Abstract
Objective
The prevalence and clinical impact on mortality of carbapenem-resistant Pseudomonas aeruginosa (CRPA) infection are unclear in elderly patients. Here, we aimed to clarify the prevalence, the clinical manifestations, antimicrobial resistance, risk factors and outcomes of elderly inpatients with CRPA infection.
Methods
A retrospective study of 600 elderly inpatients infected with P. aeruginosa was conducted at Yueyang Hospital of Integrated Traditional Chinese and Western Medicine from January 1st 2018 to December 31st 2020. All 155 patients with CRPA infection were designated as a case group. Patients with carbapenem-susceptible Pseudomonas aeruginosa (CSPA) were randomly selected from remaining 445 cases in a 1:1 ratio to case group as a control group.
Results
Of 600 P. aeruginosa isolates, the overall rates of CRPA, MDR PA (multidrug-resistance Pseudomonas aeruginosa) were 25.8% (155), 22.3% (134), respectively. The rankings of the top five resistant rates of CRPA to tested antimicrobial drugs were imipenem (87.7%), meropenem (70.3%), ciprofloxacin (51.0%), levofloxacin (48.4%), cefoperazone (43.2%). Independent risk factors for patients with CRPA infection were cerebrovascular disease (OR = 3.517, P < 0.001), foley catheter (OR = 2.073, P = 0.018), length of hospital stay ≥ 14 days (OR = 1.980, P = 0.013), albumin < 35 g/L (OR = 2.049, P = 0.020), previous antibiotic exposure to carbapenems (OR = 7.022, P = 0.004), previous antibiotic exposure to third- or fourth-generation cephalosporins (OR = 12.649, P = 0.002). Of 155 patients with CRPA infection, the mortality rate was 16.8% (26/155). Independent risk factors for mortality were receiving mechanical ventilation (OR = 3.671, P = 0.007) and neutrophil percentage ≥ 80% (OR = 2.908, P = 0.024).
Conclusion
The study revealed high rates of CRPA, MDR PA among the hospitalized elderly patient with P. aeruginosa infection. The analysis of antimicrobial susceptibility emphasizes the necessity for antimicrobial stewardship and infection control in hospitals. These findings of risk factors are practical significant to identify patients at high risk for CRPA infection and mortality that may benefit from alternate empiric treatment.
Background
Pseudomonas aeruginosa (P. aeruginosa) is an important nosocomial pathogen that causes a wide spectrum of infections, such as pneumonia, urinary tract infections, surgical site infections, and bloodstream infections.Citation1 In 2020, surveillance of nosocomial infections in China showed that P. aeruginosa was the fourth most frequently isolated Gram-negative bacilli, accounting for 8.4% of hospital-acquired infections in China Antimicrobial Surveillance Network (CHINET).Citation2 Infections caused by P. aeruginosa isolates are typically difficult to treat and eradicate due to intrinsic antibiotic resistance and a remarkable ability to acquire resistance to multiple groups of antimicrobial agents.Citation3
Recent years have witnessed an increasing prevalence of multidrug-resistant (MDR) and extensively-resistant (XDR) P. aeruginosa strains, with rates of between 15% and 30% in some geographical areas.Citation4–6 Carbapenem antibiotics reserved as agents of last resort for P. aeruginosa strains that are resistant to first-line broad-spectrum antibiotics were introduced to treat serious MDR P. aeruginosa (MDRPA) infections but eventually led to a soaring of carbapenem-resistant isolates around the global.Citation7 In 2017, the World Health Organization placed CRPA as a critical priority pathogen that desperately requires new treatment options.Citation8 The successful worldwide spread of the so-called “high-risk” clones of P. aeruginosa poses a threat to global public health that needs to be studied and managed with urgency and determination.
