Abstract
Background
It seems alternative treatments such as antioxidant intervention and anti-inflammatory intervention adjuvant to antibiotic regimens may enhance cancer prevention approaches and decrease adverse side effects related to therapeutic antibiotic regimens. So, we will evaluate the effects of concurrent omega-3 and cranberry juice supplementation along with standard antibiotic therapy on the eradication of Helicobacter pylori, gastrointestinal symptoms, some serum inflammatory and oxidative stress markers in adults with HP infection.
Methods
We will conduct a 4-week double-blinded randomized clinical trial. The subjects will be randomly stratified according to sex and BMI using a permuted block randomization procedure by Random Allocation Software (RAS). They will be assigned to one of the four study groups: (1) cranberry juice fortified with omega-3 Intervention (n=23), (2) cranberry juice intervention group (n=23), (3) placebo beverage fortified with omega-3 intervention group (n=23), and (4) placebo beverage intervention (control group) (n=23). All statistical analyses will be performed using IBM SPSS Statistics software.
Discussion
A combination of alternative therapies may have a synergistic effect compared to a single approach. It could potentially be more effective in promoting the efficacy of standard antibiotic therapy in eradicating HP infection.
Trial Registration
Iranian Registry of Clinical Trials (IRCT20151128025274N3, www.irct.ir/trial/28997).
Trial Status
This study is in the early stages of sampling.
Background
Helicobacter pylori (HP), a gram-negative bacterium, is rated as the most common human pathogen affecting approximately over half of the world’s population.Citation1,Citation2 Of course, the prevalence of this infection varies widely in different regions of the world with higher levels in developing countries, which can partly be elucidated by low socioeconomic conditions and lack of hygiene.Citation3 Adhesion HP organism to the underlying epithelium can cause inflammation and ulcer in the stomach or duodenum, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma.Citation4,Citation5 The virulence factors of HP can be divided into three major pathogenic categories based on their function, including colonization (i.e., urease, adhesins, and flagella chemotaxis system), immune escape (i.e., LPS and flagella, Cag A and T4SS, Vac A) and disease induction (Vac A, Bab A, Dup A).Citation6,Citation7 The interaction between the HP and its virulence factors, host normal mucosa, and environmental factors can induce stepwise processes: 1) chronic gastritis, inflammatory responses and stress oxidative; 2) tumor suppressor gene dysfunction and migration of bone-marrow-derived cells (BMDCs); 3) genetic alterations, epigenetic alterations; 4) oncogene activation, tumor suppressor inactivation, dysregulation of DNA repair, alteration of microRNAs; and 5) gastric carcinogenesis.Citation4,Citation8 Eradication of HP has been demonstrated to relapse HP-related diseases.Citation9 The treatment regimens of HP include clarithromycin triple therapy, bismuth quadruple therapy, concomitant therapy, sequential therapy, hybrid therapy, levofloxacin triple therapy, and fluoroquinolone sequential therapy.Citation10,Citation11 However, the effectiveness of the HP eradication treatment has reduced substantially in many countries due to antibiotic resistance.Citation12,Citation13 Indeed, several states have passed the 15–20% threshold for antibiotic resistance in the past 15 years.Citation13,Citation14 Thus, natural, non-toxic, anti-inflammatory, and alternative antimicrobial therapies, that decrease bacterial settlement and colonization, gastric inflammation, and mucosal atrophy are needed.Citation15,Citation16 It seems some of these modes can improve the effectiveness of traditional antibiotic therapy and simultaneously inhibit adverse antibiotic effects.Citation17 Among such alternative approaches, cranberry (vaccinium macrocarpon) is one of the most hopeful interventions. Extract of cranberries has been shown to exert similar inhibitory activities against HP, probably due to their high content of phenolic phytochemicals such as phenolic acids, flavonoids, and ellagic acid.Citation18 Previously, Burger et al showed the high-molecular-weight; the non-dialysable component of cranberry juice prevents specific (S-fimbriae) adhesion of HP to the human stomach epithelial cell, immobilized human mucus, and erythrocytes, in vitro.Citation19 Also, in a prospective, randomized, double-blind, placebo-controlled trial, Zhang et al concluded that daily consumption of 250 mL cranberry juice for 90 days could inhibit HP infection in afflicted adults.Citation20 However, in a randomized clinical trial by Kantiokari et al, cranberry juice for 3 months did not change the bacterial flora of the stools and gastric symptoms.Citation21
