Abstract
Purpose
This study aimed to describe trends in Klebsiella pneumoniae (KP) resistance in bloodstream infections (BSI) and to identify risk factors for a hospital-acquired carbapenem-resistant Klebsiella pneumoniae (CRKP) BSI and 28-day mortality from a hospital-acquired KP BSI.
Patients and Methods
We recorded the results of antimicrobial susceptibility testing of 396 KP-positive blood cultures from January 2016 to December 2020. A total of 277 patients with a KP BSI were included in this study, of which 171 had a hospital-acquired infection and 84 had a hospital-acquired CRKP BSI. Multivariate logistic regression analysis was used to identify risk factors for a hospital-acquired CRKP BSI and 28-day mortality from a hospital-acquired KP BSI.
Results
The proportion of hospital-acquired infections among KP BSI patients increased from 53.1% in 2016 to 72.8% in 2020. The detection rate of CRKP among KP BSI patients increased from 18.8% in 2016 to 37.7% in 2020. Multivariate logistic regression showed that β-lactam/β-lactamase inhibitor combinations (BLBLIs) exposure (P = 0.022, OR 2.863), carbapenems exposure (P = 0.007, OR 3.831) and solid organ transplantation (P <0.001, OR 19.454) were independent risk factors for a hospital-acquired CRKP BSI. Risk factors for a 28-day mortality from hospital-acquired KP BSI were CRKP BSI (P =0.009, OR 5.562), septic shock (P =0.002, OR 4.862), mechanical ventilation>96 hours (P =0.020, OR 8.765), and platelet counts <100×109/L (P =0.003, OR 4.464).
Conclusion
The incidence of hospital-acquired KP BSI continues to rise and the proportion of CRKP BSI is also increasing. We believe that the use of the BLBLIs needs to be carefully evaluated in hospital-acquired infection. Hospital-acquired KP BSI Patients with CRKP BSI, septic shock, mechanical ventilation and deficiency of platelets are more likely to have a poor prognosis.
Introduction
Given the widespread use of carbapenems worldwide, carbapenem-resistant Enterobacteriaceae (CRE) has emerged all over the world.Citation1–3 Carbapenemase types of Enterobacteriaceae have changed greatly, Klebsiella pneumoniae carbapenemase (KPC) producers have an increasing prevalence in hospital acquired pathogens, most prominently among Klebsiella pneumoniae (KP).Citation4 Transmission of carbapenem-resistant Klebsiella pneumoniae (CRKP) is largely nosocomial.Citation5,Citation6 Urine is the main source of CRKP, which may be related to many of elderly patients.Citation7,Citation8 However, the overall in-hospital mortality of urinary tract CRE infection is low,Citation9 while the mortality of bloodstream infection (BSI) caused by CRE is significantly higher.Citation10,Citation11 BSI has become one of the major medical burdens worldwide,Citation12 the incidence of sepsis and BSI has risen in recent years.Citation13 The incidence of Gram-negative BSI is increasingCitation14,Citation15 and CRKP BSI can prolong the length of hospitalization and increase patient mortality significantly,Citation16,Citation17 which is an important clinical threat.
Previous studies have suggested that the real-time Whole Genome Sequencing (WGS) method may be helpful to monitor CRKP and improve the prognosis of patients.Citation18 The positive rate of CRKP culture in rectal swab samples is high,Citation19,Citation20 which is helpful for the prediction of CRKP infection.Citation21 In addition to strict contact isolation,Citation22,Citation23 previous studies have suggested that strict antibiotics restrictions can also help reduce the prevalence of CRE infection.Citation24,Citation25 However, the specific formulation of antibiotic management plan and its impact on limiting CRE dissemination need to be more widely studied,Citation26 and the control of CRKP is still challenging due to the rapid transfer of horizontal gene of carbapenemase expressing plasmids.Citation27
This study focused on hospital-acquired KP BSI, in particular CRKP BSI patients, described the results of antimicrobial susceptibility testing and clinical characteristics, and analyzed the risk factors for hospital-acquired CRKP BSI transmission and 28-day mortality from a hospital-acquired KP BSI.
Study Design
This retrospective study was performed at The First Affiliated Hospital of Anhui Medical University, a 4990-bed tertiary-care teaching hospital in Anhui Province in east-central China from January 2016 to December 2020. KP BSI patients over 18 years old were included in this study. Hospital-acquired KP BSI was defined as the appearance of fevers, chills, or other infectious symptoms more than 48 hours after hospital admission, and confirmation of KP BSI by blood culture.Citation28 Patients with a history of hospital admission for BSI in the 14 days prior to the KP BSI admission were not included, and patients with a history of a KP BSI admission in the past 1 month were also excluded. The antibiotic use plan for all KP BSI patients was adjusted according to the results of antimicrobial susceptibility testing. If the patient had multiple positive blood cultures during the study, only the first positive result was recorded. Exclusion criteria included patients younger than 18 years old, pregnant women, polybacteremia, hospitalization less than 48 hours and patients who withdrew from treatment and were difficult to follow. KP antimicrobial susceptibility testing was performed at the microbiology laboratory of the hospital. Antimicrobial resistances of KP isolated from blood samples from 2016 to 2020 were recorded. Patient clinical characteristics were collected from the medical record review system to further analyze risk factors for hospital-acquired CRKP BSI. Multivariate analysis was used to identify risk factors for developing a hospital-acquired CRKP BSI, and for 28-day mortality due to a hospital-acquired KP BSI.
