Abstract
Helicobacter pylori is one of the most common pathogenic bacterium worldwide, infecting about 50% of the world’s population. It is a major cause of several upper gastrointestinal diseases, including peptic ulcers and gastric cancer. The emergence of H. pylori resistance to antibiotics has been a major clinical challenge in the field of gastroenterology. In the course of H. pylori infection, some bacteria invade the gastric epithelium and are encapsulated into a self-produced matrix to form biofilms that protect the bacteria from external threats. Bacteria with biofilm structures can be up to 1000 times more resistant to antibiotics than planktonic bacteria. This implies that targeting biofilms might be an effective strategy to alleviate H. pylori drug resistance. Therefore, it is important to develop drugs that can eliminate or disperse biofilms. In recent years, anti-biofilm agents have been investigated as alternative or complementary therapies to antibiotics to reduce the rate of drug resistance. This article discusses the formation of H. pylori biofilms, the relationship between biofilms and drug resistance in H. pylori, and the recent developments in the research of anti-biofilm agents targeting H. pylori drug resistance.
Introduction
Helicobacter pylori is a Gram-negative microaerobic spiral rod-shaped bacterium that was first cultured and identified by Professors Marshall and Dr. Warren.Citation1 This bacterium primarily colonizes the gastric mucosal surface and has been linked to chronic gastritis, peptic ulcer, gastric mucosa-associated tissue lymphoma (MALT), gastric cancer, and other upper gastrointestinal disorders.Citation2 Standard triple therapy (proton pump inhibitor + two antibiotics) and bismuth quadruple therapy (proton pump inhibitor + bismuth + two antibiotics) are the most commonly used during eradication therapies for H. pylori.Citation3
H. pylori secrete proteins, polysaccharides, extracellular DNA (eDNA), and other molecules to create extracellular polymeric substances (EPS) after colonizing the gastric mucosa, which are wrapped and adhered to each other by bacteria to form biofilms.Citation4 Unlike planktonic bacteria, colonies that form biofilm structures are highly resistant to the harsh external environment, including antibiotic exposure.Citation5 It is well established that when bacteria develop biofilms, their resistance to antibiotics increases by up to 10–1000 times.Citation6 Therefore, the formation of H. pylori biofilms is most likely the primary cause of long-term chronic infection, multiple drug resistance, and treatment failure.
Consequently, strategies targeting biofilms can be applied to alleviate H. pylori drug resistance. Research should be directed at developing anti-biofilm agents/molecules and determine their minimum effective concentration that can completely eradicate biofilm with maximum potency without causing unwanted side effects to the host.
Helicobacter pylori Biofilm
The formation of H. pylori biofilms decreases efficacy of conventional eradication treatments.Citation7 Individual planktonic bacteria which grow on agar plates or broths are often used as platforms for antibiotic susceptibility testing. However, these bacteria do not exist as independent individuals, and the majority do not live as a single species.Citation8 About 80% of the world’s bacteria are known to exist as biofilms,Citation9 and this has been their major means of survival for billions of years.Citation10 Bacteria that form biofilms adhere to one another using extracellular polymeric substances (EPS) composed of polysaccharides, proteins, extracellular DNA (eDNA), and share information using quorum sensing (QS) system, allowing them to live in an organized manner similar to that of animal populations.Citation4 In the event of a threat, such as drastic changes in temperature and pH, nutrient and oxygen deficiency, antibiotic exposure, or other similar events, they are able to respond immediately.
Steps of H. pylori Biofilm Formation
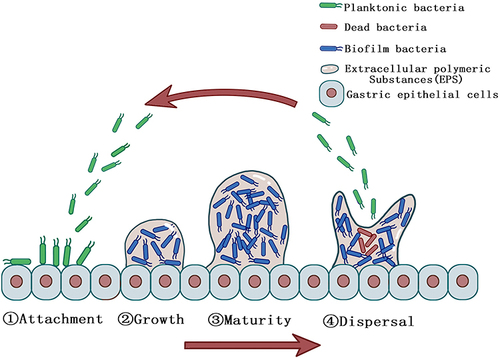
Majority of H. pylori strains are capable of forming biofilms in vivo and in vitro depending on the strain.Citation11,Citation12 Clinical strains isolated from the gastric mucosa of patients have been reported to have higher capacity to form biofilm than other strains.Citation13 The formation of biofilms by H. pylori, like other bacteria, is divided into four steps: (1) attachment, (2) growth, (3) maturity, and (4) dispersal ().Citation14 H. pylori adheres to the gastric epithelial cells in the gastric sinus. Co-adhesion is the term used to describe adhesion that occurs between the bacterial cytosol and the gastric epithelial cells.Citation15 This process is driven by bacterial structures such as flagella, pili, and lipopolysaccharides, and it is also involved in the initial step of H. pylori pathogenicity. Bacterial adhesion to a surface can upregulate the secretion of intercellular signaling molecules via a QS mechanism within minutes and co-produce EPS with surrounding bacteria to establish firm and irreversible microcolonies.Citation16,Citation17 Bacteria in microcolonies continue to proliferate and produce EPS, which promotes bacterial coaggregation and results in the formation of an early biofilm structure.Citation15 The biofilm matures after 2–4 days after initial adhesion and is maintained for some time.Citation17 When nutrients are depleted in the biofilm and waste metabolites accumulate to a specific concentration threshold, the biofilm disintegrates into the dispersal stage. This process is mediated by several mechanisms, including termination of the synthesis of biofilm matrix compounds, degradation of the matrix, and disruption of non-covalent interactions between matrix components.Citation18 After dispersal, the bacteria undergo the next stage of expanded infection and biofilm formation in a new ecological niche. In this regard, if the concentration or dose of antimicrobial agents is insufficient, or if only anti-biofilm agents without antimicrobial activity are used during the eradication of H. pylori with biofilm formation, it will not be possible to effectively eradicate all bacteria, but rather flush out only the biofilm matrix, and the dispersed flora may re-adhere to the gastric epithelium, further expanding the size of the biofilm and the scope of infection. This might be the primary reason why, in some studies, the size of the biofilm increased rather than decreased following the application of sub-inhibitory doses of antibiotics.Citation19 This also implies that some anti-biofilm agents that lack antimicrobial activity may require to be supplemented with antibiotics to improve the therapeutic effect, as they may otherwise make the infection more severe.
