Abstract
Introduction
In recent years, intermittent preventive treatment for pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) has become policy in much of sub-Saharan Africa. But resistance to SP has been spreading across sub-Saharan Africa and thus the effectiveness of IPTp-SP has been questioned. The present study therefore sought to assess the incidence of placental malaria, low birth weight, and anemia of two IPTp-SP approaches (directly observed treatment scheme versus no directly observed treatment) in Anonkoua-Kouté and Samo, Côte d’Ivoire where the reported prevalence of dfr single mutant 108 was 62% and 52.2%, respectively.
Methods
The study was a longitudinal design involving pregnant women and was conducted in Anonkoua-Kouté, a suburban area, and Samo, a rural area, from January 2008 through March 2009. Women of a pregnancy less than 28 weeks duration were randomized to receive SP (1.5 g/0.075 g SP) in a single intake twice and were followed up monthly until delivery. Doses were administered under supervision in the controlled IPTp group, while SP was given free to women in the uncontrolled IPTp group with a recommendation to take it at home. The primary end point was the proportion of low birth weight infants (body weight < 2500 g) and the secondary end point was the rate of severe anemia and placental malaria detected at delivery.
Results
A total of 420 pregnant women were enrolled (212 and 208, respectively, in the controlled and uncontrolled groups). Delivery outcome was available for 378 women. In the modified intention-to-treat analysis, low birth weight infants were born from 15.5% of women of the uncontrolled IPTp group and from 11.9% of women in the controlled IPTp group (P = 0.31). The per-protocol population analysis showed consistent results. The proportion of women with placental malaria infection, moderate anemia (hemoglobin < 11 g/dL), and severe anemia (hemoglobin < 8 g/dL) at delivery were similar between the two groups (P > 0.05).
Conclusion
The study showed that the two approaches were equivalent, suggesting that unsupervised IPTp-SP free of charge should be used in areas where implementation of the directly observed treatment scheme suffers from many constraints.
Introduction
In sub-Saharan Africa, approximately 25 million pregnant women are at risk of Plasmodium falciparum infection every year. In recent years, intermittent preventive treatment for pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) has become policy in much of sub-Saharan Africa.Citation1 IPTp consists of administration of a curative dose of an efficacious antimalarial drug at least twice during the second and third trimesters of pregnancy during routinely scheduled antenatal clinic visits irrespective of the presence of signs for a malaria infection.Citation2 Currently, IPTp-SP has been rated as having the most favorable cost–benefit profile because of its relatively low cost, high compliance, and efficacy in reducing maternal anemia and low birth weight (LBW).Citation3,Citation4 However, implementation of IPTp in most settings is limited by social, cultural, economic and operational challenges despite good coverage of antenatal services.Citation5,Citation6 In addition, a controversy has raised questions as to whether this was the most appropriate approach for malaria prevention in African pregnant women given the reported increase in parasite resistance to SP in some areas.Citation4,Citation7–Citation9 Recent studies have shown high prevalence of dhfr and dhps resistant mutant parasites in the authors’ study sites (unpublished data).Citation10,Citation11 The current national treatment guidelines and policy for malaria in Côte d’Ivoire adopted the World Health Organization’s recommendation for malaria prevention and control during pregnancy. This means the implementation of an intervention package into the antenatal care (ANC) services including the use of insecticide-treated nets (ITNs) and IPTp as well as effective case management of malaria infection in pregnant women and anemia.Citation1 Thus, since 2005, the National Malaria Control Program recommends SP as the first-line agent for IPTp and quinine for treatment of clinical malaria in all trimesters. Since this adoption, few studies have investigated the efficacy of IPTp-SP.Citation8 Compliance with the recommendation that the IPTp drug should be given under supervision in the clinic was very low.Citation12,Citation13 Studies related to IPTp use during pregnancy in Côte d’Ivoire are limited and few were found to have looked into compliance especially with the directly observed treatment (DOT) scheme. In a study conducted in 2006 in four maternities, placental parasitemia was identified in 29.26% of delivering women who received two or more doses of SP.Citation14 This ineffectiveness of IPTp could be related to the inefficacy of SP as the IPTp drug or participants did not take SP as the intake was not supervised in most participants. These findings were confirmed by Vanga-Bosson et al.Citation15 The low use of IPTp-DOT scheme and poor adherence in previous studies may be a proxy to the practice of IPTp in the whole country.