Ethics
This study protocol was approved by the institutional review committee of Anhui Medical University, the First Affiliated Hospital (Reference number: Quick-PJ 2021–09-18). The ethics committee waived informed consent because this was a retrospective study. Patient data came from the medical record system and were anonymously analyzed to preserve patient privacy. In our study, all organs were donated voluntarily with written informed consent, which was conducted in accordance with the Declaration of Istanbul.
Definitions
Multidrug- Resistant (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories.Citation29 CRKP was defined as resistance to any carbapenem. Antibiotic exposure was defined as receiving intravenous or oral antibiotics for more than 48 hours up to 90 days prior to a BSI.Citation30 The date of the collection of the first positive blood culture was considered the start of the BSI. Clinical data within 24 hours of BSI diagnosis were recorded. The Pitt bacteremia score was calculated within 24 hours of BSI diagnosis, and the highest score was recorded. Appropriate initial anti-microbial therapy was defined as the initial antibiotic regimen started after the BSI diagnosis that was consistent with the results of antimicrobial susceptibility testing.
Antimicrobial Susceptibility Testing
Isolates were confirmed using the VITEK-2 GNI (bioMerieux Vitek Inc. in Hazelwood, Missouri, USA) and Clin-ToF-II systemsCitation31 (Bioyong Technologies Inc. in Beijing, China). Antimicrobial susceptibility testing was also performed using the microdilution method or Kirby-Bauer disk diffusion method, and drug susceptibility results were interpreted according to the standards of the Clinical and Laboratory Standards Institute (CLSI).Citation32 Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as controls for antimicrobial susceptibility testing. All experiments were repeated three times.
Data Collection
The following demographic and clinical data were extracted from the hospital electronic medical record system: sex, age, length of hospital stay, previous hospitalization, complicated infection site, comorbidities (diabetes, cardiovascular, kidney disease, solid tumor, hematological malignancy, solid organ transplantation, etc.), clinical invasive procedures (catheter, central venous catheter (CVC), peripherally inserted central venous catheter [PICC], arterial catheter, blood purification, endotracheal intubation, mechanical ventilation, nasogastric tube, sputum suction, catheterization, puncture, endoscope, etc.), special treatments (corticosteroids, immunosuppressants, intravenous immunoglobulin, chemotherapy, radiotherapy), antibiotic exposure, and antibiotic treatment data. The Pitt bacteremia score was used to assess the severity of the BSI. The Charlson comorbidity index (CCI) was used to quantitatively measure comorbidities.Citation33
Statistical Analysis
SPSS version 23.0 was used for data analysis. Continuous variables with a normal distribution were expressed as mean and standard deviation (SDS), and continuous variables with a non-normal distribution were expressed as median and interquartile range (IQR). For univariate analyses, categorical variables were compared using chi-squared or Fisher’s exact tests, and continuous variables were compared using Student’s t-test or the Mann–Whitney U-test. A P value <0.05 was considered statistically significant. Significant variables in univariate analysis (P<0.05) were selected for inclusion in a multivariate logistic regression. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and P values. P values <0.05 were considered statistically significant. The Hosmer-Lemeshow test was used to evaluate the goodness of fit of the model. The logistic regression model was reliable when the P value >0.05 in Hosmer-Lemeshow test.
Results
Characteristics of Study Participants
Of the 396 patients who met BSI criteria, 277 were included in this study. Of the 277 patients included in this study, 171 (61.7%) had hospital-acquired infections and 106 (38.3%) had non-hospital-acquired infections. The mortality of the hospital-acquired infection group was 33.3% (57/171), and that of the non-hospital-acquired infection group was 12.3% (13/106), which was statistically significant (P<0.001). The mortality rate of the CRKP group was 50.0% (47/94), while that of the carbapenem susceptible Klebsiella pneumoniae (CSKP) group was 12.6% (23/183), which was statistically significant (P < 0.001). Of the patients with a CRKP BSI, 84 had a hospital-acquired infection.