Figure 1 Steps of H. pylori biofilm formation. Planktonic H. pylori (green) adhere to the surface of gastric epithelial cells and secrete molecules to create extracellular polymeric substances (EPS) to form an early biofilm structure and biofilm H. pylori (blue) are protected from the adverse external environment. After the biofilm matures, dead H. pylori (red) gradually appear due to the lack of nutrients in the biofilm and the biofilm dispersal into the next cycle.
Regulatory Pathways Affecting Biofilm Formation of Helicobacter pylori
Research on regulatory pathways affecting H. pylori biofilm formation is still in its infancy. The most extensively pathway is the intercellular communication mechanism known as the quorum sensing (QS) system.Citation20 Bacteria are capable of autonomous growth, division, sensing, and adaptation to environmental signals. The process of biofilm formation is a community behavior in which bacteria interact with one another and regulate gene expression in response to population density changes, allowing bacteria to adapt to changes in the external environment.Citation21 The transition between these two states is governed by QS signaling molecules such as N-acyl-homoserine lactones (AHL), autoinducing peptide (AIP), autoinducer-2 (AI-2), and diffusion signaling factor (DSF).Citation22,Citation23 Cole et al found that specific mutations in the cagE type IV secretion gene and quorum-sensing gene luxS may be associated with enhancing the ability of H. pylori biofilm formation.Citation24 Elsewhere, Wong et al used comparative genomics to sequence the entire genomes of 32 biofilm-forming clinical strains and found that genes involved in H. pylori biofilm formation included alpha (1,3)-fucosyltransferase, flagellar protein, 3 hypothetical proteins, outer membrane protein, and a cag pathogenicity island protein.Citation25 These genes play a role in bacterial motility, lipopolysaccharide (LPS) synthesis, Lewis antigen synthesis, adhesion, and/or the type-IV secretion system (T4SS). The outer membrane protein AlpB plays a critical role in the formation of strong biofilms by the TK1402 strain.Citation26 In ArsRS mutant strains, the outer membrane protein HomB is required for hyperbiofilm formation and aberrant regulation of this gene is sufficient to induce a hyperbiofilm phenotype.Citation27 SpoT has been implicated in biofilm formation in multi-drug resistant bacteria by upregulating the efflux pump HP1174 and the neutrophil-activating protein (NapA; HP0243).Citation28,Citation29 The transporter proteins HP0939, HP0497, and HP0471 are implicated in H. pylori biofilm formation.Citation30
Methods for Detecting Biofilms
The most frequently used staining method for the quantitative determination of in vitro biofilms grown attached in micro-titer polystyrene well plates is crystalline violet (CV).Citation31,Citation32 After staining, the scanning electron microscope is used to observe the three-dimensional structure of biofilms.Citation11 In contrast, the crystal violet staining method has its limitations, since it requires repeated washing, which invariably decreases the number of biofilm cells. Other methods for detecting biofilm formation include the tissue culture plate method,Citation33 bioluminescence analysis,Citation34 transmission percentage (%T) method,Citation35 and some other biofilm imaging techniques such as fluorescence microscopy examination, confocal laser scanning microscopy (CLSM), infrared spectroscopy, and optical fluorimetry.Citation36,Citation37
The Mechanism of Drug Resistance in Helicobacter pylori Biofilm
At present, the mechanisms of H. pylori biofilm resistance are not fully elucidated, and there is considerable room for doubt and evidence. However, according to current research, they are mostly related to the resistance mechanisms described below.