The objective of this study was to assess the incidence of placental malaria, LBW, and maternal anemia of two IPTp-SP approaches (controlled or DOT scheme versus uncontrolled IPTp-SP or no DOT). Results obtained from this study will allow for implementation of effective and knowledge-based intervention against this major cause of maternal and child morbidity and mortality in sub-Saharan Africa.
Methods
Study sites
The trial was conducted from January 2008 through March 2009 in Anonkoua-Kouté and Samo. Anonkoua-Kouté is a suburban area of Abidjan while Samo is a village near Bonoua and located 50 km north of Abidjan. In the study sites, perennial malaria transmission with seasonal peaks is mostly attributable to P. falciparum.
Ethical clearance
The study was approved by the National Ethics Committee of Life and Health Sciences of Côte d’Ivoire. All study participants were informed in their local language about the study objectives and procedures. For each study participant, written informed consent was obtained and the participant was free to withdraw consent at any time of the study without influencing their access to health services.
Enrollment
An identification number was given to each potentially eligible woman who attended the antenatal clinic and a first questionnaire was completed to assess eligibility.
Eligibility criteria were: at least 15 years old, living in the study area or the surrounding regions for at least 6 months, availability for follow-up during the pregnancy and willingness to deliver at the study site, a pregnancy of less than 28 weeks duration, willingness to come back for a planned visit and be visited by a field worker during pregnancy, and willingness to comply with study procedures.
Women who reported living 10 km from the site, who were severely ill (had medical conditions requiring hospital admission), or who reported prior adverse drug reaction to sulfa-containing medications or any other antimalarial medication were excluded.
For each study participant, a Case Report Form at inclusion was completed, which provided information on age, district of residence, sociodemographic characteristics, education, previous and current obstetric history, previous illness, treatment during the current pregnancy, and measures for malaria prevention. Gestational age was estimated using the date of the last menstrual period and/or by measurement of uterine fundal height. Obstetrical ultrasound was also used to determine the gestational age. Venous blood samples were collected for determination of the hemoglobin (Hb) level, and thick and thin blood smears were performed to detect malaria. Human immunodeficiency virus (HIV) screening was routinely undertaken in the study area after informed consent. Women who were known to be HIV infected before enrollment were also recruited.
Randomization and treatment
Study participants were randomly assigned to one of the two treatment groups using a computer generated random list. The study pharmacist, who did not take part in any other activities in the study, prepared an individual randomization code envelope from the randomization list. The sealed envelopes were sequentially numbered. When an eligible woman joined the study, the envelope with the lowest available number was opened, revealing the assignment of the participant to the appropriate treatment group.
In the controlled IPTp group, all women received DOT while in the uncontrolled IPTp group the treatment administration was not supervised (no DOT). Each woman in the no DOT group was given medication for self administration at home. Efforts were made in this group to sensitize each participant regarding the importance in taking the drugs to avoid the consequences of malaria for herself and her baby. The empty sachets were verified by a field worker as evidence of taking the drug 72 hours after.
In the two groups, the IPTp regimen consisted of a single curative dose of 1.5 g/0.075 g SP at each of the ANC visits (ANC1 and ANC2). Doses were given twice from the second trimester, at least 1 month apart. Regardless of their gestational age, all women at recruitment received a long lasting ITN (LLITN).
Follow-up
Enrolled pregnant women were followed up to delivery. At enrollment and subsequent visits, the study midwife collected the study data using a standardized questionnaire.
A physical examination was performed to measure axillary temperature, blood pressure, weight, and uterine height.