Trends in the Prevalence of KP BSI Over the Five-Year Period
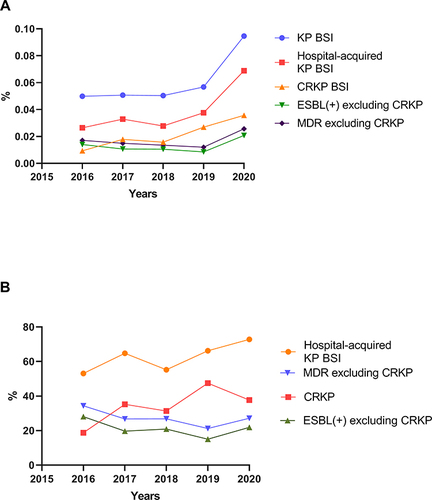
As shown in , the prevalence of KP BSI, hospital-acquired KP BSI, and CRKP BSI in inpatients increased from 0.050%, 0.026%, and 0.0094% in 2016 to 0.095%, 0.069%, and 0.036% in 2020. Except for patients with CRKP BSI, there was smaller increase in the proportion of ESBL positive KP BSI and MDR KP BSI in the total number of hospitalized patients. The proportion of hospital-acquired infections and CRKP among KP BSI patients had an upward trend over the 5-year period, from 53.1% and 18.8% in 2016 to 72.8% and 37.7% in 2020. Except for patients with CRKP BSI, the proportion of ESBL positive KP BSI and MDR KP BSI among KP BSI had a downward trend, from 28.1% and 34.4% in 2016 to 21.9% and 27.2% in 2020.
Figure 1 (A) Proportion of different categories of KP BSI in total hospitalized patients from 2016 to 2020. (B) Proportion of different categories of KP in total KPBSIs from 2016 to 2020. The detection rate of KP BSI and CRKP BSI and the proportion of hospital-acquired KP BSI among total KP BSI increased over time. Except for patients with CRKP BSI, there was smaller increase in the proportion of ESBL positive KP BSI and MDR KP BSI in the total number of hospitalized patients, while the proportion of ESBL positive KP BSI and MDR KP BSI among KP BSI had a downward trend.
Detection Distribution of KP BSI by Department
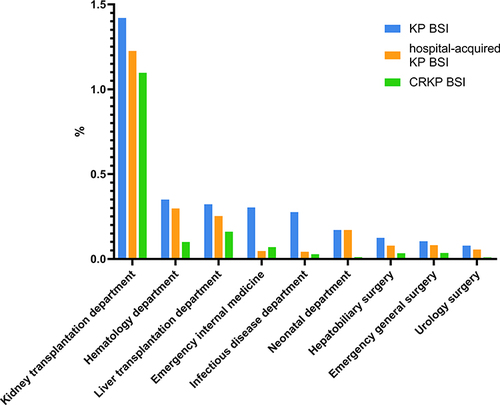
KP BSI was most commonly detected in the Intensive care unit (ICU), the department of hematology, and the department of infectious disease department. The top three departments with the highest detection rates of hospital-acquired KP BSI and CRKP BSI were the ICU, the department of hematology and the department of kidney transplantation. Details are shown in . The detection rates of KP BSI, hospital acquired KP BSI, and CRKP BSI in various departments are shown in . Except ICU, the highest hospital-acquired infection rates of KP BSI, hospital acquired KP BSI, and CRKP BSI were in the department of kidney transplantation, with rates of 1.42%, 1.22%, and 1.09%, respectively, followed by the department of hematology and the department of liver transplantation.
Figure 2 Department distribution of KP BSI (A), hospital-acquired KP BSI (B) and CRKP BSI (C) from 2016 to 2020. The primary setting of KP BSI, hospital-acquired KP BSI and CRKP BSI was the ICU.
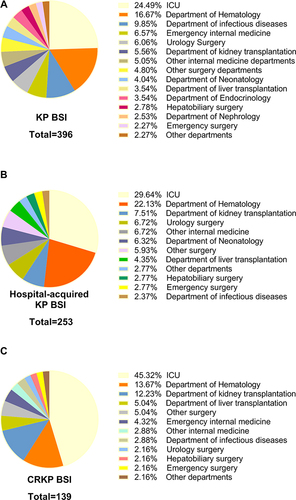
Figure 3 Hospital-acquired infection rates of KP BSI, hospital-acquired KP BSI, and CRKP BSI by various departments. The number of ICU inpatients was difficult to determine, so they were not included in this figure.
Antimicrobial Susceptibility of KP BSI
There was an upward trend in the drug resistance of KP to various antibiotics. The drug resistance rate of cephalosporins, β-lactam/β-lactamase inhibitor combinations (BLBLIs), and quinolones increased significantly in the past 5 years (details are shown in ).
Table 1 Antimicrobial Resistances of Klebsiella pneumoniae Isolated from Blood Samples from 2016 to 2020
Antimicrobial Susceptibility of CRKP BSI
There was an high rate of the drug non-susceptibility of CRKP to various antibiotics. The non-susceptibility rates of CRKP to aminoglycoside antibiotics and fosfomycin are high (details are shown in ).
Table 2 Antimicrobial Non-susceptibility of Carbapenem-Resistant Klebsiella pneumoniae Isolated from Blood Samples from 2016 to 2020
Antibiotic Exposure and Use in the Setting of Hospital-Acquired and Non-Hospital-Acquired KP BSI
As shown in , patients with hospital-acquired KP BSI had more antibiotic exposures, especially to carbapenems, glycopeptides,cephalosporins, BLBLIs, Trimethoprim-sulfamethoxazole (TMP-SMZ) and antifungal agents. The non-hospital-acquired KP BSI group was more likely to receive the treatment regimen containing BLBLIs (P=0.004). The non-hospital-acquired group was more likely to receive early appropriate therapy (P < 0.001).
