Abstract
Background
Meningitis, which is mostly caused by infectious agents, occurs more commonly during the first month of life. Bacterial meningitis is an important source of mortality and morbidity, especially in neonates of resource-limited countries. We aimed to determine the prevalence and etiological agents of bacterial meningitis and their antibiotic susceptibility pattern in neonates at the University of Gondar Comprehensive Specialized Hospital.
Methods
We collected retrospective data from bacteriological results of cerebrospinal fluid of meningitis suspected neonates from 2013 to 2019. Sample collection, culture preparation, bacterial identification, and susceptibility testing were performed using standard microbiological techniques. We extracted data on socio-demographic characteristics and culture and antibiotic susceptibility testing results. We inputted the data using Epi-info version 7 and exported it to SPSS version 20 for analysis.
Results
In this study, 1101 cerebrospinal fluid samples, 595 (54%) male and 506 (46%) female neonates, were cultured to look for meningitis-causing bacteria. Of 1101 cerebrospinal fluid, 19 (1.73%) were culture positive for meningitis-causing bacteria. The common etiological agents were Klebsiella pneumoniae 36.8% (7), non-lactose-fermenter Gram-negative rods 21% (4), and Group B streptococcus 15.8% (3). The overall resistance rate among cephalosporin, cotrimoxazole, penicillin, and aminoglycosides classes were 90%, 88.9%, 77.3%, and 54.54%, respectively. Of all isolates, 58% (11) were multidrug-resistant, including all the non-lactose-fermenter Gram-negative rods and 71.4% of the Klebsiella pneumonia isolates.
Conclusion
The prevalence of neonatal bacterial meningitis was 1.73%. Klebsiella pneumonia and other Gram-negative rods, with a high multidrug-resistant rate, were the leading cause of neonatal bacterial meningitis. Further studies are needed to explore the source of infection, incidence, and risk factors of neonatal bacterial meningitis.
Background
Meningitis is an inflammation of the meninges; it can be caused by infectious or non-infectious agents.Citation1,Citation2 Of all the different types of meningitis, bacterial meningitis (BM) is responsible for the highest global burden, Citation1 with several etiologic agents that vary with age groups and geographical areas.Citation3,Citation4 Meningitis-causing bacteria can spread from one person to another through droplets of respiratory secretions, contaminated food, or from mothers to babies during pregnancy or delivery.Citation1,Citation5 In the world, the common bacterial etiologic agents of meningitis are S. pneumoniae, N. meningitidis, H. influenzae, and S. agalactiae,Citation6,Citation7 with > 40%, >35%, and >5.5% of BM in Africa is due to S. pneumoniae, N. meningitidis, and H. influenzae, respectively.Citation3
In neonates, the etiologies of BM are usually different from other populations. Group B streptococci (GBS), Gram-negative rods (GNR), and L. monocytogenes are frequently reported.Citation7–Citation11 Neonates are at greater risk of BM due to immature humoral and cellular immunity, phagocytic function, or alternative complement pathway, which results in ineffective clearance of microorganisms. Several factors in the mother can also contribute to the development of BM among neonates.Citation4,Citation12 Neonatal bacterial meningitis (NBM) can be classified as early-onset when the ≤ 72 hours age neonate CSF is positive for bacteria and late-onset when the >72 hours age neonate CSF is positive for bacteria.Citation9,Citation13
Bacterial meningitis can rapidly be fatal or lead to severe disability, with neurologic and systemic complications, including behavioral problems, seizure disorder, or focal neurologic deficits are reported.Citation14,Citation15 Death and neuropsychological sequelae from BM is higher in developing countries,Citation16,Citation17 where delayed presentation, poor general condition, low socioeconomic status, and limited access to medical resources are reported.Citation9,Citation18 In addition to those problems, the non-specific signs and symptoms of meningitis and cardiorespiratory instability in neonates may delay the diagnosis and treatment measures, which results in an increased incidence of morbidity and mortality.Citation9,Citation19,Citation20
Currently, the increasing rate of antibiotic resistance among bacterial isolates causing nosocomial infections makes the treatment choice challenging.Citation21 For long, penicillin, aminoglycosides, cephalosporin, or fluoroquinolones were the choice of antibiotics to treat BM. However, bacterial isolates are becoming resistant to these antibiotics.Citation22 Moreover, initial treatment of BM in most parts of the developing world, including those in the African meningitis belt, is often empirical. Even though Ethiopia is one of the countries in the African meningitis belt, published data regarding epidemiology of BM and antibiotic susceptibility pattern among neonates is still limited. As a result, studies are valuable to design appropriate treatment as well as preventive policies in the country and act accordingly.