Omega-3 polyunsaturated fatty acids (which including ALA, EPA, and DHA) have antioxidant, anti-inflammation, and anticancer properties.Citation22–Citation24 Omega-3 fatty acids, particularly EPA, are the substrates of the third series of Prostaglandins (PGE3), which have an anti-inflammatory property, inhibit platelet aggregation, improve blood flow.Citation24 The n-3 fatty acids can suppress various proinflammatory mediators, such as IL-1β, IL-6, IL-8, IL-12, TNF-alpha, NF-κB, and PGE1.Citation25,Citation26 Gastric epithelial cells that were cocultured with DHA and HP displayed a fourfold decrease in IL-8 generation and a reduction in expression of COX-2 and iNOS compared to non-DHA treated bacteria.Citation27 It is shown that Fish oil, which contains 33.5% n-3-fatty acids, has a bacteriostatic effect on HP in vitro and invitro.Citation28 Still, in contrast to these pieces of evidence, Meier et al concluded omega-3 is unlikely to be useful in HP eradication regimens.Citation29 It is likely, the combination of alternative therapies compared to a single approach exerts a synergic effect. It can potentially be more effective in promoting the efficacy of standard antibiotic therapy in eradicating HP infection. Thus, we aimed for the first time to evaluate the effects of concurrent omega-3 and cranberry juice supplementation along with standard antibiotic therapy on the eradication of Helicobacter pylori, gastrointestinal symptoms, some serum inflammatory and oxidative stress markers in adults with HP infection.
Methods
Study Design
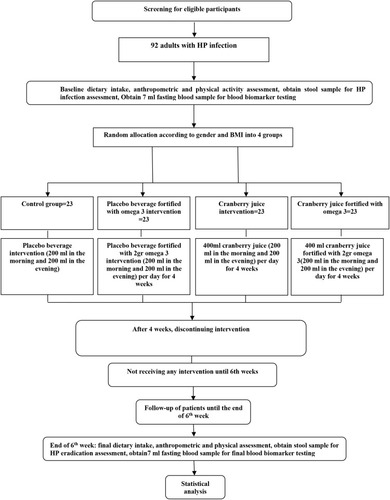
We will conduct a 4 week double-blinded randomized clinical trial. The proposed clinical trial will be held at the Nutritional Research Center, Department of Nutrition, the Ahvaz Jundishapur University of Medical Science for 4 weeks to assess the efficacy of daily concurrent omega-3 and cranberry juice supplementation in subjects with HP infection ().
Figure 1 Protocol flow diagram; We will conduct a 4-week parallel-group, randomized controlled trial to determine the effects of concurrent omega-3 and cranberry juice consumption on eradication of Helicobacter pylori, gastrointestinal symptoms, some serum inflammatory and oxidative stress markers in adults with Helicobacter pylori infection.
Aims and Study Hypotheses
The primary aim of the current trial is to examine the effect of 4 weeks’ omega-3 supplementation, cranberry juice supplementation, cranberry juice fortified with omega-3 supplementation on the eradication of Helicobacter pylori, gastrointestinal symptoms, serum levels of IL-17, TGFβ1, INF-γ, MDA and TAC in adults with HP infection. The secondary aim is to determine the associations between changes in biochemistry variables and clinical others. It is hypothesized that 4 weeks’ concurrent supplementation with omega-3 and cranberry will improve measures of investigation.
Participants
By referring to the Soroush clinic in the city of Ahvaz, 92 adults meeting the inclusion criteria will be recruited in this trial after obtaining their written consent.
The following inclusion criteria will be applied: Age 18–65 years old, having HP infection, gastrointestinal complaints, BMI range of 18.5 to 35 kg/m2, absence of lactation, pregnancy, history of food allergies, suffering from any type cancer, diabetes, as well as hepatic, renal, thyroid, and other gastrointestinal disorders, no gastrointestinal surgery, taking no antibiotics, PPIs, and H2 blockers over the past 2 months, taking no herbs and omega-3 supplements as well as vitamin-mineral supplements over the past 6 months. The subjects with any of the following criteria will be excluded from the study: Changes in diet during the study period, become pregnant during the study, unwilling to continue, and no consumption of supplements exceed 20% of total administered supplements.