EPS Barrier Protection
EPS plays a primary role in H. pylori biofilm resistance. Because the target of antibiotics is typically situated within the bacterial cell and EPS is located in the outermost layer of the biofilm, EPS wraps around the bacterium, avoiding direct interaction of the body’s immune cells with the bacteria and decreasing antibiotic penetration.Citation38 Furthermore, because EPS is generally negatively charged and some of antibiotics are positively charged, the EPS component of the biofilm also forms a natural charge barrier, limiting antimicrobial agents transport.Citation39
H. pylori Coccoid Formation
There are two forms of viable H. pylori, the spiral form, which is highly culturable and colonizable, and the viable but non-culturable (VBNC) coccoid form, also known as the persistent form, which is a dormant state of the bacterium.Citation40,Citation41 When the external environment is unfavorable for H. pylori growth and reproduction, such as lack of nutrients, changes in oxygen concentration or pH, and antimicrobial drug intervention, H. pylori form biofilms and undergoes transformation from spiral to coccoid forms.Citation42 In general, antimicrobial drugs have excellent inhibitory and bactericidal effects only on bacteria in their active phase, while dormant bacteria located deep inside the biofilm are difficult to kill. Lewis observed that while most cells in the biofilm are sensitive to antibiotics, a small proportion of persistent cells survive, independent of the antibiotic concentration.Citation43 Biofilm-protected cells can withstand large dosages of antibiotics as well as immunological defense mechanisms. When antibiotic concentrations are reduced, coccoid H. pylori transform back into a reproducible spiral and repopulate the biofilm or disperse out of it to form a new biofilm.Citation43,Citation44
Involvement of Efflux Pumps
The efflux pump is a multidrug transporter protein that is found on the bacterial cell membrane. The pump transports antimicrobial drugs out of the bacterium, thereby decreasing the intracellular concentration of antimicrobial drugs which promotes drug resistance. It is the primary cause of multi-drug resistance in H. pylori. A previous study showed that when biofilms were exposed to clarithromycin, they developed substantial levels of resistance compared to planktonic cells, and significant expression of efflux pump genes was detected in these biofilm cells.Citation45 Other studies have revealed that the expression of efflux pump genes Hp605, Hp971, Hp1327, Hp1489, Hp118, and Hp1174 is remarkably higher in bacteria that form biofilms than in planktonic bacteria,Citation5,Citation28 implying that efflux pumps and biofilms can work synergistically to increase drug resistance.
Other Drug Resistance Mechanisms
Research has revealed that increased propagation of antibiotic resistance genes in biofilms through horizontal gene transfer, integration of conjugative elements, and natural transformation leads to drug resistance.Citation46–48 Point mutations at positions 2142 or 2143 in the V structural domain of 23S rRNA in biofilms results in development of drug resistance.Citation45 Hathroubi et al found that biofilm formation causes changes in outer membrane proteins associated with antibiotic resistance and that increasing proteinase K levels can alleviate clarithromycin resistance.Citation49 Furthermore, eDNA in biofilms promotes microbial adhesion, inhibits antibiotic diffusion, and chelates cations.Citation50 Some extracellular enzymes in the biofilm may have hydrolytic effects on antibiotics.Citation51
Anti-Biofilm Agents Against H. pylori
Natural Products
As indicated in , most antibiofilm agents are mainly isolated from natural products, many of which are “secondary” metabolites and can be produced by microorganisms,Citation52 such as phytochemicals, biosurfactants, antimicrobial peptides, and microbial enzymes, etc.Citation53 In addition, several quorum sensing inhibitors and probiotics have been found to show anti-biofilm activity.Citation54,Citation55 It is interesting to note that all natural products in have anti-H. pylori biofilm activity, and nearly all of them also have antibacterial ability. These natural products have good anti-biofilm and antibacterial abilities in vitro, whereas some of the natural products such as Pistacia vera L. oleoresin, Dihydrotanshinone I (DHT), Amu-ru 7, and Casearia sylvestris leaf derivatives are effective against H. pylori both in vitro and in vivo.Citation56–59 It is noteworthy that some of the natural products tested for anti-biofilm and antibacterial ability were carried out using H. pylori strains that were resistant to one or more drugs.Citation12,Citation56,Citation60 This suggests that some natural products have the potential to alleviate H. pylori multidrug resistance. Evidence from prior studies has indicated that H. pylori eradication therapy requires a combination of different antibiotics such as clarithromycin (CLR), levofloxacin (LVX), amoxicillin (AMX), metronidazole (MTZ), and tetracycline (TET).Citation61–63 A combination of classically used antibiotics with natural products can synergistically fight against H. pylori. Pistacia vera L. oleoresin synergizes with levofloxacin to suppress drug-resistance in H. pylori strains.Citation56 When Lactobacillus salivarius LN12 cell-free supernatant (CFS) was used in combination with AMX and CLR, they disrupted the biofilm structure of some strains much more effectively than when each agent was applied alone.Citation64 Myricetin was the only natural product that synergized with all five traditional anti-H. pylori antibiotics to disrupt the transition of H. pylori from spiral to coccoid forms.Citation65 In comparison, several anti-biofilm agents appear to be more effective in eradicating H. pylori than a combination of some antibiotics. Armeniaspirol A (ARM1) exerted potent antibacterial activity against H. pylori (including multidrug-resistant strains). Moreover, a combination of ARM1 and omeprazole more effectively killed H. pylori in vivo compared to standard triple therapy in a mouse model of multidrug-resistant H. pylori infection.Citation12 The combination of DHT and omeprazole also showed superior H. pylori-killing effect than standard triple therapy, suggesting that DHT may be suitable anti-H. pylori drug when combined with a proton pump inhibitor.Citation57 From the above, it follows that natural products have great potential to combat H. pylori biofilms and to address the problem of drug resistance in H. pylori.