Table 3 Antibiotic Exposure and Use Histories of Patients with Hospital-Acquired and Non-Hospital-Acquired KP BSI
Risk Factors for CRKP BSI in Hospital-Acquired KP BSI
Compared with the CSKP group, the CRKP group had a longer hospital stay time, more antibiotic exposure, were more prone to altered mental status and septic shock, and received more invasive operations. CSKP patients were older and had more 90-day re-admissions. The Pitt bacteremia score of the CRKP group was higher. There were more patients with a history of chronic kidney disease, intracerebral hemorrhage, liver cirrhosis and solid organ transplantation in the CRKP group while there were more patients with hematological malignancies and solid tumors in the CSKP group. Detailed data are shown in .
Table 4 Clinical Characteristics of the CRKP and CSKP Groups of Hospital-Acquired KP BSI
The results of the multivariate analysis are shown in . The independent risk factors for CRKP BSI in the hospital were the BLBLIs exposure (P = 0.022, OR 2.863), Carbapenems exposure (P = 0.007, OR 3.831) and solid organ transplantation (P <0.001, OR 19.454).
Table 5 Multivariate Logistic Regression Analysis of Risk Factors for CRKP BSI in Patients with a Hospital-Acquired Infection
Risk Factors for 28-Day Mortality in Patients with Hospital-Acquired KP BSI
Univariate analysis of risk factors associated with 28-day mortality in patients with hospital-acquired KP BSI is shown in . Patients in the death group had more antibiotic exposures and received more invasive procedures. Comorbidities do not significantly increase the 28-day risk of death from hospital-acquired KPBSI.
Table 6 Univariate Analysis of Risk Factors Associated with 28-Day Mortality in Patients with Hospital-Acquired KP BSI
The results of the multivariate analysis are shown in . Multivariate analysis suggested that the risk factors for 28-day mortality after a hospital-acquired KP BSI were CRKP BSI (P =0.009, OR 5.562), septic shock (P =0.002, OR 4.862), and mechanical ventilation >96 hours (P =0.020, OR 8.765), platelet (PLT) count <100×109/L (P =0.003, OR 4.464).
Table 7 Multivariate Analysis of 28-Day Mortality in Patients with Hospital-Acquired KP BSI
Discussion
The incidence of KP BSI has gradually increased over the past few years, especially among hospital-acquired infections.Citation34 The overall resistance rate of various antibiotics to KP BSI is rising, as is the proportion of CRKP BSI.Citation35 Previous studies have shown that healthcare-associated risk factors are independently associated with gram-negative bloodstream infections.Citation15 We therefore collected the clinical data of patients with a KP BSI, recorded the results of antimicrobial susceptibility testing of KP BSI, and identified risk factors for hospital-acquired CRKP BSI and 28-day mortality from hospital-acquired KP BSI so as to provide guidance for the prevention and reasonable treatment of hospital infections.
Carbapenems are still the most commonly used antibiotics in the treatment of hospital acquired KP BSI at our hospital, and the BLBLIs are commonly used in the treatment of non-hospital-acquired KP BSIs. Although carbapenems are one of the first choice drugs for the treatment of complex urinary tract infections and severe gram-negative bacterial infections,Citation36,Citation37 many previous studies have suggested that carbapenems exposure history is a risk factor for CRKP infection.Citation38,Citation39 Our multivariate analysis supported these hypotheses. Our multivariate analysis also found that exposure to the BLBLIs was an independent risk factor for hospital-acquired CRKP BSI, a correlation rarely mentioned in previous studies. There is an antibiotic restriction strategy (including carbapenems, Polymyxin, tigecycline and ceftazidime-avibactam) at our hospital, which leads to the use of the BLBLIs has significantly increased. A previous study suggested that a history of BLBLIs exposure may increase the risk of CRKP colonization.Citation40 A larger number of clinical studies have sought alternatives to carbapenems. Some studies have suggested that BLBLIs are not inferior to carbapenems,Citation41 and may be cost-effective.Citation42 However, these studies rarely involve hospital acquired infections and BSI. A previous randomized clinical trialCitation43 did not support piperacillin-tazobactam as an alternative to meropenem in the treatment of ceftriaxone resistant Escherichia coli and KP bloodstream infections. Moreover, our univariate analysis suggested that exposure to the BLBLIs may increase the risk of 28 day mortality from hospital-acquired KP BSI. We therefore believe that the use of the BLBLIs also needs to be carefully evaluated, especially in hospital-acquired infections.