Patients with infections due to CRPA pathogens usually lead to worse clinical outcomes and higher mortality ranging between 20.0% and 30.8%.Citation9 It is reported that this opportunistic pathogen has emerged as one of the leading causes of nosocomial infections among the immunocompromised and elderly patients undergoing invasive procedures or receiving mechanical ventilation, where the risk of infection with P. aeruginosa is elevated. The growing number of elderly patients usually produces an even greater number of immunocompromised individuals at risk of these infections. Hu Y also found CRPA was more prevalent among inpatients (aged ≥ 60 years).Citation10 However, previous studies mainly focused on particular populations, such as patients with hematologic malignancies,Citation11 kidney transplant recipients,Citation12 bacteremia.Citation13 So far, the prevalence, risk factors and clinical impact on mortality of CRPA infection are unclear in elderly patients. Here, we aimed to clarify the prevalence, the clinical manifestations, antimicrobial resistance, risk factors and outcomes of elderly inpatients with CRPA infection. It is anticipated that an improving understanding of antimicrobial resistance in P. aeruginosa may help ensure appropriate empirical therapy whilst minimizing the potential development of resistance and specific interventions may be designed according to the identified risk factors to curb the spread of CRPA in China.
Materials and Methods
Patients and Data Collection
A retrospective cohort study was conducted at Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, a tertiary-care teaching hospital with around 1200-bed, located in the east region of China. All hospitalized patients diagnosed with P. aeruginosa nosocomial infection, aged ≥ 65 years, from January 1st 2018 and December 31st 2020 were eligible for inclusion in the study. During the study period, all P. aeruginosa infection patients were consecutively recruited and non-duplicated, only the first episode was reviewed and recorded. Patients infected with CRPA were designated as case group. For each CRPA case, one control (1:1 basis) that was positive for carbapenem-susceptible P. aeruginosa (CSPA) infection was randomly selected from patients admitted during the study period, which was set to control group for risk factor analysis of CRPA infection.
The definition of P. aeruginosa nosocomial infection was referred to diagnostic criteria for nosocomial infection released by the Ministry of Health, People’s Republic of China in 2001.Citation14 Clear Criteria used for patient inclusion/exclusion with details was provided as Supplementary Table 1, in briefly: (1) P. aeruginosa isolate was cultured from qualified clinical specimens, (2) presence symptoms or signs of relevant organs’ or tissues’ infection, (3) elevated leukocyte count, neutrophil percentage, C-reactive protein and/or procalcitonin level, abnormal imaging examination, (4) the patient’s condition significantly improved after receiving treatment with sensitive antibiotics for 3–5 days.
Definitions
Previous antibiotic exposure was defined as any exposure to an antibiotic for at least 48 hours within the preceding 30 days. Co-carriage was defined as detection of both P. aeruginosa and one of the following bacterial pathogens in the same sample, including Klebsiella pneumoniae, Acinetobacter baumannii, carbapenem resistant Enterobacteriaceae, Staphylococcus aureus, and Escherichia coli. The survivor group was defined as patients who were discharged from the hospital alive and the non-survivor group who died during hospitalization.
Based on the antimicrobial susceptibility testing (AST) results, P. aeruginosa was classified as follows: CRPA was defined as an isolate with a MIC ≥ 4 μg/mL for imipenem, or disk zone diameter ≤ 19 mm for meropenem in accordance with the breakpoints of 2020 Clinical and Laboratory Standards Institute (CLSI) guidelines.Citation15 Additionally, MDR PA was defined as non-susceptibility to at least one agent in three or more antimicrobial categories, XDR PA as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (ie, bacterial isolates remain susceptible to only one or two categories), and PDR PA as non-susceptibility to all antimicrobial agents tested.Citation16
Clinical Data Collection
The following information was retrieved from medical records and included in the database: Date of admission, basic demographics (age and gender), comorbidities (cerebrovascular disease, hypertension, diabetes mellitus, coronary artery disease, cardiac dysfunction, renal dysfunction, biliary tract disease, malignancy), invasive operation (nasogastric tube, foley catheter, mechanical ventilation, central venous catheter), co-carriage with other bacterium (frequently isolated species including Klebsiella pneumoniae, Acinetobacter baumannii, Staphylococcus aureus, Escherichia coli), prior surgery (within 90 days), length of hospital stay (LOS), admitted to ICU, duration of P. aeruginosa infection occurrence (days from admission to positive culture), previous antibiotic exposure (within 30 days), patient’s clinical outcomes (survivor or no-survivor), and laboratory examination findings (the blood routine, C-reactive protein (CRP), and albumin (Alb)).