Methods
Study Area, Design and Period
A hospital-based retrospective study was conducted among BM suspected neonates from 1st Jan 2013 to 31st Dec 2019 at the University of Gondar Comprehensive Specialized Hospital (UoGCSH), Northwest Ethiopia. In Gondar town, there are eight health centers, about twenty private clinics, and one specialized hospital. The hospital is one of the biggest tertiary level referral and teaching hospitals in the Amhara region. An estimated 5 million people from the surrounding zones and nearby regions visit this hospital for different medical services. The data for this study was collected from 15th Jan to 22nd Feb, 2020.
Study Population
We identified all neonates (<29 days old) suspected with BM infection cases from the UoGCSH bacteriology culture registers within the specified period. Bacterial meningitis suspected neonates were defined as any neonate with sudden onset of fever (usually > 38.5 °C rectal) and one of the meningeal signs, such as neck stiffness, altered consciousness, or others.Citation23 We used a pre-coded data sheet, which contains sex, age, isolated organism, and antibiotic susceptibility pattern result, to extract patient information. In this study, we included all BM suspected neonates who had complete laboratory documents.
Laboratory Method
Cerebrospinal fluid samples from neonates were collected in sterile containers by attending physicians and delivered to the microbiology laboratory within one hour from the time of collection. The samples were processed following the standard microbiological procedures, where a sterile loop was used to inoculate the sample on Blood and Chocolate agar plate (OXOID, UK), then MacConkey agar plate (OXOID, UK) for Gram-negative bacteria isolation. The inoculated culture plates were incubated at 35–37°C overnight in candle extinction jars to provide appropriate carbon dioxide.Citation24 The grown culture were processed and identified by standard bacteriological techniques, including colony morphology, Gram reaction, and biochemical tests. Catalase, Christie, Atkins, and Munch-Peterson (CAMP), Bile solubility, Bacitracin and Optochin susceptibility tests were used to differentiate Gram-positive isolates. However, indole production, carbohydrate fermentation, urease, motility, and Methyl Red and Voges-Proskauer tests were used to differentiate Gram-negative isolates.Citation25 The bacterial suspension was made; its turbidity was adjusted to a 0.5 McFarland turbidity standard before inoculated over the entire surface of Mueller Hinton agar (OXOID, UK), with 5% sheep blood supplemented for fastidious bacteria. Finally, the antibiotic susceptibility profile of isolates was determined by a modified Kirby-Bauer disk diffusion technique and results were interpreted based on the 28th Clinical Laboratory Standards Institute guideline.Citation26,Citation27
Data Quality, Analysis and Interpretation
The quality of data was assured using a structured data collection checklist, asking laboratory personnel about how the test was done and registered. Data were entered into EPI-Info version-7 to check data completeness and clearance, then transferred to SPSS version-20 for analysis. Frequency distribution, and percentages were used to describe the results.
Results
Over the seven-year (2013–2019) period, 1101 CSF samples from BM suspected neonates were analyzed in the microbiology laboratory at the UoGCSH. Of these, 19 (1.73%) of them were bacterial culture positive. The male to female ratio of neonates was 1:0.85, with 595 (54%) of them were male. Based on the onset type, 82.7% (910) suspected cases were late onset, with 1.87% (17) of them were CSF culture positive. About 93% of the neonates sample were from NICU. There was no significant difference in prevalence of BM between sex, onset type, and admission site ().
Table 1 Characteristics of Bacterial Meningitis Suspected Neonates Against the CSF Culture Result
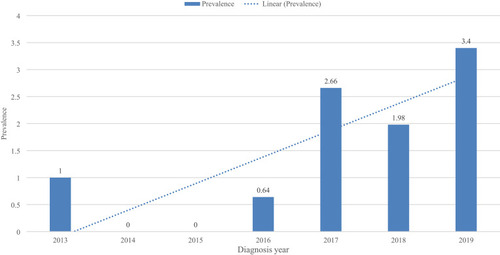
Both Gram-negative and Gram-positive bacteria were isolated, with Gram-negative bacteria were responsible for 73.7% (14) of the NBM. The predominant CSF isolate was K. pneumoniae 36.84%, followed by NLF-GNR 21.05%, and GBS 15.8%. Of all isolates, 36.8%, 26.3%, and 26.3% were isolated in 2019, 2018, and 2017, respectively. Of all the isolates, 100% of the NLF-GNR, and 71.4% of the K. pneumoniae isolates were MDR, with an overall MDR prevalence of 58% (). The highest prevalence of NBM was seen in 2019 (3.4%), followed by 2017 (2.66%), and 2018 (1.98%), while the lowest prevalence (0.0) was recorded both in 2014 and 2015 A positive year-to-year linear trend line of the prevalence of NBM was seen ().