Table 1 Natural Anti-Biofilm Agents Targeting H. pylori Infection
Nanoparticles
In recent years, nanomaterials have also been used to eradicate H. pylori biofilms and minimize drug resistance.Citation66–68 New Synthesized Silver Ultra-NanoClusters (SUNCs) alone or in combination with metronidazole exhibit good anti-biofilm and antibacterial activity.Citation69 Nanodrugs made of berberine derivatives and rhamnolipids (RHL) penetrated the mucus layer and effectively cleared H. pylori biofilms in vitro and in vivo.Citation70
Acetylcysteine
N-acetylcysteine is the only molecule in clinical trials that has been found to be effective against H. pylori biofilms.Citation71 It is an antioxidant that breaks down of mucus and is most frequently used to treat chronic respiratory tract infections.Citation72 Numerous studies have demonstrated that NAC inhibits bacterial adhesion, decreases the viability of sequestered cells, disrupts mature biofilms of a variety of bacteria, and inhibits the production of extracellular polysaccharide substrates.Citation73–75 NAC pretreatment improves the outcome of patients with refractory Helicobacter pylori infection before initiating triple therapy.Citation71
Other
A previous study showed that Extremely Low-Frequency Electromagnetic Fields (ELFs) can reduce H. pylori biofilm adhesion and formation.Citation76 A combination of curcumin and blue light irradiation for more than 6 minutes disrupted H. pylori mature biofilms by more than 50% and enhanced the antimicrobial effect.Citation77,Citation78 An Electrolyzed Superoxidized Solution exerted antibacterial and anti-biofilm effects against H. pylori.Citation79
Shortcomings of the Current Study
First, researchers have made significant progress in understanding biofilms for opportunistic pathogenic bacteria, particularly those found in hospitals. Many of these bacteria are typically sequestered on surfaces of indwelling medical devices.Citation80 For example, Pseudomonas aeruginosa, which can form biofilms on medical equipments such as catheters, implants, and contact lenses,Citation81 Escherichia coli, which can also form biofilms on surfaces of indwelling catheters,Citation82 Acinetobacter baumannii, which has been reported to cause ventilator-associated and catheter-associated biofilm infections.Citation83 Staphylococcus, which can result in mechanical heart valves-associated and central venous catheter-associated biofilm infections,Citation84 and Candida, which can form biofilms on medical devices such as vascular catheters, joint prostheses, and dialysis catheters,Citation85 etc. To date, there are few studies on H. pylori biofilms, and research is still at the preliminary stage. Given that biofilms may be an important cause of H. pylori drug-resistant and long-term infections, significant research attention should be directed at H. pylori biofilms. Second, most anti-biofilm agents targeting H. pylori have been mainly tested in vitro using only standard strains such as SS1, ACTC43503, NCTC11639, and G27, which are not geographic- or strain-specific.Citation7,Citation86 Even when clinical strains were used, the majority of them were isolated from infected patients and cultured in vitro to form biofilms. This does not perfectly reflect the biofilm formation process in the in vivo environment.Citation87 Currently, there is no clinical guideline and therapeutic agent for biofilm infections. In vivo and clinical trials must be improved and practiced. Finally, rapid and accurate diagnostic tools for H. pylori biofilms should be developed for effective treatment and prevention of long-term chronic infection. In addition, conventional microbial culture, molecular biology, and other tests, should carried out in the early stages of biofilm infection, for accurate diagnosis and subsequent anti-biofilm treatment.
Conclusion and Perspectives
The emergence of drug resistance in H. pylori has become a unique clinical challenge. The formation of H. pylori biofilms is considered an important factor contributing to antibiotic resistance in humans. Thus, anti-biofilm agents should be developed because they have strong antagonistic effect against bacterial biofilms. This will decrease drug resistance hence increase the eradication rate of H. pylori and providing us with a new approach to address the antibiotic resistance problem. Unconfirmed speculation suggests that anti-biofilm agents will most likely become the new treatment approach in addressing the failure of H. pylori eradication therapy and multi-drug resistance in the near future.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273–1275.
- Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi:10.1111/apt.14561
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288
- Rather MA, Gupta K, Mandal M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol. 2021;52(4):1701–1718. doi:10.1007/s42770-021-00624-x
- Yonezawa H, Osaki T, Hojo F, Kamiya S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb Pathog. 2019;132:100–108. doi:10.1016/j.micpath.2019.04.030
- Chen L, Wen YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3(2):66–73. doi:10.4248/IJOS11022
- Yonezawa H, Osaki T, Kamiya S. Biofilm formation by helicobacter pylori and its involvement for antibiotic resistance. Biomed Res Int. 2015;2015:914791. doi:10.1155/2015/914791
- Eick S. Biofilms. Monogr Oral Sci. 2021;29:1–11. doi:10.1159/000510184
- Flemming HC, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol. 2019;17(4):247–260. doi:10.1038/s41579-019-0158-9
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi:10.1038/nrmicro821
- Attaran B, Falsafi T, Moghaddam AN. Study of biofilm formation in C57Bl/6J mice by clinical isolates of Helicobacter pylori. Saudi J Gastroenterol. 2016;22(2):161–168. doi:10.4103/1319-3767.178529
- Jia J, Zhang C, Liu Y, et al. Armeniaspirol A: a novel anti-Helicobacter pylori agent. Microb Biotechnol. 2021;15(2):442–454. doi:10.1111/1751-7915.13807
- Yonezawa H, Osaki T, Kurata S, Zaman C, Hanawa T, Kamiya S. Assessment of in vitro biofilm formation by Helicobacter pylori. J Gastroenterol Hepatol. 2010;25 Suppl 1:S90–S94. doi:10.1111/j.1440-1746.2009.06213.x
- Chandki R, Banthia P, Banthia R. Biofilms: a microbial home. J Indian Soc Periodontol. 2011;15(2):111–114. doi:10.4103/0972-124X.84377
- Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8(7):471–480. doi:10.1038/nrmicro2381
- Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176(2):269–275. doi:10.1128/jb.176.2.269-275.1994
- Hall MR, Mcgillicuddy E, Kaplan LJ. Biofilm: basic principles, pathophysiology, and implications for clinicians. Surg Infect (Larchmt). 2014;15(1):1–7. doi:10.1089/sur.2012.129
- Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Curr Opin Microbiol. 2014;18:96–104. doi:10.1016/j.mib.2014.02.008
- Vinod KK, Lall C, Vimal RR, Vedhagiri K, Sunish IP, Vijayachari P. Can subminimal inhibitory concentrations of antibiotics induce the formation of biofilm in leptospira? Microb Drug Resist. 2018;24(7):1040–1042. doi:10.1089/mdr.2017.0409
- Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43(1):197–222. doi:10.1146/annurev-genet-102108-134304
- Muhammad MH, Idris AL, Fan X, et al. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol. 2020;11:928. doi:10.3389/fmicb.2020.00928
- Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31(2):224–245. doi:10.1016/j.biotechadv.2012.10.004
- Zhou L, Yu Y, Chen X, et al. The multiple DSF-family QS signals are synthesized from carbohydrate and branched-chain amino acids via the FAS elongation cycle. Sci Rep. 2015;5(1):13294. doi:10.1038/srep13294
- Cole SP, Harwood J, Lee R, She R, Guiney DG. Characterization of monospecies biofilm formation by Helicobacter pylori. J Bacteriol. 2004;186(10):3124–3132. doi:10.1128/JB.186.10.3124-3132.2004
- Wong EH, Ng CG, Chua EG, et al. Comparative genomics revealed multiple helicobacter pylori genes associated with biofilm formation in vitro. PLoS One. 2016;11(11):e166835. doi:10.1371/journal.pone.0166835
- Yonezawa H, Osaki T, Fukutomi T, et al. Diversification of the AlpB outer membrane protein of helicobacter pylori affects biofilm formation and cellular adhesion. J Bacteriol. 2017;199(6). doi:10.1128/JB.00729-16
- Servetas SL, Doster RS, Kim A, et al. ArsRS-dependent regulation of homB contributes to Helicobacter pylori biofilm formation. Front Microbiol. 2018;9:1497. doi:10.3389/fmicb.2018.01497
- Ge X, Cai Y, Chen Z, et al. Bifunctional enzyme SpoT is involved in biofilm formation of helicobacter pylori with multidrug resistance by upregulating efflux pump hp1174 (gluP). Antimicrob Agents Chemother. 2018;62(11). doi:10.1128/AAC.00957-18
- Zhao Y, Cai Y, Chen Z, et al. SpoT-mediated NapA upregulation promotes oxidative stress-induced Helicobacter pylori biofilm formation and confers multidrug resistance. Antimicrob Agents Chemother. 2021;65(5). doi:10.1128/AAC.00152-21
- Cai Y, Wang C, Chen Z, et al. Transporters HP0939, HP0497, and HP0471 participate in intrinsic multidrug resistance and biofilm formation in Helicobacter pylori by enhancing drug efflux. Helicobacter. 2020;25(4):e12715. doi:10.1111/hel.12715
- Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. doi:10.1128/jcm.22.6.996-1006.1985