Although there is an antibiotic restriction strategy at our hospital, we found that the incidence of CRKP BSI has increased over time. Antibiotic-resistant KP cases at our hospital have gradually concentrated on CRKP. We therefore speculate that besides carbapenem abuse there are other reasons for the CRKP epidemic. In general, patients with solid tumors and hematologic malignancies are prone to infections. Our study noted similar findings. There were significantly more KPBSI patients with hematologic diseases and solid tumors, and the proportion of CSKP BSI in these patients was higher than CRKP BSI. We hypothesize that this is related to the shorter hospital stays of these patients. Patients with hematologic diseases and solid tumors are often hospitalized for chemotherapy, which are short hospital stays. Our univariate analysis suggested that the CRKP BSI group had longer hospital stays, which was similar to the results obtained in previous studies.Citation44,Citation45
We also believe that the high incidence of CRKP BSI is related to CRKP colonization. Previous studies have confirmed the association between KP colonization and bloodstream infection,Citation46,Citation47 but the number of studies on KP nasopharynx colonizationCitation48 is significantly less than that of rectal colonization.Citation49,Citation50 The longer the hospital stay, the greater the risk of infection from colonizing bacteria. Our study found that patients with hospital-acquired CRKP BSI often also had a respiratory tract infection, which has been previously reported.Citation51 Many previous studies have shown that the use of nasogastric tubes is a risk factor for CRKP colonization and infection,Citation52,Citation53 and that the risk of a pulmonary infection has increased significantly in these patients. Our univariate analysis found that patients who had sputum aspiration events were more likely to suffer from CRKP BSI, which was rarely reported by prior works. Sputum aspiration events can damage the respiratory mucosa, allowing CRKP colonizing the nasopharynx to enter the bloodstream. Previous retrospective studies also have shown that the lungs are prone to nosocomial infections.Citation54 Although it is difficult to ascertain if the source of the BSI in these patients is respiratory, we believe that nasopharyngeal colonization of KP merits further study in hospital-acquired infections.
In addition to polymyxin, ceftazidime-avibactam, and other new antibiotics,Citation55 previous studies proposed the feasibility of adding aminoglycosidesCitation56 or fosfomycinCitation57 to treat CRKP infection. However, the antimicrobial susceptibility testing results at our hospital suggested that the rates of non-susceptibility to both drugs are high. We noted that the non-susceptibility of TMP-SMZ in blood samples was lower than that of aminoglycosides and fosfomycin. The relationship between the use of TMP-SMZ and the resistance rate in KP infection is controversial,Citation58,Citation59 which may be related to the frequency of TMP-SMZ use.Citation60 Although TMP-SMZ is not usually used as a therapeutic drug for BSI, we propose the possibility of using TMP-SMZ in CRKP BSI patients with urinary and respiratory infections. The resistance rate of minocycline to CRKP varies greatly in different regions,Citation61,Citation62 but the intravenous minocycline and the treatment regimen of minocycline combined with other antibioticsCitation63 have great potential for the treatment of CRKP infection. The role of minocycline in the treatment of CRKP BSI needs to be further studied.
Our univariate analysis found that central venous catheters (CVC) may be more strongly associated with hospital-acquired CRKP BSI and hospital death from a hospital-acquired KPBSI than peripherally inserted central venous catheter (PICC). Previous studies have suggested that PICCs have a lower risk of bloodstream infections than CVCs.Citation64 However, the risk of lower extremity venous thrombosis may be higher after a PICC.Citation65 Previous studies have suggested that intravenous catheterization increased the risk of a poor prognosis in the setting of a BSI.Citation66 The choice of PICC or CVC requires further research.
According to the results of our univariate analysis, comorbidities increased the risk of a hospital-acquired CRKP BSI but did not significantly increased the 28-day mortality risk from a hospital-acquired KPBSI. The 28-day mortality of patients with hospital acquired KPBSI was significantly different in patients who underwent invasive operations or had liver, kidney or hematologic dysfunction. The results of our multivariate analysis suggest that the risk factors for 28-day mortality after a hospital-acquired KP BSI were CRKP BSI, septic shock, mechanical ventilation >96 hours, and platelet counts <100×109/L, similar results have been reported in previous studies.Citation67,Citation68 Our univariate analysis suggested that a high coefficient of variation of red blood cell distribution width (RDW-CV) was a risk factor for the 28-day mortality of KP BSI patients. Red blood cell distribution width (RDW) has been recently found to be a potential marker of cardiovascular disease. Studies have shown that a high RDW may be associated with adverse consequences of heart failure,Citation69 in particular long-term prognosis. With respect to infection, previous studies have shown that RDW can predict the prognosis of sepsis in patients with various non-hematologic diseases to a certain extent.Citation70 RDW-CV index is easy to obtain, and its value in infectious diseases merits further study.