Table 2 Distribution of Meningitis Causing Bacteria from Neonates Within the Sex, Onset Type, Admission Site, and Diagnosis Year
Between 2013 and 2019, 23 individual antibiotics and 10 antibiotic classes were used to identify the susceptibility pattern of bacterial isolates. The overall resistance rate among Cephalosporin, Cotrimoxazole, Penicillin, and Aminoglycosides class was 90% (18/20), 88.9% (8/9), 77.3% (17/22), and 54.54% (12/22), respectively. All the isolates tested for ceftazidime (7/7), amoxicillin (6/6), piperacillin (4/4), cefazolin (3/3), Augmentin (2/2), and cefotaxime (2/2) were resistant to the antibiotics, while all isolates tested for oxacillin (3/3), amikacin (3/3), and vancomycin (3/3) were sensitive ().
Table 3 Antimicrobial Susceptibility Profile of Meningitis Causing Bacteria from Neonates at the UoGCSH
Discussion
Although worldwide deaths from BM have been decreased in the last 15–20 years, principally due to immunization, it is still higher in Africa.Citation6 In 2015, WHO recorded an estimated 193,871 deaths due to BM in Africa, which comprises 67% of the worldwide BM death recorded in the same year, with an estimated 61,905 (39/100,000) 1–59 months aged infant died.Citation28 Bacterial meningitis epidemics put around 430 million people living in sub-Saharan Africa countries at risk. In this area, between 1995 and 2014, over 900,000 cases of BM were reported, with 10% deaths and another 10–20% neurological sequelae.Citation29
The overall prevalence of NBM at the UoGCSH was 1.73% (19/1101). A previous retrospective study (2003–2013) from the same study area, which focused on the prevalence of BM among the pediatric population, reported an almost similar prevalence of NBM, which was 1.86%.Citation30 However, our finding is higher than the prevalence, which was 0.48%, reported ten years ago from the same study area (2006–2010).Citation31 On the other hand, our result is lower than the prevalence, which was 4.7%, from a ten-year retrospective study (2001–2010) in Addis Ababa.Citation32 Nowadays, with advanced bacterial detection methods are introduced into the world, reporting the epidemiology of BM using culture methods solely underestimates its prevalence. For example, a study from Addis Ababa, Ethiopia, reported that GBS was detected by Polymerase Chain Reaction (PCR) from 64% of culture-negative CSF samples of neonates.Citation33 Another study from Mozambique also showed that the prevalence of acute BM among under-five patients was quite different when different methods are used, with 52.3% by quantitative PCR and 7.3% by culture method.Citation34
According to the results of our study, the prevalence of NBM was higher among late-onset cases 1.87% (17/910) than early-onset 1.05% (2/191). Moreover, 89.5% of positive CSF cultures were from late-onset neonates. Since late-onset BM is usually due to organisms acquired from the environment or caregivers, it is advisable to strengthen the infection control and prevention practices in the hospital environment.Citation9,Citation13
The results of our study showed that both Gram-negative (73.7%) and Gram-positive (26.3%) bacterial isolates were responsible for NBM, with K. pneumoniae (36.8%), NLF-GNR (21%), and GBS (15.8%) were the leading bacterial isolates. Similar to our finding, hospital-based studies from MexicoCitation35 and IranCitation36,Citation37 reported K. pneumoniae as the leading cause of NBM. Bacteria in the Enterobacteriaceae group, including K. pneumoniae, are known to cause nosocomial infections in neonates. In 2017, WHO classified carbapenemase and extended-spectrum b-lactamase (ESβL) producing Enterobacteriaceae as one of the priority 1, or critical pathogens needing new antibiotics urgently.Citation38 In contrast to our finding, a retrospective study (2001–2010) from Addis Ababa, Ethiopia, reported S. pneumoniae (23%) as the leading cause of NBM, followed by E. coli (16%) and Acinetobacter (13%).Citation32 A review paper in sub-Saharan Africa reported GBS (25%) as the leading cause of NBM, followed by S. pneumoniae (17%) and S. aureus (12%).Citation39 We also reported GBS as one of the leading causes of neonatal meningitis. Neonates may acquire GBS during pregnancy or delivery as 10–30% of pregnant women are already colonized with GBS.Citation40 Geographical differences may explain the differences in pathogen prevalence or distribution between and within regions.Citation9