Ethics and Trial Registration
The participants who meet the inclusion criteria will be fully explained about the study’s protocol. This protocol, approved by the Medical Ethics Committee of Ahvaz University of Medical Sciences, is in accordance with the Declaration of Helsinki (approval number: IR.AJUMS.REC.1396.819). Each participant will sign an informed consent form. This randomized clinical trial was registered on the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20151128025274N3, www.irct.ir/trial/28997).
Sample Size
Sample size calculated based on studies by Kim et alCitation30 and Santra et alCitation31 according to compare the rate of disease recovery in four groups considering 5%, 14%, 53%, and 60% rate of disease recovery. It was calculated 95% confidence interval and 80% power (α=0.05 and β=0.2), using the NCSS software. Finally, considering the 20% sample loss, 23 subjects were recruited for each group.
Randomization
The subjects will be randomly stratified according to sex and BMI using a permuted block randomization procedure by Random Allocation Software (RAS). They will be assigned to one of the four study groups ().
(1) Cranberry juice fortified with omega-3 Intervention+ standard antibiotic therapy (n=23).
(2) Cranberry juice intervention group+ standard antibiotic therapy (n=23).
(3) Placebo beverage fortified with omega-3 intervention group + standard antibiotic therapy (n=23).
(4) Placebo beverage intervention + standard antibiotic therapy (control group) (n=23).
The standard antibiotic therapy will comprise 14-day 4 drug therapy, omeprazole (20mg, one tablet at the morning and one tablet at night), clarithromycin (500mg, one capsule at the morning and one capsule at night), amoxicillin (500mg, two capsules at the morning and two capsules at night), and bismuth (2 tablets at the morning and two capsules at night).
Intervention
Cranberry juice will be prepared from cranberry concentrate by dilution (4%, v/v) in potable tap water; 0.25 g/L of sucralose will be added as a sweetener. The placebo beverage will have the same taste and flavor as the cranberry juice containing vitamin C (0.5 g/L), sucralose (0.2 g/L), 1.8 g/L of citric acid, malic acid (0.3 g/L), benzoic acid (0.5 g/L), and natural cranberry flavoring (0.5g/L) and coloring (0.8 g/L).Citation32,Citation33 Tak Daneh Company, Iran, will supply both the cranberry juice and the placebo beverage. Moreover, that company will carry out the process of fortifying cranberry juice with omega-3.
The cranberry juice fortified with omega-3 was produced in pilot research, and after several days, the final product will be investigated in terms of organoleptic and antioxidant capacity. Every 400 mL of cranberry juice is fortified with 2 gr omega-3 (1200 EPA and 800 DHA). Omega-3 capsules will be supplied by Karen Pharma & Food Supplement Co., Tehran, Iran.
All the subjects will be requested to consume regularly supplements every day for 4 weeks. Each intervention group 1 subjects will receive 400 mL cranberry juice fortified with 2 gr omega-3 daily. Each intervention group 2 and 3 will consume 400 mL cranberry juice and placebo beverage fortified with 2 gr omega-3 capsules per day, respectively. The control group will receive 400 mL placebo beverage. Cranberry juice or corresponding placebo beverage will be consumed twice daily, 200 mL in the morning and 200 mL in the evening. To check compliance, subjects will be asked to record the time and date of supplement intake. Moreover, to ensure that the intervention group subjects regularly consume the supplement, they will be contacted every 3 days by a dietitian, or if it was not possible to call them, they would be followed through SMS. The study subjects will be asked not to change their dietary habits and physical activity during the study (6 weeks).
