Abstract
Shewanella algae, a Gram-negative bacillus found in warm saltwater environments, has been increasingly recognized as a human pathogen that can cause infection of the skin and soft tissue, ear, blood, and intra-abdominal. In this case, we report a Shewanella algae infection that caused sepsis, renal insufficiency, cardiac dysfunction, fistula and massive pleural effusion after surgery in a 73-year-old man with cancer of the esophagus and cardia.
Introduction
Shewanella algae are heterotrophic facultative anaerobes and exist throughout the world, mainly in marine environments.Citation1 The infection of Shewanella has been dominated by the infection of S. algae. The most common source for human infection is exposure to seawater, especially patients with exposure of cutaneous ulcers to seawater.Citation2 The common clinical syndrome includes skin and soft tissue infection, otitis, and a variety of other disorders, with or without bacteremia.Citation2 Immune-compromised population, those with malignancy, tourists, swimmers, fishermen, and sailors with underlying liver disease are at high risk of Shewanella infection.Citation3 The previous fulminant bacteremia reports of S. putrefaciens were in patients with severe hepatobiliary disease and malignancy.Citation4 Most of the course about S. algae is benign;Citation5 we present a case with fulminating course of S. algae.
Case Report
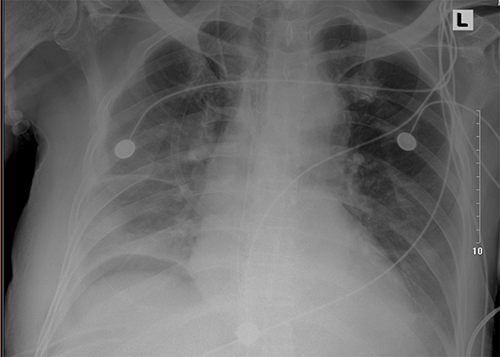
A 73-year-old man with cancer of the esophagus and cardia and a 3-week history of progressive dysphagia. He had undergone cholecystostomy for gall stones 13 years previously. After robot-assisted esophagectomy and combined thoracoscopy- and laparoscopy-assisted esophagectomy surgery, he was transferred to ICU, and the APACHE II score was 8 with normal vital signs. On the day after admission, the patient was extubated when the oxygenation index was more than 300. In the night of extubation, the patient had a blood pressure of 85/54 mmHg accompanied with massive drainage of pleural effusion, and subsequently with oliguria, cardiac dysfunction, and atrial fibrillation. The drainage of closed thoracic in the right was 1550 mL with dusky red color within 24 hours. The laboratory evaluation was significant for blood urea nitrogen of 16.4 mmol/L (normal 3.6–9.5), creatinine of 184 μmol/L (normal 52–101), cystatin C of 1.98 ng/L (normal 0.51–1.09), N-terminal fragment brain natriuretic peptides (NT-pro-BNP) of 2677.00 pg/mL (normal < 125), and procalcitonin (PCT) of 16.4 ng/mL (normal < 0.05 ng/mL). Treatment with continuous renal replacement therapy (CRRT), high-flow oxygen therapy, and piperacillin-tazobactam was started. Piperacillin-tazobactam was used in the initial empiric therapy for sepsis. The culture of drainage fluid and sputum grew Shewanella putrefaciens, but the result of next-generation sequencing technology (NGS, Genskey Medical Technology Co., Ltd, Beijing, China) with blood sample was S. algae with 58.96% relative abundance. The result of susceptibility testing is shown in . Chest radiography and computed tomography (CT) scan showed bilateral pleural effusions ().
Table 1 Antibiotic Susceptibility Test of Shewanella Algae in the Two Admissions to ICU
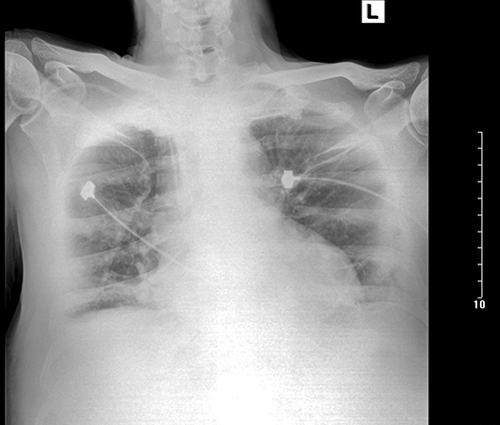
After 8 days in the ICU, the patient was transferred to the ward of gastrointestinal surgery. On the ward, his condition progressed with the drainage of the mediastinum and his temperature increased. The drainage of mediastinum was more than an average of 500 mL each day while on the ward (). The initial antibiotic therapy was cefoperazone/sulbactam, and additional coverage with imipenem/cilastatin when the patient’s temperature was more than 38 °C. The highest temperature was 39.9 °C on the ward. In 6 days, he developed dyspnea with hypoxia and progressed to narcolepsy; blood-gas analysis showed pH was 7.15, O2 was 69 mmHg, CO2 was 69 mmHg.