According to the results of our study, there was a high antimicrobial-resistant rate of neonate CSF bacterial isolates among Cephalosporin 90% (18/20), Cotrimoxazole 89% (8/9), Penicillin 77.3% (17/22), and Aminoglycosides 54.5% (12/22). The antibiotic choices for NBM, including ampicillin, gentamicin, or cefuroxime,Citation13 are also becoming ineffective. Our paper reported a higher resistance to β-lactam (around 77%) and Aminoglycoside (54.5%) antibiotics than a review paper in the sub-Saharan African countries, with 68% and 27% of bacterial isolates from neonates were resistant to β-lactam and aminoglycoside antibiotics, respectively.Citation39 In the present study, Meropenem-resistant NLF-GNR were also isolated from meningitis suspected neonates. Findings reported by different scholars support our result.Citation41,Citation42
In the present study, the overall prevalence of MDR among neonatal CSF isolates was 58% (11/19). About 78.6% (11/14) of Gram-negative isolates and none of the Gram-positive isolates (0/5) were MDR. Moreover, all of the NLF-GNR (4) and 71.4% (5/7) of the K. pneumoniae isolates were MDR. In the world, there is a continuous rise in the incidence of MDR Gram-negative bacteria in the neonatal population, with ESβL and carbapenem-resistant Enterobacteriaceae are the leading MDR pathogens.Citation43 In Ethiopia, drug-resistant K. pneumoniae is commonly isolated from different clinical samples, with 61.8% of the isolates were reported as ESβL producers.Citation44 Recent data from three hospitals in Amhara region, Ethiopia, also showed that 37.2% and 16.7% of K. pneumoniae isolates were ESβL and carbapenemase producers, respectively.Citation45
Conclusions and Recommendations
The overall prevalence of culture-proven BM among neonates was 1.78%, with a positive year-to-year linear trend line. Klebsiella pneumoniae was the leading cause of neonatal BM, followed by NLF-GNRs. Gram-negative isolates were resistant to common antibiotics used for neonatal BM, and more than two-thirds of them were MDR. Moreover, bacteria resistant to the last-resort antibiotic agent, meropenem, were isolated in the CSF sample of neonates. Therefore, infection preventive measures,Citation46,Citation47 including strict hand hygiene by health-care workers, disinfection and sterilization of equipment, and antibiotic prophylaxis of the mother, should strictly be followed in the study area. Further research is needed to explore the epidemiology and risk factors of bacterial meningitis in neonates.
Abbreviations
BM, Bacterial Meningitis; CSF, Cerebrospinal Fluid; GBS, Group B streptococcus; LF-GNR, Lactose-fermenter Gram-negative rods; MDR, multidrug resistant; NBM, Neonatal Bacterial Meningitis; NICU, Neonatal Intensive Care Unit; NLF-GNR, Non-lactose-fermenter Gram-negative rods; UoGCSH, University of Gondar Comprehensive Specialized Hospital; WHO, World Health Organization.
Data Sharing Statement
All data generated or analyzed during this study were included in this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
Before the commencement of the study, we obtained ethical clearance from the University of Gondar, School of Biomedical and Laboratory Sciences ethical review committee, and an official letter of co-operations was provided to UoGCSH. Before data collection, we explained the study objectives to the heads of the hospital director and laboratory personnel who worked in the hospital. Since we used secondary data for this study and didn’t require the patient’s informed consent. We conducted the study following the Declaration of Helsinki.Citation48 To ensure confidentiality of information from participant’s record, we didn’t record any personal identifiers on the data collection sheet, and secured data from participant records were not available to anyone except for the investigators.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We thank all the Medical Microbiology staff working at the University of Gondar, School of Biomedical and Laboratory Science, College of Medicine and Health Sciences for their unreserved support. We like to thank the UoGCSH and its bacteriology laboratory staff for their support during the data collection period.
Disclosure
The authors declare that they have no competing interests in this work.