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179. doi:10.1016/S0167-7012(00)00122-6
- Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis. 2011;15(4):305–311. doi:10.1016/S1413-8670(11)70197-0
- Sanchez MC, Llama-Palacios A, Marin MJ, et al. Validation of ATP bioluminescence as a tool to assess antimicrobial effects of mouthrinses in an in vitro subgingival-biofilm model. Med Oral Patol Oral Cir Bucal. 2013;18(1):e86–e92. doi:10.4317/medoral.18376
- Dhale RP, Ghorpade MV, Dharmadhikari CA. Comparison of various methods used to detect biofilm production of Candida species. J Clin Diagn Res. 2014;8(11):C18–C20. doi:10.7860/JCDR/2014/10445.5147
- Gomes F, Teixeira P, Cerca N, Azeredo J, Oliveira R. Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Curr Microbiol. 2011;63(4):354–359. doi:10.1007/s00284-011-9984-3
- Zufferey J, Rime B, Francioli P, Bille J. Simple method for rapid diagnosis of catheter-associated infection by direct acridine Orange staining of catheter tips. J Clin Microbiol. 1988;26(2):175–177. doi:10.1128/jcm.26.2.175-177.1988
- Penesyan A, Paulsen IT, Gillings MR, Kjelleberg S, Manefield MJ. Secondary effects of antibiotics on microbial biofilms. Front Microbiol. 2020;11:2109. doi:10.3389/fmicb.2020.02109
- Tseng BS, Zhang W, Harrison JJ, et al. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol. 2013;15(10):2865–2878. doi:10.1111/1462-2920.12155
- Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. Fems Microbiol Lett. 2004;230(1):13–18. doi:10.1016/S0378-1097(03)00856-5
- Krzyzek P, Gosciniak G. A proposed role for diffusible signal factors in the biofilm formation and morphological transformation of Helicobacter pylori. Turk J Gastroenterol. 2018;29(1):7–13. doi:10.5152/tjg.2017.17349
- El ML, Tejada-Arranz A, Rifflet A, et al. A peptide of a type I toxin-antitoxin system induces Helicobacter pylori morphological transformation from spiral shape to coccoids. Proc Natl Acad Sci U S A. 2020;117(49):31398–31409. doi:10.1073/pnas.2016195117
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. doi:10.1038/nrmicro1557
- Zhang Y. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect. 2014;3(1):e3. doi:10.1038/emi.2014.3
- Yonezawa H, Osaki T, Hanawa T, Kurata S, Ochiai K, Kamiya S. Impact of Helicobacter pylori biofilm formation on clarithromycin susceptibility and generation of resistance mutations. PLoS One. 2013;8(9):e73301. doi:10.1371/journal.pone.0073301
- Bae J, Oh E, Jeon B. Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob Agents Chemother. 2014;58(12):7573–7575. doi:10.1128/AAC.04066-14
- Madsen JS, Burmolle M, Hansen LH, Sorensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. 2012;65(2):183–195. doi:10.1111/j.1574-695X.2012.00960.x
- Merod RT, Wuertz S, Spormann AM. Extracellular polymeric substance architecture influences natural genetic transformation of Acinetobacter baylyi in biofilms. Appl Environ Microbiol. 2014;80(24):7752–7757. doi:10.1128/AEM.01984-14
- Hathroubi S, Zerebinski J, Clarke A, Ottemann KM. Helicobacter pylori biofilm confers antibiotic tolerance in part via a protein-dependent mechanism. Antibiotics. 2020;9(6). doi:10.3390/antibiotics9060355
- Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41(3):341–352. doi:10.3109/1040841X.2013.841639
- Ma JF, Hager PW, Howell ML, Phibbs PV, Hassett DJ. Cloning and characterization of the Pseudomonas aeruginosa zwf gene encoding glucose-6-phosphate dehydrogenase, an enzyme important in resistance to methyl viologen (paraquat). J Bacteriol. 1998;180(7):1741–1749. doi:10.1128/JB.180.7.1741-1749.1998
- Clardy J, Fischbach MA, Currie CR. The natural history of antibiotics. Curr Biol. 2009;19(11):R437–R441. doi:10.1016/j.cub.2009.04.001
- Melander RJ, Basak AK, Melander C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat Prod Rep. 2020;37(11):1454–1477. doi:10.1039/d0np00022a
- Carradori S, Di Giacomo N, Lobefalo M, Luisi G, Campestre C, Sisto F. Biofilm and quorum sensing inhibitors: the road so far. Expert Opin Ther Pat. 2020;30(12):917–930. doi:10.1080/13543776.2020.1830059
- Maccelli A, Carradori S, Puca V, et al. Correlation between the antimicrobial activity and metabolic profiles of cell free supernatants and membrane vesicles produced by lactobacillus reuteri DSM 17938. Microorganisms. 2020;8(11):1653. doi:10.3390/microorganisms8111653
- Di Lodovico S, Napoli E, Di Campli E, et al. Pistacia vera L. Oleoresin and levofloxacin is a synergistic combination against resistant Helicobacter pylori strains. Sci Rep. 2019;9(1):4646. doi:10.1038/s41598-019-40991-y