Assessment of Dietary Intake, Anthropometric Parameters, and Physical Activity
A demographic questionnaire will be applied at the beginning of the study. Dietary intake will be evaluated by 3 days food record approach (two weekdays and one weekend day) at the beginning and end of the study. Participants will be explained about the food recording by a trained dietician. Dietary intake data will be analyzed by Nut IV (the Hearst Corporation, San Bruno, CA). The same dietitian will measure anthropometric variables such as body weight, height, and BMI. Bodyweight will be measured with the accuracy of 100 gr using a Seca scale at the baseline and end of the study. Height will be assessed in a relaxed position by a Seca stadiometer with an accuracy of 0.5 cm. Then, BMI will be computed as body weight (kg) divided by the square of height (m), the beginning and end of the study. To acquire physical activity levels of participants, the International Physical Activity Questionnaire (IPAQ) will be applied at the baseline, and the end of the study in an interview, and the results will be represented as “high,” “moderate,” and “low” activity. The Persian translation of the short form IPAQ has been validated by Dashti et al (Cronbach’s alpha=0.7 and test–retest reliability coefficient=0.9).Citation34
Assessment of Gastrointestinal Complaints
To assess the Gastrointestinal common symptoms, a Persian version of the gastrointestinal symptom rating scale (GSRS) will be used. This instrument contains 15 items, with five subscales (symptom clusters) including reflux, abdominal pain, indigestion, diarrhea, and constipation. This disease-specific tool is graded on a 7-point Likert scale, that 1 expresses no discomfort at all, and 7 represents very severe discomfort. Previously, in an Iranian population with functional gastrointestinal disorders, Cronbach α values of the GSRS and the subscales of diarrhea, abdominal pain, constipation, indigestion were equivalent to 0.81, 0.70, 0.70, 0.63, 0.76, respectively.Citation35
Assessment of HP Eradication
At the baseline and sixth weeks, stool samples (1–2 mL) will be collected using a kit consisting of a plastic spoon. The samples will be frozen at a temperature below −20°C until tested. The quantitative and qualitative measurement of HP organisms in the stool will be determined by microplates-based enzyme immunoassay technique (DIA.PRO, Diagnostic Bioprobes Srl, Milano, Italy). The test principle as follows: monoclonal highly purified HP antibody will be coated onto the wall of microwells. An aliquot of a diluted fecal specimen will be added to microtiter wells of a microplate, and the HP antigens will bind to the antibody after an incubation period. The unbound material will be washed away. After adding enzyme conjugate, it will bind to the antibody-antigen complex and will form the immunocomplex of “HP antibody–HP antigen–HRP-conjugated enzyme vonjugate.” The unbound enzyme conjugate will be washed off in the subsequent step. HRP-conjugated enzyme conjugate bound to the microwell will then be incubated with a substrate solution in a timed reaction and then measured in a spectrophotometric microwell reader compared in a parallel manner with calibrator and controls. The enzymatic activity of the enzyme conjugate antibody bound to HP proteins captured on the wall of each microtiter well will be proportional to the amount of antigen in each test sample. The minimal concentration of HP determined by this kit will be <0.015 mg/g of stool. The suggested positive cut-off for fecal HP antigen is 3 ng/mL.
Assessment of Biochemistry Variables
At baseline and end of the study, 7 mL of venous blood samples (in regular tubes) will be collected after 12 hours of overnight fasting. ELISA kits (IBL International GmbH) will be used to determine serum levels of IL-17, TGFβ1, and INF-γ. Moreover, serum levels of TAC and MDA will be measured by ELISA kits (Zell Bio GmbH).
Statistical Analysis
All statistical analyses will be performed using the IBM SPSS Statistics software (Version 18) (IBM SPSS Statistics, Armonk, USA). All data will be presented as mean ± SD. The normality of the variables will be confirmed using the Kolmogorov–Smirnov test. The percent change of each variable will be also calculated by the formula [(E − B)/B × 100], where E is the end of treatment values and B is the baseline values. A chi-square test will be used to compare the categorical data between treatment groups at the baseline. ANOVA and Kruskal Wallis test will be applied to compare parametric continuous and nonparametric data between the groups, respectively. Paired sample t-test or Wilcoxon signed-rank test will be applied to compare data within the groups. To control confounding variables, analysis of covariance (ANCOVA) test will be used to determine the differences between the groups post-intervention, while adjusting for baseline measurements and covariates. A p-value of less than 0.05 will be regarded to be statistically significant.
Discussion
The International Agency for Research on Cancer has classified the HP infection as a type I carcinogen.Citation36 Traditional HP infection eradication includes pharmacological therapy, which is based on at least two known antibiotics combined with PPI. However, the eradication rate of this organism has been reducing because of the rising prevalence of antibiotic resistance, particularly clarithromycin resistance. It seems that a combination of omega-3 fatty acids and an antioxidant increase anti-inflammatory and antioxidant effects of omega-3 fatty acids.Citation37 Since polyunsaturated long-chain fatty acids are susceptible to oxidation, the combination of omega-3 fatty acids and antioxidants have earlier been used in previous evidences.Citation37,Citation38 In addition, studies have indicated that solely fish oil supplementation increases oxidative stress, which could be due to the effect of fish oil on the decrease of antioxidant enzyme expression.Citation39 It seems that antioxidants prevent the oxidative stress that may be induced by omega-3 fatty acids. Thus, their co-supplementations may be more effective than single supplementation.Citation38
Abbreviations
HP, Helicobacter pylori; MALT, mucosa-associated lymphoid tissue; LPS, lipopolysaccharide; Cag A, cytotoxin-associated gene A; Vac A, vacuolating cytotoxin gene A; Bab A, blood group antigen-binding adhesin A; Dup A, duodenal ulcer promoter gene A; T4SS, type IV secretion systems; ALA, α-Linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; PG, prostaglandins; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin8; IL-12, interleukin 12; TNF-alpha, tumor necrosis factor-alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IL-17, interleukin 17; TGFβ1, transforming growth factor beta-1; INF-γ, interferon gamma; MDA, malondialdehyde; TAC, total antioxidant capacity; IPAQ, International Physical Activity Questionnaire.