ANC visits
At each ANC visit, information on previous illnesses and treatments were collected and fundal height and axillary temperature were measured. The number of tablets of SP-DOT and other medicines handed to mothers were recorded. A blood sample was obtained by fingerprick for thick and thin blood films. Similar information was collected at unscheduled visits.
Delivery
During labor, venous blood samples were collected for determination of Hb concentration and malaria diagnosis. Immediately after delivery, babies were weighed using a hanging weighing scale (Model 180; Salter Brecknell, West Midlands, United Kingdom). Data regarding newborn characteristics (vital status at birth, birth weight, sex, and the presence of twins or malformation) were collected. Blood smears were made with blood collected from the maternal side of the delivered placenta and the umbilical vein cord. In addition, pieces of placental tissues were used to prepare impression smears after swabbing it on blotting paper. If women delivered outside and attended the study site immediately, Hb level, sex, Apgar index, and birth weight were assessed.
Sample size calculation
An absolute difference of 15% in the prevalence of placental malaria between the uncontrolled and controlled groups was considered to be of interest. It has been reported that about 29% of placental malaria occurs in the study sites;Citation14 and the sample size calculation for a 5% type I error and a 10% type II error yielded 169 subjects in each group. For practical reasons, it was finally decided to include 400 participants (200 in each group).
Laboratory procedures
All participants received routine prenatal tests which included blood group and antibody screening, rubella, toxoplasma, syphilis, and midstream urine test, Hb electrophoresis, blood urea nitrogen, glycemia, and creatinemia screening, and obstetrical ultrasound.
Thick films and placental impression smears were stained with 10% Giemsa for 15 minutes. To determine the percentage of malaria parasitemia from placental impression smears, malaria parasite-infected red cells were counted against 2000 erythrocytes.
Placental infection status was categorized as infected (presence of any asexual parasite stages in the placenta) and noninfected (parasite negative smear). Smears with malaria pigment but with no asexual parasite stages were declared as unknown and were not included in the analysis. Microscopic examination of blood smears was done under oil immersion for parasite detection and 200 high-power fields were examined before the smear was considered negative. Parasites were enumerated using thick film, as previously described.Citation16 The parasite density (per 1 μL of blood) was calculated, assuming a normal leukocyte level of 8000/1 μL. The thin film was used to speciate the parasites. Each blood film was independently examined by two microscopists. In cases of discrepancy, a third microscopist counted the number of parasites in the films. The average of two counts that agreed was used as the final level of parasitemia.
Venous blood (5 mL) was collected using butterfly needles into ethylenediaminetetraacetic acid Vacutainer® tubes (BD Diagnostics, Franklin Lakes, NJ) for measurement of Hb levels using an automated hematology analyzer (Mythic 22; Orphee SA, Geneva, Switzerland).
Management of anemia and malaria cases
Study participants found to be anemic at ANC visits (Hb level <11 g/dL) were treated, based on national guidelines, with ferrous sulfate (200 mg) and folic acid (0.25 mg) given as a single combined tablet daily for 30 days. Confirmed cases of malaria (axillary temperature ≥ 37.5°C and with a positive smear test) were given treatment with quinine, based on the national malaria treatment guidelines for pregnant women.
Definitions
LBW was defined as a birth weight less than 2500 g, prematurity as a gestational age of less than 37 weeks (determined by Ballard examination), and maternal anemia as moderate to severe for Hb <11 g/dL and Hb <8 g/dL, respectively.
Statistical analysis
The primary efficacy endpoint was the proportion of LBW infants. The secondary end points were the proportions of women with placental malaria (ie, presence of asexual-stage parasites in the placental impression smear), moderate maternal anemia (Hb <11 g/dL), and severe maternal anemia (Hb <8 g/dL) at delivery as well as the mean birth weight and mean maternal Hb level at delivery. For the primary end point, the analysis was performed on the modified intention-to- treat (ITT) population of women who completed the study (and for the women whose infants’ information on birth weight was collected). The analysis was also conducted for the per-protocol (PP) population, excluding women who were lost to follow-up. For secondary endpoints, the analyses were performed in the PP population and they included data on stillbirths and spontaneous abortion. Differences in frequencies were compared by either chi-squared or Fisher’s exact tests as appropriate, and continuous variables by Student’s t-test when the data were normally distributed. Nonparametric tests were used for nonnormally distributed data. In the multivariable analysis, the factors associated with the dependant variable (LBW or placental malaria or anemia) based on univariable analysis were included. Statistical analyses were performed using Stata® version 10.0 (StataCorp LP, College Station, TX).