He was then re-admitted to the ICU with the diagnosis of anastomotic fistula, respiratory acidosis, pulmonary infections, renal insufficiency, and empyema. The culture of drainage fluid and sputum was positive for Shewanella putrefaciens again. At this time, the laboratory investigations were abnormal with NT-pro-BNP of 2554.00 pg/mL, blood urea nitrogen of 17.52 mmol/L, creatinine of 142 μmol/L, and cystatin C of 2.54 ng/L. The therapy included CRRT and meropenem was started. The drainage of the mediastinum had the same color as the biliary fluid (). In the 7 days re-admitted to the ICU, the patient developed severe intraperitoneal hemorrhage. His severe intraperitoneal hemorrhage required emergency laparotomy for hemostasis, but the surgery had a high risk. After we informed the potential risk, his relatives finally chose to forgo the next treatment. The patient finally died on the day of discharge.
Discussion
Shewanella species are ubiquitous, non-fermenting Gram-negative rod-shaped bacteria widely distributed in nature, especially in the marine environment.Citation6 The genus is increasingly reported in human infection, with Shewanella putrefaciens and S. algae responsible for the majority. Approximately 80% of human isolates attributing to Shewanella are S. algae.Citation5 The hemolytic activity, formation of biofilms, enzymatic activity, exotoxin production and the ability to adhere to human epithelial cells contribute to the virulence of S. algae.Citation7 In this case, we first performed culture and molecular identification of bacteria of drainage fluid and sputum, which resulted in Shewanella putrefaciens. This was the first time we had seen the rarer pathogen, so we performed the NGS again according to the advice of the clinical laboratory. The result of NGS was S. algae, which was different from the result of culture. The identification of S. algae in our clinical laboratory was difficult because the automated identification systems cannot distinguish between these two species, since S. algae is not included in the databases. The 16S rRNA sequence analysis is the most recommended method for identifying Shewanella species.Citation8 So, the true result should be infection with S. algae.
Shewanella algae is the most relevant human pathogen in the genus Shewanella, causing skin and soft tissue infections, bacteremia, otitis, and a variety of other diseases.Citation2 Death from Shewanella infection was 8% and 13%, respectively, and bacteremia was 18% and 28%, respectively in the two articles.Citation8,Citation9 The infection of S. algae is usually benign but may be fulminant in immunocompromised hosts. Through retrieving the PubMed and one article previously published,Citation10 we found at least 48 cases infected with S. algae. Shewanella algae was misidentified for many years as S. putrefaciens or Pseudomonas putrefaciens, and cases of S. algae infection in detailed regions have not been included.Citation11,Citation12 So, the number of S. algae infection has been largely under-reported.Citation12 Detailed cases had been reported by Jacob-Kokura et al. and Lee et al.Citation10,Citation13,Citation14 Although S. algae infection remains rare in recent years, the incidence is increasing. But among the articles we searched, cases about S. algae infection that were cancer patients without typical preceding exposure were few. In a retrospective study about risk factors, clinical manifestation,s and outcome of Shewanella infection, they analyzed 29 patients. However, only seven had definite infection, six cases were infected by S. algae among the 29 patients, and two cases separately had lung cancer and hepatocellular carcinoma.Citation11 Meanwhile, four cases had been reported, among these four cases of S. algae infection, one patient was diagnosed with chronic osteomyelitis with a history of exposure,Citation15 and another was intraductal papillary mucinous tumor.Citation16 The other two patients were both diagnosed with gastric cancer.Citation17,Citation18
Our patient had no typical preceding exposure. Clinical strains were isolated from stool, blood, bile, and sputum. The source of Shewanella infection includes exposure of skin breakdown to seawater, traumatic events with contamination of injured tissues, via the gastrointestinal tract by food consumption, and through the ear canal.Citation19 Cases of gastroenteritis and peritonitis caused by Shewanella also have been reported.Citation20,Citation21 The infection is commonly seen among patients with underlying diseases such as renal failure, neutropenia, hepatobiliary disease, diabetes, and trauma accidents.Citation8 The ability of S. algae to adapt to bile salt is an important factor for its survival and subsequent infection of the hepatobiliary tract.Citation22 The hepatobiliary tract is also a major source of bloodstream infection of S. algae.Citation22 In this case, it was suspected that the malignancy and surgery both impaired the immune system and the surgery increased the opportunity of S. algae exposure to the thorax and mediastinum. Then the virulence factors of S. algae led to the massive hydrothorax, increasing the incidence of anastomotic fistula and finally contributing to the intraperitoneal hemorrhage.
