with HBVsvp+ LPS. Capital letters represent the statistical significance difference between normal control and chronic patient at different treatments and/or within each treatment. Error bars represent standard error (SE).
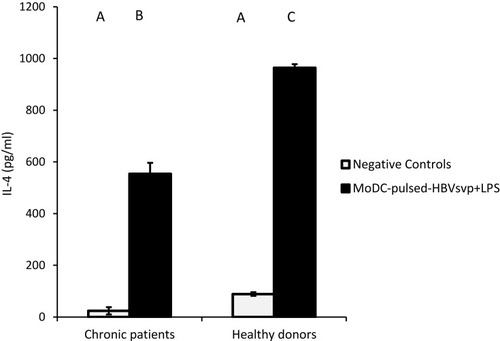
Figure 5 Showed MoDCs from healthy donors and chronic patients were secreted significantly larger amounts of the effector cytokine IL-12 into the culture medium when co-cultured with HBVsvp+ LPS. Capital letters represent the statistical significance difference between normal control and chronic patient at different treatments and/or within each treatment. Error bars represent standard error (SE).
MoDCs-Pulsed-HBVsvp Efficiently Induced Autologous T Cell Polarization into Th1/Tc in vitro
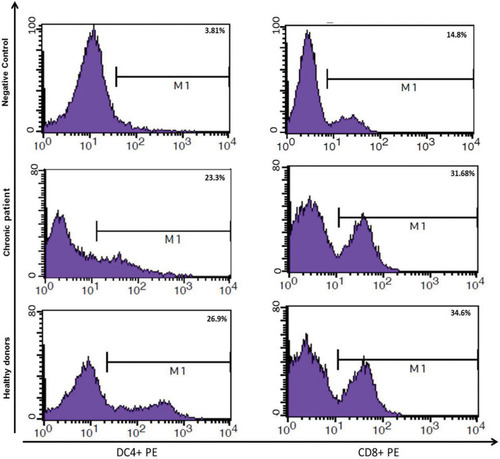
As shown in , MoDCs-pulsed-HBVsvp were able to induce Th1 and Tc in vitro at a MoDCs: T cell ratio (1:5). The Th1 and Tc frequencies were highly induced by MoDCs-pulsed-HBVsvp, indicating the ability of MoDCs-pulsed-HBVsvp to induce an autologous Th1/Tc polarization response in vitro for both chronic HBV patients and healthy donors and confirmed the ability of HBVsvp to up-regulate the stimulatory capacity of the MoDCs. Our data indicated that MoDCs induced efficiently autologous T cell polarization into Th1 and Tc cells.
Figure 6 Showed ability of MoDCs-pulsed-HBVsvp to induce an autologous Th1/Tc polarization response in vitro for both healthy donors and chronic patients.
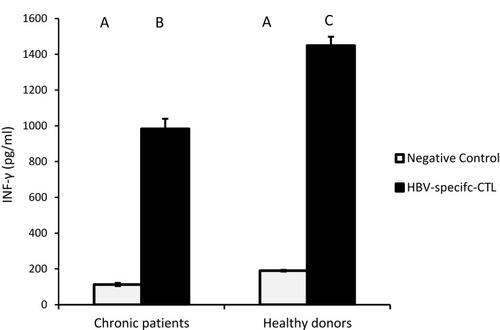
HBV-specific-CTL from chronic HBV patients and healthy donors secreted significantly elevated amounts of IFN-γ, the effector cytokine, into the culture medium upon co-culture with MoDCs-pulsed-HBVsvp. Our results showed that HBV-specific-CTL produced significantly elevated amounts of IFN-γ after co-culturing with MoDCs-pulsed-HBVsvp for chronic HBV patients (p < 0.05) and healthy donors (p < 0.001), in vitro ().
Figure 7 Showed HBV-specific-CTL from healthy donors and chronic patients were secreted significantly larger amounts of the effector cytokine IFN-γ into the culture medium when co-cultured with MoDCs-pulsed-HBVsvp. Capital letters represent the statistical significance difference between normal control and chronic patient at different treatments and/or within each treatment. Error bars represent standard error (SE).