References
- WHO. Meningitis; 2020. Available from: https://www.who.int/health-topics/meningitis#tab=tab_1. Accessed 1219, 2020.
- CDC. Meningitis Home CDC; 2020. Available from: https://www.cdc.gov/meningitis/. Accessed 1215, 2020.
- Oordt-Speets AM, Bolijn R, Van Hoorn RC, Bhavsar A, Kyaw MH. Global etiology of bacterial meningitis: a systematic review and meta-analysis. PLoS One. 2018;13(6). doi:10.1371/journal.pone.0198772
- Pong A, Bradley JS. Bacterial meningitis and the newborn infant. Infect Dis Clin North Am. 1999;13(3):711–733. doi:10.1016/S0891-5520(05)70102-110470563
- CDC. Meningitis about bacterial meningitis infection CDC; 2020. Available from: https://www.cdc.gov/meningitis/bacterial.html. Accessed 1215, 2020.
- Soeters HM, Diallo AO, Bicaba BW, et al. Bacterial meningitis epidemiology in five countries in the meningitis belt of Sub-Saharan Africa, 2015–2017. J Infect Dis. 2019:220. doi:10.1093/infdis/jiz358.
- Grandgirard D, Leib SL. Meningitis in neonates: bench to bedside. Clin Perinatol. 2010;37(3):655–676. doi:10.1016/j.clp.2010.05.00420813277
- Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/S1474-4422(18)30499-X30879893
- Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015;42(1):29–45, vii–viii. doi:10.1016/j.clp.2014.10.004
- Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. 1999;23(3). doi:10.1016/S0146-0005(99)80066-4
- Ouchenir L, Renaud C, Khan S, et al. The epidemiology, management, and outcomes of bacterial meningitis in infants. Pediatrics. 2017;140(1):e20170476. doi:10.1542/peds.2017-047628600447
- Hsu M-H, Hsu J-F, Kuo H-C, et al. Neurological complications in young infants with acute bacterial meningitis. Front Neurol. 2018;9:9. doi:10.3389/fneur.2018.0090329434565
- Bundy LM, Noor A. Neonatal meningitis. In: StatPearls [Internet]. StatPearls Publishing LLC; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532264/. Accessed 1223, 2020.
- Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental impairment in children after Group B streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017:65. doi:10.1093/cid/cix663.
- Barichello T, Fagundes GD, Generoso JS, Elias SG, Simões LR, Teixeira AL. Pathophysiology of neonatal acute bacterial meningitis. J Med Microbiol. 2013;62(PART):12. doi:10.1099/jmm.0.059840-0
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328. doi:10.1016/S1473-3099(10)70048-720417414
- Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:7. doi:10.1186/1741-7015-7-4719228370
- Pelkonen T, Roine I, Monteiro L, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in Sub-Saharan Africa. Clin Infect Dis. 2009;48(8):1107–1110. doi:10.1086/59746319275501
- Gordon SM, Srinivasan L, Harris MC. Neonatal meningitis: overcoming challenges in diagnosis, prognosis, and treatment with omics. Front Pediatr. 2017;5:5. doi:10.3389/fped.2017.0013928168186
- Ogunlesi T. Diagnosis and treatment of bacterial meningitis in the newborn. Niger J Paediatr. 2012;40(1). doi:10.4314/njp.v40i1.2
- Talebi Bezmin Abadi A, Rizvanov AA, Haertlé T, Blatt NL. World Health Organization report: current crisis of antibiotic resistance. Bionanoscience. 2019;9(4):778–788. doi:10.1007/s12668-019-00658-4
- Kim KS. Neonatal bacterial meningitis. Neoreviews. 2015;16(9):e535–e543. doi:10.1542/neo.16-9-e535
- World Health Organization. WHO – recommended standards for surveillance of selected vaccine-preventable diseases. WHO Vaccines Biol. 2003;03.
- WHO. Laboratory Methods for the Diagnosis of Meningitis Cused by Neisseria Meningitidis, Streptococcus Pneumoniae, and Haemophilus Influenzae: WHO Manual. 2nd ed. Vol. 40; 2011. doi:10.1093/ije/dyq165
- Tille PM. Bailey & Scott’s Diagnostic Microbiology. 14th ed. Elsevier; 2017:205–247.
- American Society of Microbiology. Manual of Clinical Microbiology. 11th Vol. 1. Jorgensen JH, Carroll KC, Funke G, et al. editor. ASM Press; 2015. doi:10.1128/9781555817381
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. Wayne: PA Clin Lab Stand Institute. Published online; 2018.