Shewanella species are generally susceptible to third-generation cephalosporins, beta-lactam/beta-lactamase inhibitors, aminoglycosides, and fluoroquinolones, and have resistance to colistin, sulfonamides, trimethoprim, and polymyxin B; the ampicillin and cephalosporins susceptibility varied.Citation5 Antibiotic resistance to Shewanella species is on the rise. Shewanella has been considered a reservoir and vehicle of different antibiotic resistance genes due to the several determinants of antibiotic resistance detected in the genus.Citation8 The oxacillinase class D β-lactamase encoding genes (blaOXA) confer resistance to carbapenems, qnr genes to quinolones, class C beta-lactamases (blaAmpC) to cephalosporins, and eptA gene to colistin.Citation8,Citation23 The susceptibility testing results guided us to the antimicrobial therapy, which used the minimum inhibitory concentration (MIC) to define the resistance of specific strains to the relative antibiotic. The broth microdilution method was used to determine the MIC of antimicrobial agents. The breakpoints were identified by Vitek 2 Compact (bioMerieux, France) automated identification system. The first result of the antibiotic susceptibility test was pan sensitivity. We first initiated piperacillin-tazobactam in the first admission to the ICU. During the period on the ward, the antibiotics were imipenem/cilastatin and cefoperazone/sulbactam. However, in the second admission to the ICU, the culture and sensitivity test showed that S. algae was resistant to the imipenem and piperacillin-tazobactam, thus we switched to meropenem. Resistance to piperacillin-tazobactam, imipenem, and meropenem had also been reported in some cases.Citation24
Conclusion
Shewanella species has been considered an emerging cause of human infections. Although its infection has a strong relationship with exposure to seawater and contact with seafood, several cases that had no exposure to aquatic sources have been reported, so clinical staff need to consider the rare pathogen for the early diagnosis and prompt initiation of treatment, especially for patients with malignancy. Also, further study needs to explore the precise guidelines for the antibiotic choice and duration for Shewanella species.
Ethics Statement
This case had obtained the consent of the ethics committee of Xijing Hospital, and written informed consent was obtained from the patient’s relatives for the publication of the case details and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lemaire ON, Méjean V, Iobbi-Nivol C. The Shewanella genus: ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol Rev. 2020;44(2):155–170. doi:10.1093/femsre/fuz031
- Tellgren-Roth C, Thorell K, Galperin MY, et al. Complete genome sequence and methylome of the type strain of Shewanella algae. Microbiol Resour Announc. 2021;10(31):e0055921. doi:10.1128/MRA.00559-21
- Weiss TJ, Barranco-Trabi JJ, Brown A, Oommen TT, Mank V, Ryan C. Case report: Shewanella algae pneumonia and bacteremia in an elderly male living at a long-term care facility. Am J Trop Med Hyg. 2021;106:60–61. doi:10.4269/ajtmh.21-0614
- Otsuka T, Noda T, Noguchi A, Nakamura H, Ibaraki K, Yamaoka K. Shewanella infection in decompensated liver disease: a septic case. J Gastroenterol. 2007;42(1):87–90. doi:10.1007/s00535-006-1957-0
- Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect. 2005;11(5):347–352. doi:10.1111/j.1469-0691.2005.01108.x
- Tamez AM, McLaughlin RW, Li J, Wan X, Zheng J. Searching for putative virulence factors in the genomes of Shewanella indica and Shewanella algae. Arch Microbiol. 2021;203(2):683–692. doi:10.1007/s00203-020-02060-1