Conclusion
CRKP threatens the control and treatment of hospital-acquired infection seriously. Hospital-acquired KP BSI Patients with CRKP BSI, septic shock, mechanical ventilation and deficiency of platelets are more likely to have a poor prognosis. Although an antibiotic restriction strategy is used, the incidence of hospital-acquired KP BSI continues to rise and the proportion of CRKP BSI is also increasing. For patients admitted to ICU, organ transplant wards and hematology department, more resources need to be invested in the prevention of hospital-acquired KP BSI. In addition to reducing the length of hospital stay and the number of invasive operations, we believe that the use of the BLBLIs needs to be carefully evaluated in hospital-acquired infections. According to the monitoring of local antimicrobial susceptibility results, TMP-SMZ and minocycline may be potential treatment options for CRKP infection. We also believe that more attention should be paid to the relationship between respiratory tract infection and bloodstream infection.
Disclosure
The authors report no conflicts of interest related to this work.
Acknowledgments
We thank all of the authors and people who helped us to accomplish this work. This study was supported by the National Natural Science Foundation of China (No. 81973983), the Borrowing and Transferring Subsidy Project in 2019, Hefei (No. J2019Y04), Collaborative Tackling and Public Health Collaborative Innovation Project in Anhui Province (No. GXXT-2020-018), the joint construction project of clinical medicine university and hospital (No. 2021lcxk006), and Natural Science Research Project of Universities in Anhui Province (No. KJ2020A0176).
References
- van Duijn PJ, Dautzenberg MJ, Oostdijk EA. Recent trends in antibiotic resistance in European ICUs. Curr Opin Crit Care. 2011;17(6):658–665. doi:10.1097/MCC.0b013e32834c9d87
- Hu F, Zhu D, Wang F, et al. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–s134. doi:10.1093/cid/ciy657
- Humphries RM, Yang S, Kim S, et al. Duodenoscope-related outbreak of a carbapenem-resistant Klebsiella pneumoniae identified using advanced molecular diagnostics. Clin Infect Dis. 2017;65(7):1159–1166. doi:10.1093/cid/cix527
- Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521–s528. doi:10.1093/cid/ciz824
- David S, Reuter S, Harris SR, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nature Microbiol. 2019;4(11):1919–1929. doi:10.1038/s41564-019-0492-8
- Zong Z, Wu A, Hu B. Infection control in the era of antimicrobial resistance in china: progress, challenges, and opportunities. Clin Infect Dis. 2020;71(Suppl 4):S372–s378. doi:10.1093/cid/ciaa1514
- Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
- Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314(14):1479–1487. doi:10.1001/jama.2015.12480
- Guh AY, Limbago BM, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther. 2014;12(5):565–580. doi:10.1586/14787210.2014.902306
- Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi:10.1111/j.1469-0691.2011.03478.x
- Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
- Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536–1551. doi:10.1007/s00134-020-06106-2
- Fleischmann-Struzek C, Mikolajetz A, Schwarzkopf D, et al. Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between National Health Systems: secular trends in sepsis and infection incidence and mortality in Germany. Intensive Care Med. 2018;44(11):1826–1835. doi:10.1007/s00134-018-5377-4
- Zhu Q, Zhu M, Li C, et al. Epidemiology and microbiology of Gram-negative bloodstream infections in a tertiary-care hospital in Beijing, China: a 9-year retrospective study. Expert Rev Anti Infect Ther. 2021;19(6):769–776. doi:10.1080/14787210.2021.1848544
- Mitchell E, Pearce MS, Roberts A. Gram-negative bloodstream infections and sepsis: risk factors, screening tools and surveillance. Br Med Bull. 2019;132(1):5–15. doi:10.1093/bmb/ldz033
- Stewardson AJ, Marimuthu K, Sengupta S, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19(6):601–610. doi:10.1016/S1473-3099(18)30792-8
- Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033. doi:10.1128/AAC.01020-07
- Fasciana T, Ciammaruconi A, Gentile B, et al. Draft genome sequence and biofilm production of a carbapenemase-producing Klebsiella pneumoniae (KpR405) sequence type 405 strain isolated in Italy. Antibiotics. 2021;10(5):560.
- Wiener-Well Y, Rudensky B, Yinnon AM, et al. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect. 2010;74(4):344–349. doi:10.1016/j.jhin.2009.07.022
- Yan L, Sun J, Xu X, et al. Epidemiology and risk factors of rectal colonization of carbapenemase-producing Enterobacteriaceae among high-risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155. doi:10.1186/s13756-020-00816-4
- Glisovic S, Eintracht S, Longtin Y, et al. Rectal swab screening assays of public health importance in molecular diagnostics: sample adequacy control. J Infect Public Health. 2018;11(2):234–237. doi:10.1016/j.jiph.2017.07.009
- Lledo W, Hernandez M, Lopez Eet al. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep. 2009;58(10):256–260.