Results
Characteristics of the study participants at enrollment
Study population
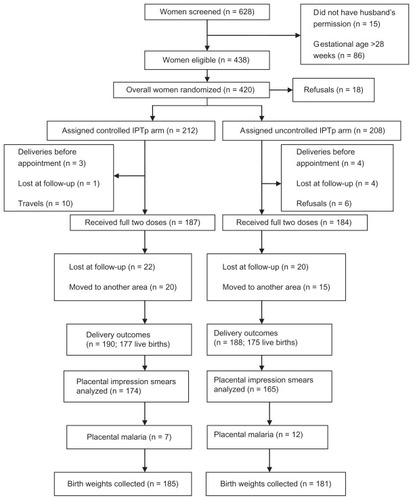
A total of 628 pregnant women were screened. Overall, 420 women were randomized to receive IPTp-SP (212 in controlled IPTp group and 208 in uncontrolled IPTp group). The reasons for ineligibility were unwillingness to consent to the study (no permission from husband), gestational age more than 28 weeks, low probability of completing follow-up (planning to travel before delivery), and allergies to sulfonamides.
Endpoint was reached in 366 and 339 women for modified ITT and PP analysis, respectively, for both treatment groups. The trial profile is shown in .
Characteristics of the mothers are summarized in . The mean age of the participants was 25.86 and 26.13 years in the uncontrolled and controlled groups, respectively. Primigravidae and secundigravidae represent 50.2% of the total sample size. There was more secundigravidae in the uncontrolled group than the controlled group (31.3% versus 21.7%; P = 0.02).
Table 1 Sociodemographic characteristics at inclusion
Follow-up
The second dose was not administered to 12% of women in the controlled group and 11% of women in the uncontrolled group. The main reasons for failure to administer the second dose (controlled versus uncontrolled) included travel (5% versus 2%), refusal because of the occurrence of an adverse event after the first dose (2% versus 3%), and delivery before administration of the second dose (1% versus 2%) (). Overall, 25 (6%) of 420 women (nine in controlled group and 16 in uncontrolled group) delivered outside the study maternity clinics. For those women, birth weights were recorded from ANC cards. A total of 366 birth weights were collected (including weights for stillborn babies). The mean birth weight was 2898.13 ± 37.39 g and 2967 ± 33.52 g in the uncontrolled and controlled groups, respectively, regarding the modified ITT population. There was no significant difference between the two groups for the mean birth weight or the rate of LBW (P = 0.31). In the PP population, the mean weight was 2894 ± 38.15 g (uncontrolled group) and 2966.70 ± 35.47 g (controlled group) (P = 0.16). In the modified ITT analysis, LBW infants were born to 15% of women in the uncontrolled IPTp group and to 12% of women in the controlled IPTp group (P = 0.31). The PP analysis showed consistent results (). The factors associated with LBW in the multivariable analysis were placental malaria (adjusted odds ratio = 6.9, 95% confidence interval 2.6–18), maternal malaria at delivery (odds ratio = 0.6, 95% confidence interval 0.08–3.9), and moderate anemia (odds ratio = 1.7, 95% confidence interval 0.8–3.5) ().
Table 2 Proportion of women with low birth weight infants (<2500 g)
Table 3 Factors associated with low birth weight or placental malaria: logistic regression model
By stratifying according to gravidity, there was no significant difference between groups in terms of LBW either in primigravidae, secundigravidae, or in multigravidae, but sample sizes had a low power to detect a difference between groups ().