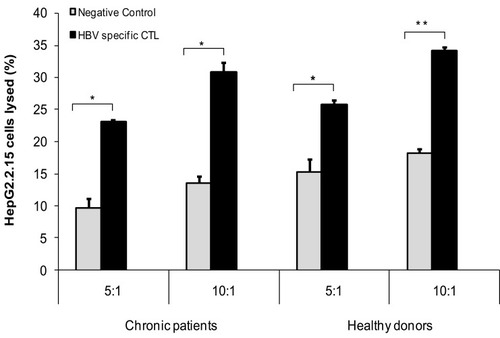
MoDCs-Pulsed-HBVsvp More Efficiently Induced HBV-Specific CTL Responses in vitro
Our results showed that MoDCs-pulsed-HBVsvp were able to activate specific HBV-specific-CTL and led to very strong cytolytic capacity against HepG2.2.15, in vitro. As presented in , the cytolytic capacity of HBV-specific CTL against HepG2.2.15 at different ratios (5:1 or 10:1) was significantly increased for either chronic HBV patients (p < 0.05) or healthy donors (p < 0.001) compared to negative control. These data indicated that MoDCs-pulsed-HBVsvp efficiently induced an HBV-specific CTL which had specific cytotoxicity in healthy donors and CHB patients, in vitro.
Discussion
The molecular mechanisms required by viruses to escape host-specific immune responses and result in chronic states still need clarification. Many viruses have developed a broad range of molecular changes to escape the host-specific immune response.Citation29,Citation30 Many viral immune evade mechanisms target the function of DCs.Citation13–Citation17 Previous reports showed that in chronic HBV infection, DCs are more sensitive to HBV and the presence of HBV particles is associated with impaired functions of MoDCs.Citation23 Earlier studies confirmed that the response of CHB patients to antiviral treatments was correlated with the predominant Th1 immunity and associated with enhanced CTL activity, indicating Th1 immunity could be essential regulator for treatment of CHB patients.Citation31,Citation32 In the present study, we investigated the ability of MoDCs-pulsed-HBVsvp to polarize Th1 cells and induce HBV-specific Cytotoxic T-Lymphocytes responses in both chronic HBV patients and healthy volunteers.
There is clear evidence that pathogens contribute to the production of defensive Th1 cells by their impacts on APCs.Citation33–Citation35 In our previous studies, we showed that mouse DC can specifically be activated in vitro against HBV and trigger immune responses, and the tolerance of HBV transgenic mice was broke down using DC-based immunization.Citation21,Citation22 In this study, we were able to prove that MoDCs can be triggered effectively with HBVsvp in vitro, which expressed high levels of costimulatory molecules such as CD86 and HLA-DR than negative control in healthy donors and chronic patients. Moreover, activation of MoDCs was highly significant in the presence of the co-stimulatory factor LPS. This finding was in consent with preceding studies that recorded higher levels of surface markers expression in HB-Ag-pulsed MoDCs,Citation24 and others have shown that the impaired DCs function could to some extent be restored by treating DC with pathogenic antigen in vitro.Citation36 In the present study, the HBsvp-pulsed DC vaccine developed much higher amounts of IL-4 and IL-12 than the DC control and was able to secrete IL-12 much more strongly and primarily induce polarization of Th cells into Th1 and Tc cells. This result coincided with the previous study, showing that HB-Ag-pulsed MoDCs expressed higher cytokine levels.Citation24
It is known that naive T cells can be primed by DC through three signals.Citation37,Citation38 The first signal results from antigen (peptide)–MHC complexes and assess immune antigen specificity, the second signal comes from costimulatory markers on the DC surface, the third signal is mediated by the cytokine balance or the OX40 ligand, and may decide polarization of naïve Th cells into Th1 or Th2 immune cells.Citation37,Citation38 HBV infection has been reported to have compromised MoDCs antigen-presenting function associated with Th1 immunity impairment, and that may play an important role in the processes of viral immune escape that results in persistent infection.Citation12 HBsAg pulsed DC vaccine has the potential to improve adaptive immunity against HBV, including a specific Th-cell and CTL response, to effectively control HBV infection. We found that both Th1 and Tc frequencies were strongly influenced by MoDCs-pulsed-HBVsvp, indicating the ability of MoDCs-pulsed-HBVsvp to induce an autologous Th1/Tc polarization response in vitro for both healthy subjects and chronic cases and confirmed the ability of HBVsvp to up-regulate the stimulatory capacity of the MoDCs. This finding agreed with previous studies that showed DC pulsed with HBV particles has been reported to effectively break CTL tolerance in transgenic HBV mice,Citation39–Citation41 and inducing immune response among healthy donors.Citation42,Citation43