- Bravenec CA, Pandit RT, Beaver HA. Shewanella algae keratitis. Indian J Ophthalmol. 2019;67(1):148–150. doi:10.4103/ijo.IJO_617_18
- Yousfi K, Bekal S, Usongo V, Touati A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur J Clin Microbiol Infect Dis. 2017;36(8):1353–1362. doi:10.1007/s10096-017-2962-3
- Vignier N, Barreau M, Olive C, et al. Human infection with Shewanella putrefaciens and S. algae: report of 16 cases in Martinique and review of the literature. Am J Trop Med Hyg. 2013;89(1):151–156. doi:10.4269/ajtmh.13-0055
- Jacob-Kokura S, Chan CY, Kaplan L. Bacteremia and empyema caused by Shewanella algae in a trauma patient. Ann Pharmacother. 2014;48(1):128–136. doi:10.1177/1060028013517630
- To KKW, Wong SSY, Cheng VCC, et al. Epidemiology and clinical features of Shewanella infection over an eight-year period. Scand J Infect Dis. 2010;42(10):757–762. doi:10.3109/00365548.2010.490562
- Torri A, Bertini S, Schiavone P, et al. Shewanella algae infection in Italy: report of 3 years’ evaluation along the coast of the northern Adriatic Sea. New Microbes N Infect. 2018;23:39–43. doi:10.1016/j.nmni.2018.01.002
- Lee ST, Lee SJ, Yun MJ, et al. A case of Shewanella algae bacteremia accompanying cellulitis in both legs of a patient on hemodialysis: case report and literature review. Infect Chemother. 2012;44(3):193. doi:10.3947/ic.2012.44.3.193
- Takata T, Chikumi H, Morishita S, et al. Shewanella algae bacteremia in an end-stage renal disease patient: a case report and review of the literature. Intern Med. 2017;56(6):729–732. doi:10.2169/internalmedicine.56.7616
- Sumathi BG, Kumarswamy SR, Amritam U, Arjunan R. Shewanella algae: first case report of the fast emerging marine pathogen from squamous cell carcinoma patient in India. South Asian J Cancer. 2014;03(03):188–189. doi:10.4103/2278-330X.136819
- Kim DM, Kang CI, Lee CS, et al. Treatment failure due to emergence of resistance to carbapenem during therapy for Shewanella algae bacteremia. J Clin Microbiol. 2006;44(3):1172–1174. doi:10.1128/JCM.44.3.1172-1174.2006
- Tan CK, Lai CC, Kuar WK, Hsueh PR. Purulent pericarditis with greenish pericardial effusion caused by Shewanella algae. J Clin Microbiol. 2008;46(8):2817–2819. doi:10.1128/JCM.01018-08
- Zhang F, Fang Y, Pang F, et al. Rare Shewanella spp. associated with pulmonary and bloodstream infections of cancer patients, China: a case report. BMC Infect Dis. 2018;18(1):454. doi:10.1186/s12879-018-3354-8
- Janda JM, Abbott SL. The genus Shewanella: from the briny depths below to human pathogen. Crit Rev Microbiol. 2014;40(4):293–312. doi:10.3109/1040841X.2012.726209
- Dey S, Bhattacharya D, Roy S, Nadgir SD, Patil A, Kholkute SD. Shewanella algae in acute gastroenteritis. Indian J Med Microbiol. 2015;33(1):172–175. doi:10.4103/0255-0857.148442
- Shanmuganathan M, Goh BL, Lim C, NorFadhlina Z, Fairol I. Shewanella algae peritonitis in patients on peritoneal dialysis. Perit Dial Int. 2016;36(5):574–575. doi:10.3747/pdi.2015.00287
- Tseng SY, Tung KC, Cheng JF, et al. Genome characterization of bile-isolated Shewanella algae ACCC. Gut Pathog. 2018;10:38. doi:10.1186/s13099-018-0267-4
- Zago V, Veschetti L, Patuzzo C, Malerba G, Lleo MM. Shewanella algae and Vibrio spp. strains isolated in Italian aquaculture farms are reservoirs of antibiotic resistant genes that might constitute a risk for human health. Mar Pollut Bull. 2020;154:111057. doi:10.1016/j.marpolbul.2020.111057
- Bernshteyn M, Ashok Kumar P, Joshi S. Shewanella algae - a novel organism causing bacteremia: a rare case and literature review. Cureus. 2020;12(9):e10676. doi:10.7759/cureus.10676