All women who were parasitemic at enrollment received antimalarial treatment using quinine. All patients recovered completely and were microscopically confirmed negative. The rate of malaria at inclusion was 9.6% and 8.5% in the uncontrolled and controlled group, respectively. During the follow-up period, 18 (8.7%) and eleven (5.2%) clinical episodes of P. falciparum malaria were observed in the uncontrolled and controlled groups, respectively (). Most of the clinical episodes occurred in the second trimester. The proportion of women with peripheral malaria at delivery was 4.9% in the uncontrolled group and 4% in the controlled group (PP analysis). There were no significant differences in the prevalence of placental infection (impression smear positive) between the two groups (7.3% versus 4%; P = 0.19) (). Women with clinical malaria during pregnancy had a high risk (odds ratio = 7.9) of placental malaria.
Table 4 Rates of parasitemia, reported fever, and anemia among participants
Table 5 Comparative efficacy of uncontrolled and controlled intermittent preventive treatment during pregnancy: secondary end points in crude analysis
At inclusion, 65 (31.3%) and 56 (26.4%) participants had moderate anemia (Hb <11 g/dL) in the uncontrolled and controlled groups, respectively. The mean Hb concentration at delivery was 9.87 ± 0.1419 g/dL in the uncontrolled group and 10.22 ± 0.1301 g/dL in the controlled group. In the uncontrolled and controlled groups, the rate of moderate anemia (Hb <11 g/dL) was 50.9% and 46%, respectively, while the proportion of severe anemia (Hb <8 g/dL) was 14.6% and 9.8%, respectively ().
Pregnancy outcome
Information on pregnancy outcome and on the survival of babies born from women enrolled in this study is summarized in . Delivery was observed in 188 and 190 women, respectively, in the uncontrolled and controlled groups ().
Table 6 Pregnancy outcomes
Adverse events: mothers
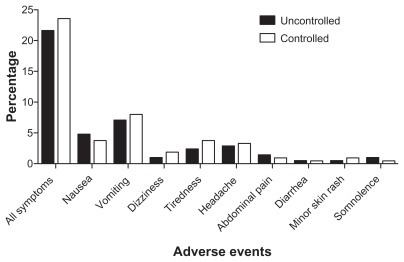
Moderate adverse events occurred in 24% and 22% of the participants, respectively, in the controlled and uncontrolled groups. The most common complaints were nausea, vomiting, tiredness, and headaches; and no significant difference between the two groups was observed (P > 0.05) ().
Discussion
Currently, the World Health Organization recommends, for prevention of malaria during pregnancy, a package of interventions for pregnant women living in areas where malaria is endemic, including IPT, ITNs, case management of clinical malaria, and diagnosis and treatment of anemia.Citation1 Clinical trials have demonstrated the positive impact of IPTp-SP or other effective antimalarials, such as mefloquine, on placental parasitemia, anemia, and LBW.Citation17,Citation18 In recent years, IPTp-SP has been the cornerstone of malaria control in pregnant women living in stable transmission areas.
The comparison of efficacy (mainly on birth weight) between pregnant women receiving IPTp-SP, either under supervision or without any control, in Côte d’Ivoire was assessed in this study. Birth weight was used as the primary end point, because it is a good public health indicator of the consequences of malaria in pregnancy, and it correlated with the efficacy of IPTp in several studies.Citation18,Citation19
Maternal anemia has been proposed as an indicator for monitoring malaria control in sub-Saharan Africa,Citation20 based on the excess of risk of anemia in primigravidae compared to multigravidae.