- Borer A, Eskira S, Nativ R, et al. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in Southern Israel. Infect Control Hosp Epidemiol. 2011;32(12):1158–1165. doi:10.1086/662620
- Ghafur A, Nagvekar V, Thilakavathy S, et al. “Save Antibiotics, Save lives”: an Indian success story of infection control through persuasive diplomacy. Antimicrob Resist Infect Control. 2012;1(1):29. doi:10.1186/2047-2994-1-29
- Giacobbe DR, Del Bono V, Mikulska M, et al. Impact of a mixed educational and semi-restrictive antimicrobial stewardship project in a large teaching hospital in Northern Italy. Infection. 2017;45(6):849–856. doi:10.1007/s15010-017-1063-7
- Bogan C, Marchaim D. The role of antimicrobial stewardship in curbing carbapenem resistance. Future Microbiol. 2013;8(8):979–991. doi:10.2217/fmb.13.73
- Martin J, Phan HTT, Findlay J, et al. Covert dissemination of carbapenemase-producing Klebsiella pneumoniae (KPC) in a successfully controlled outbreak: long- and short-read whole-genome sequencing demonstrate multiple genetic modes of transmission. J Antimicrob Chemother. 2017;72(11):3025–3034. doi:10.1093/jac/dkx264
- Redder JD, Leth RA, Møller JK. Analysing risk factors for urinary tract infection based on automated monitoring of hospital-acquired infection. J Hosp Infect. 2016;92(4):397–400. doi:10.1016/j.jhin.2015.12.009
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
- Zhang P, Wang J, Hu H, et al. Clinical characteristics and risk factors for bloodstream infection due to carbapenem-resistant Klebsiella pneumoniae in patients with hematologic malignancies. Infect Drug Resist. 2020;13:3233–3242.
- Di Gaudio F, Indelicato S, Indelicato S, et al. Improvement of a rapid direct blood culture microbial identification protocol using MALDI-TOF MS and performance comparison with SepsiTyper kit. J Microbiol Methods. 2018;155:1–7.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Third Informational Supplement, M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- Vaquero-Herrero MP, Ragozzino S, Castaño-Romero F, et al. The Pitt bacteremia score, Charlson comorbidity index and chronic disease score are useful tools for the prediction of mortality in patients with Candida bloodstream infection. Mycoses. 2017;60(10):676–685. doi:10.1111/myc.12644
- Watson D, Spaulding AB, Dreyfus J. Risk-set matching to assess the impact of hospital-acquired bloodstream infections. Am J Epidemiol. 2019;188(2):461–466. doi:10.1093/aje/kwy252
- Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59(10):5873–5884. doi:10.1128/AAC.01019-15
- Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799. doi:10.1001/jama.2018.0438
- Kim SW, Yoon JS, Park J, et al. Empirical treatment with carbapenem vs third-generation cephalosporin for treatment of spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2021;19(5):976–986.e975. doi:10.1016/j.cgh.2020.06.046
- Xiao T, Zhu Y, Zhang S, et al. A retrospective analysis of risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteremia in nontransplant patients. J Infect Dis. 2020;221(Suppl 2):S174–s183. doi:10.1093/infdis/jiz559
- Hsu JY, Chuang YC, Wang JT, et al. Healthcare-associated carbapenem-resistant Klebsiella pneumoniae bloodstream infections: risk factors, mortality, and antimicrobial susceptibility, 2017–2019. J Formosan Med Assoc. 2021;120:1994–2002. doi:10.1016/j.jfma.2021.04.014
- Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Suppl 2):S206–s214. doi:10.1093/infdis/jiz622
- Zhang H, Liang B, Wang J, et al. Non-carbapenem β-lactam/β-lactamase inhibitors versus carbapenems for urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae: a systematic review. Int J Antimicrob Agents. 2021;58(4):106410. doi:10.1016/j.ijantimicag.2021.106410
- Dong Y, Li Y, Zhang Y, et al. Clinical efficacy and cost-effectiveness of β-Lactam/β-Lactamase inhibitor combinations and carbapenems in liver cirrhosis patients with gram-negative bacteria bloodstream infection. Infect Drug Resist. 2020;13:1327–1338. doi:10.2147/IDR.S241648
- Harris PNA, Tambyah PA, Lye DC, et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320(10):984–994. doi:10.1001/jama.2018.12163
- Landman D, Babu E, Shah N, et al. Transmission of carbapenem-resistant pathogens in New York City hospitals: progress and frustration. J Antimicrob Chemother. 2012;67(6):1427–1431. doi:10.1093/jac/dks063
- Adams DJ, Susi A, Nylund CM. Clinical characteristics, risk factors, and outcomes of patients hospitalized in the US military health system with carbapenem-resistant Enterobacteriaceae infection. Am J Infect Control. 2020;48(6):644–649. doi:10.1016/j.ajic.2019.10.006
- Parm Ü, Metsvaht T, Sepp E, et al. Mucosal surveillance cultures in predicting Gram-negative late-onset sepsis in neonatal intensive care units. J Hosp Infect. 2011;78(4):327–332. doi:10.1016/j.jhin.2011.03.025