Preferably, HBV particles trigger a cellular response consisting of Th1 cells.Citation44 In the previous study, it was shown that a DC vaccine pulsed with HBcAg instead HBsAg efficiently induces HBV-specific CTLs in HBV-associated HCC patients;Citation36 therefore, in the present study, pulsation of MoDCs with HBVsvp from healthy donors and CHB patients developed an HBV-specific DC vaccine. In our study, MoDCs-pulsed-HBVsvp were effectively induced specific HBV-specific-CTL and led to very strong cytolytic capacity against target cell in vitro; however, the frequency of specific CTLs of CHB patients was lower than healthy donors. Further, MoDCs-pulsed-HBVsvp also induced the T cells to release high levels of IFN-γ to inhibit the HBV infection through their non-cytolytic action. Those findings indicate that priming of the CD8+ T cells from CHB patients and healthy donors with HBVsvp-loaded DCs recovered its cellular role of destruction of target cells infected with HBV. This result conformed to the previous study, which indicated that HB-Ag-pulsed MoDC can effectively induce specific CTL of HBV and recovered its function against the HBV-infected cells in vitro and secrete much more of IFN-γ from stimulated the T cells.Citation24
However, our data showed that MoDCs activation, Th1 and Tc frequencies, and cytokines secretion was significantly higher in the presence of LPS combined with HBVsvp. As adjuvant, LPS stimulant immune system to extremely trigger immune responses and could be indispensable to fully activate the mononuclear cells, in vitro.Citation21,Citation22,Citation45,Citation46 We had to choose LPS because it has been shown a potential in vaccination, wide application, easy storage, and with both carrier and adjuvant functions that activate MoDCs.Citation47 LPS frequently used in humans and different animal models with its safety consideration. Additionally, the use of LPS as a non-infectious stimulant adjuvant for activation of MoDCs is much less risk than using (adeno-) viral vectors.Citation41,Citation48
To summarize, our findings demonstrate that MoDCs activation by HBVsvp can resolve the functionally impaired DCs and T cell in chronic HBV patients, suggesting that vaccination with ex vivo activated MoDCs could be a promising tool for therapeutic or prophylactic approaches against the HBV.
Abbreviations
Ags, antigens; APCs, antigen‐presenting cells; CD86, cluster of differentiation 86; CHB, chronic hepatitis B virus; CTL, cytotoxic T-lymphocytes; DC, dendritic cells; ELISA, enzyme linked immunosorbent assay; FACS, fluorescence-activated cell sorting; GMCSF, granulocyte macrophage colony-stimulating factor; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBVsvp, hepatitis B virus subviral particles; HLA-DR, human leukocyte antigen-DR isotype; IFN-γ, interferon gamma; IL-4, interleukin 4; IL-12, interleukin 12; LPS, lipopolysaccharide; MoDCs, monocyte-derived DCs.
Ethics Approval and Consent
Informed written consent was obtained from all participants included in the study. The consent form and the research proposal were approved by the ethical committee at the CCHE and the liver institute(s) that the CHB patients were recruited, and the research was conducted in accordance with the principles of the Declaration of Helsinki.
Author Contributions
All authors contributed to study design, execution, acquisition of data, analysis and interpretations, drafting and revising the manuscript, giving final approval of the version to be published, and agree to be accountable for all aspects of the work and individually responsible for its content.