The prevalence of malaria infection during pregnancy was lower in the controlled group than in the uncontrolled group with no statistical difference. The results showed that the presence of malaria parasites in the peripheral blood of clinically symptomatic pregnant women is an indication of ongoing placental infection, as previously shown.Citation21 Other studies have shown that peripheral parasitemia at any stage of the pregnancy was significantly correlated with placental infection at delivery.Citation22,Citation23 Additionally, studies have indicated that peripheral and placental parasitemia decrease with increasing parity among pregnant women.Citation24,Citation25 It has been observed that pregnant women, especially primigravidae and secundigravidae, are more susceptible to malaria infections.Citation22 Although there is increasing evidence that prevalence of malaria infections is parity-related and primigravidae women are more susceptible to malaria than multigravidae women, this study did not show any association between malaria infection and parity. This finding may have been caused by the failure of a small sample size and the presence of LLITNs to show any association between parity or gravidity and malaria infection.
It is important to note that women with microscopically detectable placental P. falciparum malaria had a higher risk of delivering an LBW baby than those without detectable placental P. falciparum corresponding to a relative risk of 6.9, supporting the strong association between P. falciparum malaria and the risk of LBW.Citation24 There was no difference in terms of LBW between the two groups, although more pregnant women with malaria episodes were seen in the uncontrolled group.
The results showed no statistically significant difference between the controlled and uncontrolled groups regarding the prevalence of LBW and anemia at delivery. The results are consistent with efficacy results reported from several sub- Saharan African countriesCitation17,Citation18,Citation26–Citation28 in regard to the prevalence of LBW and severe anemia in the controlled IPTp group.
In contrast, results from Gambia show a lower prevalence of LBW compared to the present study.Citation29 The lack of difference between the two groups in the prevalence of LBW and severe anemia could be explained by the fact that SP was provided to the women in the uncontrolled group with a recommendation to take the drug at home. However, it has been demonstrated that a highly efficacious intervention given under strict trial conditions rarely translates into an equally effective intervention when implemented under programmatic conditions.Citation30–Citation32 There are many factors that have the potential to influence the relative implementability of interventions including: the complexity of the interventions,Citation33 the capacity of the health system and health providers to deliver the interventions at the scale required, and the acceptability of the interventions to providers and users.
During the study, SP was not always available at pharmacy sites and clean cups and water were also not available to administer the drugs. The women should buy their drugs at an external pharmacy and take the drugs at home. Regular availability of SP in the health facility is an important factor in improving the use of IPTp and implementation of the DOT scheme.Citation12,Citation13 SP for IPTp in public hospitals in Côte d’Ivoire was supplied by the Ministry of Health. However, periodic supply shortages were frequently reported. The other main constraints to practicing DOT were lack of clean water and cups at ANC clinics.Citation12 Good quality services at ANC clinics are necessary to promote high coverage and to achieve actual and perceived health benefits for mothers and their children. Allowing pregnant women to take the IPTp drug unsupervised, either at the clinic or at home, makes compliance uncertain and undermines the essence of IPTp.
HIV infection is known to be associated with an increased risk of malaria and LBW, and it may have affected the occurrence of LBW and maternal anemia.Citation34 HIV may have acted as a confounder in the present study. In the clinical trial sites, HIV screening was routinely undertaken by the National HIV Control Program during the trial, but the HIV status was only determined for 60% of the women. However, the overall prevalence of HIV infection in the present study area was 4%.Citation35 Even though bed nets are not traditionally used in this area, the delivery of LLITN through ANC was well accepted and compliance with their use was very high according to the questionnaire taken at enrollment. These results cannot be attributed to IPTp-SP alone as ITNs appeared to be an important variable in determining the efficacy of IPTp. Some studies on the combined use of ITNs and IPTp demonstrated the evidence of effectiveness of these interventions when used in combination. Citation36,Citation37 It is possible that if an effective ITN is used regularly, IPTp may not be required in some populations.Citation38 Although SP is generally considered to be a safe drug when used for IPTp, it can rarely cause serious skin reactions and hematological side effects.Citation39 No serious adverse events attributable to the administration of SP were seen in this study for which the proportion of women who reported an adverse event was 24% and 22% in the controlled and uncontrolled groups.
The present study was a small, regional prospective study and therefore had certain limitations, namely, the small sample size and lack of a control group. Larger studies are needed to compare the two approaches with a control arm in the context of high resistance to SP in the country.