Ethics Approval and Consent to Participate
This protocol, approved by the Medical Ethics Committee of Ahvaz University of Medical Sciences, is in accordance with the Declaration of Helsinki (approval number: IR.AJUMS.REC.1396.819). Each participant will sign an informed consent form. This randomized clinical trial was registered on the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20151128025274N3).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgment
We hereby express our gratitude to the Ahvaz Jundishapur University of Medical Sciences for funding this trial.
Disclosure
The authors declare that they have no competing interests in this work.
References
- Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta‐analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi:10.1111/apt.1456129430669
- Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi:10.1053/j.gastro.2017.04.02228456631
- Hunt R, Xiao S, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterol Organ Global Guidelines. 2010;1–5.
- Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi:10.1016/j.canlet.2013.08.01623981572
- Kalisperati P, Spanou E, Pateras IS, et al. Inflammation, DNA damage, Helicobacter pylori and gastric tumorigenesis. Front Genet. 2017;8:20. doi:10.3389/fgene.2017.0002028289428
- Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629. doi:10.1038/nrgastro.2010.15420938460
- Sicinschi LA, Correa P, Bravo LE, et al. Non‐invasive genotyping of Helicobacter pylori cagA, vacA, and hopQ from asymptomatic children. Helicobacter. 2012;17(2):96–106. doi:10.1111/j.1523-5378.2011.00919.x22404439
- Chang W-L, Yeh Y-C, Sheu B-S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci. 2018;25(1):68. doi:10.1186/s12929-018-0466-930205817
- Chey WD, Wong BC. American college of gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808. doi:10.1111/j.1572-0241.2007.01393.x17608775
- Khaleghi S, Naghibi S, Naghibi S. Comparison of sequential and routine four drugs therapeutic regiments in Helicobacter pylori eradication. J Gorgan Univ Med Sci. 2013;15(3):1–6.
- Moradimoghadam F, Khosravi Khorashad A, Mokhtarifar A. Comparison between quadruple therapy and triple therapy for eradication of Helicobacter pylori in patients with chronic dyspepsia. Horiz Med Sci. 2009;14(4):13–18.
- Farzi N, Yadegar A, Sadeghi A, et al. High prevalence of antibiotic resistance in Iranian Helicobacter pylori isolates: importance of functional and mutational analysis of resistance genes and virulence genotyping. J Clin Med. 2019;8(11):2004. doi:10.3390/jcm8112004
- Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372–1382. e1317. doi:10.1053/j.gastro.2018.07.00729990487
- Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: a recent literature review. World J Methodol. 2015;5(3):164. doi:10.5662/wjm.v5.i3.16426413490
- Hołubiuk Ł, Imiela J. Diet and Helicobacter pylori infection. Prz Gastroenterol. 2016;11(3):150.27713775
- Park S-H, Kangwan N, Park J-M, Kim E-H, Hahm KB. Non-microbial approach for Helicobacter pylori as faster track to prevent gastric cancer than simple eradication. World J Gastroenterol. 2013;19(47):8986. doi:10.3748/wjg.v19.i47.898624379623
- Han Y-M, Park J-M, Jeong M, et al. Dietary, non-microbial intervention to prevent Helicobacter pylori-associated gastric diseases. Ann Transl Med. 2015;3(9).
- Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr. 2005;14(2):120.15927928
- Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42(S3):279–284. doi:10.1080/1040839020935191612058986
- Zhang L, Ma J, Pan K, Go VLW, Chen J, You W. Efficacy of cranberry juice on Helicobacter pylori infection: a double‐blind, randomized placebo‐controlled trial. Helicobacter. 2005;10(2):139–145. doi:10.1111/j.1523-5378.2005.00301.x15810945
- Kontiokari T, Salo J, Eerola E, Uhari M. Cranberry juice and bacterial colonization in children—a placebo-controlled randomized trial. Clin Nutr. 2005;24(6):1065–1072. doi:10.1016/j.clnu.2005.08.00916194582
- Moro K, Nagahashi M, Ramanathan R, Takabe K, Wakai T. Resolvins and omega three polyunsaturated fatty acids: clinical implications in inflammatory diseases and cancer. World J Clin Cases. 2016;4(7):155. doi:10.12998/wjcc.v4.i7.15527458590
- Park J-M, Kwon S-H, Han Y-M, Hahm K-B, Kim E-H. Omega-3 polyunsaturated fatty acids as potential chemopreventive agent for gastrointestinal cancer. J Cancer Prev. 2013;18(3):201. doi:10.15430/JCP.2013.18.3.20125337547
- Ianiro G, Franceschi F, Bibbo S, Gasbarrini A. Omega-3 fatty acids: a novel resort against gastrointestinal injury. Eur Rev Med Pharmacol Sci. 2014;18(20):3086–3090.25392109
- Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009;91(6):791–795. doi:10.1016/j.biochi.2009.01.00819455748
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–1115. doi:10.1042/BST2016047428900017
- Correia M, Michel V, Osório H, et al. Crosstalk between Helicobacter pylori and gastric epithelial cells is impaired by docosahexaenoic acid. PLoS One. 2013;8(4):e60657. doi:10.1371/journal.pone.006065723577140
- Thompson L, Cockayne A, Spiller R. Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: a possible explanation of the effect of diet on peptic ulceration. Gut. 1994;35(11):1557–1561. doi:10.1136/gut.35.11.15577828972
- Meier R, Wettstein A, Drewe J, Geiser H, GROUP SHS. Fish oil (Eicosapen) is less effective than metronidazole, in combination with pantoprazole and clarithromycin, for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2001;15(6):851–855. doi:10.1046/j.1365-2036.2001.00989.x11380323
- Kim H, Surh Y-J. Helicobacter pylori-induced oxidative stress and inflammation In: Studies on Experimental Models. Totowa, NJ: Humana Press, Springer; 2011:343–370.
- Santra A, Chowdhury A, Chaudhuri S, Gupta JD, Banerjee P, Guha Mazumder D. Oxidative stress in gastric mucosa in Helicobacter pylori infection. Indian J Gastroenterol. 2000;19(1):21–23.10659483
- Shmuely H, Yahav J, Samra Z, et al. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antibiotics and a proton pump inhibitor. Mol Nutr Food Res. 2007;51(6):746–751. doi:10.1002/mnfr.20060028117487928
- Gotteland M, Andrews M, Toledo M, et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition. 2008;24(5):421–426. doi:10.1016/j.nut.2008.01.00718343637
- Dashti S, Su TT, Esfehani AJ, Esfehani RJ. Effect of physical activity level on emotional status of Iranian women. World Appl Sci J. 2014;30(7):852–857.
- Mazaheri M, SadatKhoshouei M. Comparison between psychometric characteristics of Persian version of the gastrointestinal symptoms rating scale in functional gastrointestinal disorders and normal groups. Govaresh. 2012;17(1):18–24.
- Cancer IAfRo. Schistosomes, Liver Flukes and Helicobacter pylori. Vol. 61 Lyon: IARC; 1994.
- Sepidarkish M, Akbari-Fakhrabadi M, Daneshzad E, et al. Effect of omega-3 fatty acid plus vitamin E co-supplementation on oxidative stress parameters: a systematic review and meta-analysis. Clin Nutr. 2020;39(4):1019–1025. doi:10.1016/j.clnu.2019.05.00431128941
- Sepidarkish M, Morvaridzadeh M, Akbari-Fakhrabadi M, Almasi-Hashiani A, Rezaeinejad M, Heshmati J. Effect of omega-3 fatty acid plus vitamin E co-supplementation on lipid profile: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(2):1649–1656. doi:10.1016/j.dsx.2019.03.01831336536
- Carrepeiro MM, Rogero MM, Bertolami MC, Botelho PB, Castro N, Castro IA. Effect of n-3 fatty acids and statins on oxidative stress in statin-treated hypercholestorelemic and normocholesterolemic women. Atherosclerosis. 2011;217(1):171–178. doi:10.1016/j.atherosclerosis.2010.12.01321561620