Acknowledgments
We thank Dr. Stefan Urban and his group at University of Heidelberg and for their kind gift of HepG2.2.15 cell line. We would like to thank Mahmoud Mostafa for his kind help. We would like to thank Dr. Albaraa El-Saied and Dr. Ehab Fathy for their support and assistance with statistical analysis. We would like to express our deepest gratitude to the Egyptian ministry for scientific research and Science and technology development fund (STDF) in Egypt for financing this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol. 2011;45(6):1286–1296. doi:10.1016/j.jhep.2010.12.031
- Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based- therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006;12(18):2876–2883. doi:10.3748/wjg.v12.i18.287616718812
- van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40(3):738–746. doi:10.1002/hep.2036615349914
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13(1):29–60. doi:10.1146/annurev.iy.13.040195.0003337612225
- Marinos G, Torre F, Chokshi S, et al. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology. 1995;22(4):1040–1049. doi:10.1016/0270-9139(95)90607-x7557849
- Maini MK, Boni C, Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191(8):1269–1280. doi:10.1084/jem.191.8.126910770795
- Op den Brouw ML, Binda RS, van Roosmalen MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126(2):280–289. doi:10.1111/j.1365-2567.2008.02896.x18624732
- Cesar Aguilar J, Lobaina Y. Immunotherapy for chronic hepatitis B using HBsAg-based vaccine formulations: from preventive commercial vaccines to therapeutic approach julio cesar aguilar. Euroasian J Hepatogastroenterol. 2014;4(2):92–97. doi:10.5005/jp-journals-10018-110929699355
- Maini MK, Boni C, Ogg GS, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117(6):1386–1396. doi:10.1016/s0016-5085(99)70289-110579980
- Webster GJ, Reignat S, Maini MK, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32(5):1117–1124. doi:10.1053/jhep.2000.1932411050064
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9(1):271–296. doi:10.1146/annurev.iy.09.040191.0014151910679
- Beckebaum S, Cicinnati VR, Zhang X, et al. Hepatitis B virus-induced defect of monocyte derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109(4):487–495. doi:10.1046/j.1365-2567.2003.01699.x12871214
- Knight SC, Macatonia SE, Patterson S. Infection of dendritic cells with HIV1: virus load regulates stimulation and suppression of T‐cell activity. Res Virol. 1993;144:75–80. doi:10.1016/s0923-2516(06)80015-48446782
- Auffermann‐Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97(10):3171–3176. doi:10.1182/blood.v97.10.317111342445
- Kruse M, Rosorius O, Kratzer F, et al. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T‐cell stimulatory capacity. J Virol. 2000;74(15):7127–7136. doi:10.1128/jvi.74.15.7127-7136.200010888653
- Grosjean I, Caux C, Bella C, et al. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186(6):801–812. doi:10.1084/jem.186.6.8019294135
- Engelmayer J, Larsson M, Subklewe M, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163(12):6762–6768.10586075
- Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte macrophage colony stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi:10.1084/jem.176.6.16931460426
- Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180(1):83–93. doi:10.1084/jem.180.1.838006603
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down-regulated by tumor necrosis factor a. J Exp Med. 1994;179(4):1109–1118. doi:10.1084/jem.179.4.11098145033
- Farag MM, Hoyler B, Encke J, et al. Dendritic cells can effectively be pulsed by HBVsvp and induce specific immune reactions in mice. Vaccine. 2010;29(2):200–206. doi:10.1016/j.vaccine.2010.10.05621050902
- Farag MMS, Tedjokusumo R, Flechtenmacher C, et al. Immune tolerance against HBV can be overcome in HBV transgenic mice by immunization with dendritic cells pulsed by HBVsvp. Vaccine. 2012;30(42):6034–6039. doi:10.1016/j.vaccine.2012.07.05722867720
- Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104(2):138–150. doi:10.1006/clim.2002.524512165275
- Chen W, Shi M, Shi F, et al. HBcAg-pulsed dendritic cell vaccine induces Th1 polarization and production of hepatitis B virus-specific cytotoxic T lymphocytes. Hepatol Res. 2009;39(4):355–365. doi:10.1111/j.1872-034X.2008.00468.x19889049