In conclusion, the two approaches were equivalent suggesting that unsupervised IPTp-SP free of charge should be used in areas where implementation of the DOT scheme suffers from many constraints. Encouragement of respectful communication between midwives and their patients can help to reinforce the trust relationship that exists.
Acknowledgments
The authors are grateful to the women who participated in the study and medical staff, particularly the midwives of Anonkoua- Kouté and Samo maternities. The authors would also like to acknowledge the contributions of Dr Man-Koumba Soumahoro for the multivariable analysis, Mr Ghiorghis Belai for revision of the manuscript, and Mrs Gbaguidi Martin and Kobenan Kra who undertook central reading of the slides. The authors thank the Pasteur Institute Malaria Network for financial support.
Disclosure
The authors report no conflicts of interest in this work. All authors participated in design, implementation, analysis, or interpretation of the study. OAT was involved in all phases of the study and had full access to all the data in the study. LKP, JR, and KM participated in the design and supervised the study. CMA, NTL, AABA, AE, CB, and KD were responsible for conduction of field studies and coordination of study procedures. SD was involved in the design and the statistical analysis of data. The manuscript was drafted by OAT; substantial input came from all investigators. All authors critically reviewed the report and approved the final version of the report for submission.
References
- World Health OrganizationA Strategic Framework for Malaria Prevention and Control During Pregnancy in the African RegionBrazzavilleWorld Health Organization Regional Off ice for Africa2004
- World Health OrganizationGuidelines for the Treatment of Malaria2nd editionGenevaWorld Health Organization2010
- GoodmanCAColemanPGMillsAJThe cost-effectiveness of antenatal malaria prevention in sub-Saharan AfricaAm J Trop Med Hyg2001641–2 Suppl455611425177
- NewmanRDPariseMESlutskerLNahlenBSteketeeRWSafety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan AfricaTrop Med Int Health20038648850612791054
- HoltzTHKachurSPRobertsJMUse of antenatal care services and intermittent preventive treatment for malaria among pregnant women in Blantyre District, MalawiTrop Med Int Health200491778214728610
- RogersonSJChalulukaEKanjalaMMkundikaPMhangoCMolyneuxMEIntermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997– 1999Trans R Soc Trop Med Hyg200094554955311132387
- Ter KuileFOSteketeeRWIntermittent preventive therapy with sulfadoxine-pyrimethamine during pregnancy: seeking information on optimal dosing frequencyJ Infect Dis2007196111574157618008239
- Ter KuileFOvan EijkAMFillerSJEffect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic reviewJAMA2007297232603261617579229
- DeloronPBertinGBriandVMassougbodjiACotMSulfadoxine/ pyrimethamine intermittent preventive treatment for malaria during pregnancyEmerg Infect Dis201016111666167021029522
- DjamanJAhiboHYapiFHMolecular monitoring of falciparum malaria isolates in Côte d’Ivoire: genetic markers (dhfr-ts, dhps, pfcrt, pfmdr-1) for antimalarial-drugs resistanceEur J Scientific Res2010403461470
- DjamanJAMazabraudABascoLSulfadoxine-pyrimethamine susceptibilities and analysis of the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum isolates from Côte d’IvoireAnn Trop Med Parasitol2007101210311217316496
- AkinleyeSOFaladeCOAjayiIOKnowledge and utilization of intermittent preventive treatment for malaria among pregnant women attending antenatal clinics in primary health care centers in rural southwest, Nigeria: a cross-sectional studyBMC Pregnancy Childbirth200992819589164
- MubyaziABlochPKamugishaMKituaAIjumbaJIntermittent preventive treatment of malaria during pregnancy: a qualitative study of knowledge, attitudes and practices of district health managers, antenatal care staff and pregnant women in Korogwe district, North-Eastern TanzaniaMalar J200543116033639