- World Health Organization. Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization. Who; 2018. Available from: http://www.who.int/healthinfo/global_burden_disease/estimates/en/. Accessed 414, 2021.
- WHO. Meningococcal meningitis; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis. Accessed 1220, 2020.
- Dagnaw M, Anagaw B, Motbaynor E, Getie M, Million Y, Mathewos B. Bacterial profile and antimicrobial susceptibility pattern of bacterial meningitis among neonates and children at Gondar University Hospital, Northwest Ethiopia. Int J Pharm Heal Res. 2013;01(02):46–52.
- Tegene B, Gebreselassie S, Fikrie N. Bacterial Meningitis: a five-year retrospective study among patients who had attended at University of Gondar Teaching Hospital, Northwest Ethiopia. Biomed Res Ther. 2015;2(5). doi:10.7603/s40730-015-0012-2
- Reta MA, Zeleke TA. Neonatal bacterial meningitis in Tikur Anbessa Specialized Hospital, Ethiopia: a 10-year retrospective review. Springerplus. 2016;5(1). doi:10.1186/s40064-016-3668-1
- Geteneh A, Kassa T, Alemu Y, et al. Enhanced identification of Group B streptococcus in infants with suspected meningitis in Ethiopia. PLoS One. 2020;15(11):e0242628. doi:10.1371/journal.pone.024262833211777
- Nhantumbo AA, Cantarelli VV, Caireão J, et al. Frequency of pathogenic paediatric bacterial meningitis in Mozambique: the critical role of multiplex real-time polymerase chain reaction to estimate the burden of disease. PLoS One. 2015;10(9):e0138249. doi:10.1371/journal.pone.013824926393933
- Macías-Parra M. Neonatal bacterial meningitis in a third level hospital in mexico city during a 32 years period: clinical characteristics and risk factors for mortality and neurologic sequelae. EC Paediatr. 2019;8(1):03–16.
- Aletayeb MH, Ahmad FS, Masood D. Eleven-year study of causes of neonatal bacterial meningitis in Ahvaz, Iran. Pediatr Int. 2010;52(3):463–466. doi:10.1111/j.1442-200X.2010.03107.x20202151
- Boskabadi H, Heidari E, Zakerihamidi M. Etiology, clinical findings and laboratory parameters in neonates witacute bacterial meningitis. Iran J Microbiol. 2020;12(2). doi:10.18502/ijm.v12i2.2612
- Who. Who publishes list of bacteria for which new antibiotics are urgently needed. Saudi Med J. 2017;38(4).
- Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019;19(11):1219–1234. doi:10.1016/S1473-3099(19)30414-131522858
- Randis TM, Baker JA, Ratner AJ. Group B Streptococcal Infections. Pediatr Rev. 2017;38(6):254–262. doi:10.1542/pir.2016-012728572134
- Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol. 2016;54(7):1700–1710. doi:10.1128/JCM.03264-1526912753
- Agarwal S, Kakati B, Khanduri S, Gupta S. Emergence of carbapenem resistant non-fermenting gram-negative bacilli isolated in an ICU of a tertiary care hospital. J Clin Diagnostic Res. 2017;11(1). doi:10.7860/JCDR/2017/24023.9317
- Folgori L, Bielicki J, Heath PT, Sharland M. Antimicrobial-resistant Gram-negative infections in neonates: burden of disease and challenges in treatment. Curr Opin Infect Dis. 2017;30(3):281–288. doi:10.1097/QCO.000000000000037128306563
- Abayneh M, Worku T. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacilli: a meta-analysis report in Ethiopia. Drug Target Insights. 2020;14(1):16–25. doi:10.33393/dti.2020.217033132695
- Moges F, Gizachew M, Dagnew M, et al. Multidrug resistance and extended-spectrum beta-lactamase producing Gram-negative bacteria from three Referral Hospitals of Amhara region, Ethiopia. Ann Clin Microbiol Antimicrob. 2021;20(1). doi:10.1186/s12941-021-00422-1
- Tietjen L, Bossemeyer D, McIntosh N. Infection Prevention Guidelines for Healthcare Facilities with Limited Resources. Vol. 372. JHPIEGO Corporation; 2013.
- World Health Organization. WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care; 2009.
- World Medical Association declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA - J Am Med Assoc. 2013;310(20). doi:10.1001/jama.2013.281053