- Luo P, Huang Y, Hang X, et al. Dihydrotanshinone I is effective against drug-resistant Helicobacter pylori in vitro and in vivo. Antimicrob Agents Chemother. 2021;65(3). doi:10.1128/AAC.01921-20
- Bai CL, Osaki T, Yonezawa H, et al. In vitro and in vivo effects of the Mongolian drug Amu-ru 7 on Helicobacter pylori growth and viability. Microbiol Immunol. 2010;54(9):508–515. doi:10.1111/j.1348-0421.2010.00246.x
- Sposito L, Oda FB, Vieira JH, et al. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J Ethnopharmacol. 2019;233:1–12. doi:10.1016/j.jep.2018.12.032
- Cataldi V, Di Bartolomeo S, Di Campli E, Nostro A, Cellini L, Di Giulio M. In vitro activity of Aloe vera inner gel against microorganisms grown in planktonic and sessile phases. Int J Immunopathol Pharmacol. 2015;28(4):595–602. doi:10.1177/0394632015600594
- Graham DY. Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol. 2009;7(2):145–148. doi:10.1016/j.cgh.2008.10.024
- Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20(2):280–322. doi:10.1128/CMR.00033-06
- Vagarali MA, Metgud SC, Bannur H, Karadesai SG, Nagmoti JM. Clinical significance of various diagnostic techniques and emerging antimicrobial resistance pattern of Helicobacter pylori from gastric biopsy samples. Indian J Med Microbiol. 2015;33(4):560–564. doi:10.4103/0255-0857.167349
- Jin F, Yang H. Effects of Lactobacillus salivarius LN12 in Combination with amoxicillin and clarithromycin on Helicobacter pylori biofilm in vitro. Microorganisms. 2021;9(8):1611. doi:10.3390/microorganisms9081611
- Krzyzek P, Migdal P, Paluch E, Karwanska M, Wieliczko A, Gosciniak G. Myricetin as an antivirulence compound interfering with a morphological transformation into coccoid forms and potentiating activity of antibiotics against Helicobacter pylori. Int J Mol Sci. 2021;22(5):2695. doi:10.3390/ijms22052695
- Arif M, Sharaf M, Samreen KS, Chi Z, Liu CG. Chitosan-based nanoparticles as delivery-carrier for promising antimicrobial glycolipid biosurfactant to improve the eradication rate of Helicobacter pylori biofilm. J Biomater Sci Polym Ed. 2021;32(6):813–832. doi:10.1080/09205063.2020.1870323
- Cai J, Huang H, Song W, et al. Preparation and evaluation of lipid polymer nanoparticles for eradicating H. pylori biofilm and impairing antibacterial resistance in vitro. Int J Pharm. 2015;495(2):728–737. doi:10.1016/j.ijpharm.2015.09.055
- Puca V, Traini T, Guarnieri S, et al. The antibiofilm effect of a medical device containing TIAB on microorganisms associated with surgical site infection. Molecules. 2019;24(12):2280. doi:10.3390/molecules24122280
- Grande R, Sisto F, Puca V, et al. Antimicrobial and antibiofilm activities of new synthesized silver Ultra-NanoClusters (SUNCs) against helicobacter pylori. Front Microbiol. 2020;11:1705. doi:10.3389/fmicb.2020.01705
- Shen Y, Zou Y, Chen X, et al. Antibacterial self-assembled nanodrugs composed of berberine derivatives and rhamnolipids against Helicobacter pylori. J Control Release. 2020;328:575–586. doi:10.1016/j.jconrel.2020.09.025
- Cammarota G, Branca G, Ardito F, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8(9):817–820. doi:10.1016/j.cgh.2010.05.006
- Zuin R, Palamidese A, Negrin R, Catozzo L, Scarda A, Balbinot M. High-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary disease. Clin Drug Investig. 2005;25(6):401–408. doi:10.2165/00044011-200525060-00005
- Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. Coli biofilms. Int J Antimicrob Agents. 2003;22 Suppl 2:95–100. doi:10.1016/s0924-8579(03)00232-2
- Olofsson AC, Hermansson M, Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl Environ Microbiol. 2003;69(8):4814–4822. doi:10.1128/AEM.69.8.4814-4822.2003
- Perez-Giraldo C, Rodriguez-Benito A, Moran FJ, Hurtado C, Blanco MT, Gomez-Garcia AC. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J Antimicrob Chemother. 1997;39(5):643–646. doi:10.1093/jac/39.5.643
- Di Campli E, Di Bartolomeo S, Grande R, Di Giulio M, Cellini L. Effects of extremely low-frequency electromagnetic fields on Helicobacter pylori biofilm. Curr Microbiol. 2010;60(6):412–418. doi:10.1007/s00284-009-9558-9
- Darmani H, Am SE, Mb BS. Blue light emitting diodes cripple Helicobacter pylori by targeting its virulence factors. Minerva Gastroenterol Dietol. 2019;65(3):187–192. doi:10.23736/S1121-421X.19.02593-5
- Darmani H, Smadi E, Bataineh S. Blue light emitting diodes enhance the antivirulence effects of Curcumin against Helicobacter pylori. J Med Microbiol. 2020;69(4):617–624. doi:10.1099/jmm.0.001168