- OffiananATAkoAAPenaliLKEfficacy of non-controlled intermittent preventive treatment in pregnant (IPTp) women in Côte d’IvoirePaper presented at: American Society of Tropical Medicine and Hygiene 56th Annual MeetingNovember 4–8, 2007Philadelphia, PA
- Vanga-BossonHACoffiePAKanhonSCoverage of intermittent prevention treatment with sulphadoxine-pyrimethamine among pregnant women and congenital malaria in Côte d’IvoireMalar J20111010521529344
- ShuteGTThe microscopic diagnosis of malariaWernsdorferMcGregorLPrinciple and Practice of MalariologyEdinburghChurchill Livingstone1988781814
- BriandVBotteroJNoëlHIntermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open- label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquineJ Infect Dis20092006991100119656069
- GiesSCoulibalySOOuattaraFTD’AlessandroUIndividual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weightTrop Med Int Health200914217418219171009
- MenendezCBardajiASigauqueBMalaria prevention with IPTp during pregnancy reduces neonatal mortalityPLoS One201052e943820195472
- SavageEJMsyambozaKGiesSD’AlessandroUBrabinBJMaternal anaemia as an indicator for monitoring malaria control in pregnancy in sub-Saharan AfricaBJOG2007114101222123117666098
- OforiMFStaalsoeTBamVExpression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infectionInfect Immun20037131584158612595482
- CottrellGMaryJYBarroDCotMIs malarial placental infection related to peripheral infection at any time of pregnancy?Am J Trop Med Hyg20057361112111816354822
- McGreadyRDavisonBBStepniewskaKThe effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmissionAm J Trop Med Hyg200470439840715100454
- OforiMAnsahEAgyepongIOfori-AdjeiDHviidLAkanmoriBPregnancy-associated malaria in a rural community of GhanaGhana Med J2009431131819652749
- ShulmanCEDormanEKImportance and prevention of malaria in pregnancyTrans R Soc Trop Med Hyg2003971303512886801
- TionoABOuedraogoABougoumaECPlacental malaria and low birth weight in pregnant women living in a rural area of Burkina Faso following the use of three preventive treatment regimensMalar J2009822419811649
- SirimaSBCotteAHKonateAMalaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina FasoAm J Trop Med Hyg200675220521116896120
- Le PortACottrellGDechavanneCPrevention of malaria during pregnancy: assessing the effect of the distribution of IPTp through the national policy in BeninAm J Trop Med Hyg201184227027521292898
- MbayeARichardsonKBalajoBA randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine–pyrimethamine in Gambian multigravidaeTrop Med Int Health2006117992100216827700
- AbdellaYMDeribewAKassahunWDoes insecticide treated mosquito nets (ITNs) prevent clinical malaria in children aged between 6 and 59 months under program setting?J Community Health200934210211218958607
- CrawleyJHillJYarteyJFrom evidence to action? Challenges to policy change and programme delivery for malaria in pregnancyLancet Infect Dis20077214515517251085
- YeungSWhiteNJHow do patients use antimalarial drugs? A review of the evidenceTrop Med Int Health200510212113815679555
- GerickeCAKurowskiCRansonMKMillsAIntervention complexity – a conceptual framework to inform priority-setting in healthBull World Health Organ200583428529315868020
- FillerSJKazembePThigpenMRandomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in MalawiJ Infect Dis2006194328629316826475
- HIV Control Ministry, National Institute of Statistics, RETROCIEnquête sur les Indicateurs du Sida, Côte d’Ivoire 2005Survey on AIDS indicators, Ivory Coast 2005Calverton, MDORC Macro2007 French
- KabanywanyiAMMacArthurJRStolkWAMalaria in pregnant women in an area with sustained high coverage of insecticide-treated bed netsMalar J2008713318644118
- NgandaRYDrakeleyJReyburnHMarchantTKnowledge of malaria influences the use of insecticide treated nets but not intermittent presumptive treatment by pregnant women in TanzaniaMalar J200434215541178
- MenendezCBardajiASigauqueBA randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinicPLoS One200834e193418398460
- PetersPJThigpenMCPariseMENewmanRDSafety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatmentDrug Saf200730648150117536875