- Guilhen C, Miquel S, Charbonnel N, et al. Colonization and immune modulation properties of Klebsiella pneumoniae biofilm-dispersed cells. NPJ Biofilms Microbiomes. 2019;5(1):25. doi:10.1038/s41522-019-0098-1
- Parm U, Metsvaht T, Sepp E, et al. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum Dev. 2011;87(6):391–399. doi:10.1016/j.earlhumdev.2011.02.007
- Kontopoulou K, Iosifidis E, Antoniadou E, et al. The clinical significance of carbapenem-resistant Klebsiella pneumoniae rectal colonization in critically ill patients: from colonization to bloodstream infection. J Med Microbiol. 2019;68(3):326–335. doi:10.1099/jmm.0.000921
- Souverein D, Euser SM, Herpers BL, et al. Association between rectal colonization with highly resistant gram-negative rods (HR-GNRs) and subsequent infection with HR-GNRs in clinical patients: a one year historical cohort study. PLoS One. 2019;14(1):e0211016. doi:10.1371/journal.pone.0211016
- Strich JR, Warner S, Lai YL, et al. Needs assessment for novel Gram-negative antibiotics in US hospitals: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1172–1181. doi:10.1016/S1473-3099(20)30153-5
- Kim YA, Lee SJ, Park YS, et al. Risk factors for carbapenemase-producing enterobacterales infection or colonization in a Korean Intensive Care Unit: a Case-Control Study. Antibiotics. 2020;9(10). doi:10.3390/antibiotics9100680
- Akgul F, Bozkurt I, Sunbul M, et al. Risk factors and mortality in the Carbapenem-resistant Klebsiella pneumoniae infection: case control study. Pathog Glob Health. 2016;110(7–8):321–325. doi:10.1080/20477724.2016.1254976
- Zhao GJ, Li D, Zhao Q, et al. Incidence, risk factors and impact on outcomes of secondary infection in patients with septic shock: an 8-year retrospective study. Sci Rep. 2016;6:38361. doi:10.1038/srep38361
- Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–s575. doi:10.1093/cid/ciz830
- Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, et al. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(3):905–913. doi:10.1093/jac/dku432
- Loose M, Link I, Naber KG, et al. Carbapenem-containing combination antibiotic therapy against carbapenem-resistant uropathogenic Enterobacteriaceae. Antimicrob Agents Chemother. 2019;64(1). doi:10.1128/AAC.01839-19
- Li J, Bi W, Dong G, et al. The new perspective of old antibiotic: in vitro antibacterial activity of TMP-SMZ against Klebsiella pneumoniae. J Microbiol Immunol Infect. 2020;53(5):757–765. doi:10.1016/j.jmii.2018.12.013
- Koppe U, von Laer A, Kroll LE, et al. Carbapenem non-susceptibility of Klebsiella pneumoniae isolates in hospitals from 2011 to 2016, data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob Resist Infect Control. 2018;7:71. doi:10.1186/s13756-018-0362-9
- Cusini A, Herren D, Bütikofer L, et al. Intra-hospital differences in antibiotic use correlate with antimicrobial resistance rate in Escherichia coli and Klebsiella pneumoniae: a retrospective observational study. Antimicrob Resist Infect Control. 2018;7:89. doi:10.1186/s13756-018-0387-0
- Pogue JM, Neelakanta A, Mynatt RP, et al. Carbapenem-resistance in gram-negative bacilli and intravenous minocycline: an antimicrobial stewardship approach at the Detroit Medical Center. Clin Infect Dis. 2014;59(Suppl 6):S388–393. doi:10.1093/cid/ciu594
- Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE Study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–s205. doi:10.1093/cid/ciy660
- Zhao C, Wistrand-Yuen P, Lagerbäck P, et al. Combination of polymyxin B and minocycline against multidrug-resistant Klebsiella pneumoniae: interaction quantified by pharmacokinetic/pharmacodynamic modelling from in vitro data. Int J Antimicrob Agents. 2020;55(6):105941. doi:10.1016/j.ijantimicag.2020.105941
- Yamaguchi RS, Noritomi DT, Degaspare NV, et al. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: a propensity-adjusted analysis. Intensive Care Med. 2017;43(8):1097–1104. doi:10.1007/s00134-017-4852-7
- Bonizzoli M, Batacchi S, Cianchi G, et al. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med. 2011;37(2):284–289. doi:10.1007/s00134-010-2043-x
- Reunes S, Rombaut V, Vogelaers D, et al. Risk factors and mortality for nosocomial bloodstream infections in elderly patients. Eur J Intern Med. 2011;22(5):e39–44. doi:10.1016/j.ejim.2011.02.004
- Zhang S, Yang Z, Sun L, et al. Clinical observation and prognostic analysis of patients with Klebsiella pneumoniae bloodstream infection. Front Cell Infect Microbiol. 2020;10:577244. doi:10.3389/fcimb.2020.577244
- Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–1876. doi:10.1097/00003246-200006000-00031
- Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117(2):163–168. doi:10.1161/CIRCULATIONAHA.107.727545
- Hu ZD, Lippi G, Montagnana M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: a narrative review. Clin Biochem. 2020;77:1–6. doi:10.1016/j.clinbiochem.2020.01.001