- Lucio-Sauceda DG, Urrutia-Baca VH, Gomez-Flores R, De La Garza-ramos MA, Tamez-Guerra P, Orozco-Flores A. Antimicrobial and anti-biofilm effect of an electrolyzed superoxidized solution at neutral-pH against Helicobacter pylori. Biomed Res Int. 2019;2019:6154867. doi:10.1155/2019/6154867
- Stewart PS, Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin Microbiol Infect. 2020;26(8):1034–1038. doi:10.1016/j.cmi.2020.02.027
- Thi M, Wibowo D, Rehm B. Pseudomonas aeruginosa Biofilms. Int J Mol Sci. 2020;21(22):8671. doi:10.3390/ijms21228671
- Sharma G, Sharma S, Sharma P, et al. Escherichia coli biofilm: development and therapeutic strategies. J Appl Microbiol. 2016;121(2):309–319. doi:10.1111/jam.13078
- Gedefie A, Demsis W, Ashagrie M, et al. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect Drug Resist. 2021;14:3711–3719. doi:10.2147/IDR.S332051
- Otto M, Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI. Staphylococcal biofilms. Microbiol Spectr. 2018;6(4). doi:10.1128/microbiolspec.GPP3-0023-2018
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17(2):255–267. doi:10.1128/CMR.17.2.255-267.2004
- Grande R, Di Marcantonio MC, Robuffo I, et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front Microbiol. 2015;6:1369. doi:10.3389/fmicb.2015.01369
- Fauzia KA, Miftahussurur M, Syam AF, et al. Biofilm formation and antibiotic resistance phenotype of helicobacter pylori clinical isolates. Toxins. 2020;12(8):473. doi:10.3390/toxins12080473
- Krzyzek P, Junka A, Slupski W, et al. Antibiofilm and antimicrobial-enhancing activity of Chelidonium majus and Corydalis cheilanthifolia extracts against multidrug-resistant Helicobacter pylori. Pathogens. 2021;10(8):1033. doi:10.3390/pathogens10081033
- Yu M, Wang X, Ling F, Wang H, Zhang P, Shao S. Atractylodes lancea volatile oils attenuated helicobacter pylori NCTC11637 growth and biofilm. Microb Pathog. 2019;135:103641. doi:10.1016/j.micpath.2019.103641
- Vetvicka V, Vetvickova J, Fernandez-Botran R. Effects of curcumin on Helicobacter pylori infection. Ann Transl Med. 2016;4(24):479. doi:10.21037/atm.2016.12.52
- Krzyzek P, Gosciniak G, Fijalkowski K, et al. Potential of bacterial cellulose chemisorbed with anti-metabolites, 3-bromopyruvate or sertraline, to fight against Helicobacter pylori lawn biofilm. Int J Mol Sci. 2020;21(24):9507. doi:10.3390/ijms21249507
- Zhang L, Wu WK, Gallo RL, et al. Critical role of antimicrobial peptide cathelicidin for controlling helicobacter pylori survival and infection. J Immunol. 2016;196(4):1799–1809. doi:10.4049/jimmunol.1500021
- Bugli F, Palmieri V, Torelli R, et al. In vitro effect of clarithromycin and alginate lyase against helicobacter pylori biofilm. Biotechnol Prog. 2016;32(6):1584–1591. doi:10.1002/btpr.2339
- Tran TH, Truong THH, Nguyen TTL, Nguyen VMH, Thi NM, Luong TM. Growth-inhibiting, bactericidal, antibiofilm, and urease inhibitory activities of Hibiscus rosa sinensis L. flower constituents toward antibiotic sensitive- and resistant-strains of Helicobacter pylori. ACS Omega. 2020;5(32):20080–20089. doi:10.1021/acsomega.0c01640
- Chen X, Li P, Shen Y, Zou Y, Yuan G, Hu H. Rhamnolipid-involved antibiotics combinations improve the eradication of Helicobacter pylori biofilm in vitro: a comparison with conventional triple therapy. Microb Pathog. 2019;131:112–119. doi:10.1016/j.micpath.2019.04.001
- Shen Y, Li P, Chen X, et al. Activity of sodium lauryl sulfate, rhamnolipids, and N-Acetylcysteine against biofilms of five common pathogens. Microb Drug Resist. 2020;26(3):290–299. doi:10.1089/mdr.2018.0385
- Di Fermo P, Di Lodovico S, Amoroso R, et al. Searching for new tools to counteract the helicobacter pylori resistance: the positive action of resveratrol derivatives. Antibiotics. 2020;9(12). doi:10.3390/antibiotics9120891
- Ji J, Yang H. In vitro effects of lactobacillus plantarum ln66 and antibiotics used alone or in combination on Helicobacter pylori mature biofilm. Microorganisms. 2021;9(2):424. doi:10.3390/microorganisms9020424
- Wylie MR, Windham IH, Blum FC, Wu H, Merrell DS. In vitro antibacterial activity of nimbolide against Helicobacter pylori. J Ethnopharmacol. 2022;285:114828. doi:10.1016/j.jep.2021.114828
- Grande R, Carradori S, Puca V, et al. Selective inhibition of helicobacter pylori carbonic anhydrases by carvacrol and thymol could impair biofilm production and the release of outer membrane vesicles. Int J Mol Sci. 2021;22(21):11583. doi:10.3390/ijms222111583
- Spiegel M, Krzyzek P, Dworniczek E, Adamski R, Sroka Z. In silico screening and in vitro assessment of natural products with anti-virulence activity against Helicobacter pylori. Molecules. 2021;27(1):20. doi:10.3390/molecules27010020