- Mizukoshi E, Sidney J, Livingston B, et al. Cellular immune responses to the hepatitis B virus polymerase. J Immunol. 2004;173(9):5863–5871. doi:10.4049/jimmunol.173.9.586315494540
- Szkaradkiewicz A, Jopek A, Wysocki J. Effects of IL-12 and IL-18 on HBcAg-specific cytokine production by CD4 T lymphocytes of children with chronic hepatitis B infection. Antiviral Res. 2005;66(1):23–27. doi:10.1016/j.antiviral.2004.12.00515781128
- Akbar SM, Furukawa S, Horiike N, et al. Vaccine therapy for hepatitis B virus carrier. Curr Drug Targets Infect Disord. 2004;4(2):93–101. doi:10.2174/156800504334088515180457
- Farag MM, Peschel G, Müller M, et al. Characterization of the interaction between subviral particles of hepatitis B virus and dendritc cells in vitro study. Infect Drug Resist. 2019;12:3125–3135. doi:10.2147/IDR.S22129431632101
- Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen‐specific T‐cell tolerance. Nat Med. 2000;6(12):1348–1354. doi:10.1038/8216111100119
- Bertoletti A, Sette A, Chisari FV, et al. Natural variants of cytotoxic epitopes are T‐cell receptor antagonists for anti‐viral cytotoxic T cells. Nature. 1994;369(6479):407–410. doi:10.1038/369407a08196768
- Tsai SL, Sheen IS, Chien RN, et al. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10(1):120–135. doi:10.1007/bf225600412566993
- Boni C, Bertoletti A, Penna A, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102(5):968–975. doi:10.1172/JCI37319727065
- de Jong EC, Vieira PL, Kalinski P, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168(4):1704–1709. doi:10.4049/jimmunol.168.4.170411823500
- d’Ostiani CF, Del Sero G, Bacci A, et al. Dendritic cells discriminate between yeasts and hyphae of the fungus candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191(10):1661–1674. doi:10.1084/jem.191.10.166110811860
- Vieira PL, de Jong EC, Wierenga EA, et al. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164(9):4507–4512. doi:10.4049/jimmunol.164.9.450710779751
- Shi M, Qian S, Chen WW, et al. Hepatitis B virus (HBV) antigen-pulsed monocyte-derived dendritic cells from HBV-associated hepatocellular carcinoma patients significantly enhance specific T cell responses in vitro. Clin Exp Immunol. 2007;147(2):277–286. doi:10.1111/j.1365-2249.2006.03281.x17223969
- Kapsenberg ML. Dendritic-cell control of pathogen driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–993. doi:10.1038/nri124614647480
- Reise Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–483. doi:10.1038/nri184516691244
- Shimizu Y, Guidotti LG, Fowler P, et al. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161(9):4520–4529.9794377
- Oka Y, Akbar SM, Horiike N, et al. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103(1):90–97. doi:10.1046/j.1365-2567.2001.01202.x11380696
- Huang Y, Chen Z, Jia H, et al. Induction of Tc1 response and enhanced cytotoxic T lymphocyte activity in mice by dendritic cells transduced with adenovirus expressing HBsAg. Clin Immunol. 2006;119(3):280–290. doi:10.1016/j.clim.2006.01.01516531121
- Fazle Akbar SM, Furukawa S, Onji M, et al. Safety and efficacy of hepatitis B surface antigen-pulsed dendritic cells in human volunteers. Hepatol Res. 2004;29(3):136–141. doi:10.1016/j.hepres.2004.03.00315203076
- Akbar SM, Furukawa S, Hasebe A, et al. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrier. Int J Mol Med. 2004;14(2):295–299.15254781
- Milich DR, Schödel F, Hughes JL, et al. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71(3):2192–2201. doi:10.1128/JVI.71.3.2192-2201.19979032353
- Ma XJ, Tian DY, Xu D, et al. Uric acid enhances T cell immune responses to hepatitis B surface antigen-pulsed-dendritic cells in mice. World J Gastroenterol. 2007;13(7):1060–1066. doi:10.3748/wjg.v13.i7.106017373740
- Phillipps KS, Wykes MN, Liu XQ, et al. A novel synthetic adjuvant enhances dendritic cell function. Immunology. 2009;128(1):582–588. doi:10.1111/j.1365-2567.2008.03038.x
- Gamvrellis A, Leong D, Hanley JC, et al. Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol. 2004;82(5):506–516. doi:10.1111/j.0818-9641.2004.01271.x15479436
- Qiu SJ, Lu L, Qiao C, et al. Induction of tumor immunity and cytotoxic t lymphocyte responses using dendritic cells transduced by adenoviral vectors encoding HBsAg: comparison to protein immunization. J Cancer Res Clin Oncol. 2005;131(7):429–438. doi:10.1007/s00432-004-0616-115818505