Microbiological Methods and Laboratory Tests
Bacterial species identification and antimicrobial susceptibility analysis were performed using an automated Vitek-2 system (bioMerieux, France), species identification was further confirmed with MALDI-TOF MS system (Autof, China), the antimicrobial susceptibility testing results were adopted in interpretation with the 2020 CLSI M100-S30 guidelines.Citation15 Isolates were categorized as susceptible, intermediate, or resistant. The tested antimicrobial agents were as follows: aminoglycosides (amikacin, gentamicin, tobramycin), antipseudomonal fluoroquinolones (ciprofloxacin, levofloxacin), antipseudomonal penicillins +β-lactamase inhibitors (piperacillin-tazobactam), monobactam (aztreonam), antipseudomonal cephalosporins (ceftazidime, cefepime), cefoperazone, cefoperazone-sulbactam, antipseudomonal carbapenems (imipenem, meropenem). The susceptibility of meropenem was determined by a Kirby-Bauer disk diffusion method. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were tested as quality control isolates in each antimicrobial susceptibility test. The hemoglobin (Hb), CRP, leukocytes count (WBC), neutrophil percentage, and ALB were determined as well.
Statistical Analysis
Continuous variables are described as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables are described as frequencies and percentages. The Kolmogorov–Smirnov test was conducted to evaluate the normality of variable distribution. Continuous variables were compared using an independent-samples t-test or the Mann–Whitney U-test. The χ2 test or Fisher’s exact tests were conducted to evaluate the difference between categorical variables. All reported P values were two tailed, and P values <0.05 were evaluated as statistically significant. Factors that revealed statistical significance in univariate analysis were selected into a forward stepwise logistic regression analysis, which was used to analyze the risk factors for geriatric patients with CRPA infection and mortality. All statistical analyses were performed using SPSS 26.0 for Windows software (SPSS Inc.).
Results
Descriptive Epidemiology
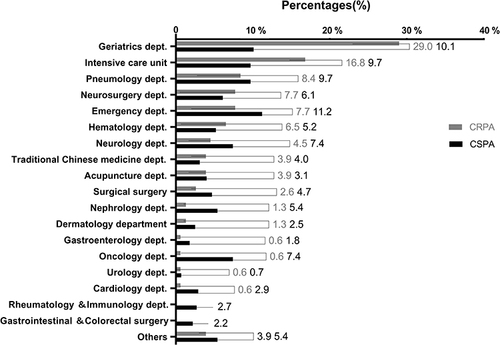
A total of 600 nonduplicated patients with P. aeruginosa infection were included during the 3-year study period. The median age was 71 years, ranging from 65 to 99 years, 311 (51.8%) of them were male. Among culture-positive specimens, lower respiratory tract accounted for 80.8% (n=485), followed by pus 9.5% (n=54), urine 5.83% (n=35), blood 2.67% (n=16), bile 1.33% (n=8), and excretion 0.33% (n=2), respectively. If we stratified the patients based on departments of admission, the highest percentage (90, 15.0%) of P. aeruginosa was obtained from the geriatrics department, followed by intensive care unit (ICU) (69, 11.5%), emergency department (62, 10.3%), department distribution of P. aeruginosa isolates was shown in and detail data in Supplementary Table 2.
Antibiotic Resistance Profiling of P. aeruginosa
showed the resistance profiles of 14 different antimicrobial agents used for treatment of cases diagnosed with P. aeruginosa. The total resistance rates of P. aeruginosa in descending order were: ciprofloxacin (27.7%), levofloxacin (24.8%), imipenem (22.7%), cefoperazone (19.5%), aztreonam (18.7%), meropenem (18.2%), gentamicin (14.5%), piperacillin (14.3%), ceftazidime (13.3%), tobramycin (12.2%), cefepime (9.5%), cefoperazone-sulbactam (8.2%), piperacillin-tazobactam (7.3%), and amikacin (6.2%).
Table 1 Susceptibility of P. aeruginosa to Antimicrobial Agents During the Period from 2018 Through 2020
The overall rates of CRPA, MDR PA, and XDR PA were 25.8% (155), 22.3% (134), and 6.5% (39), respectively. As shown in , the resistant rates of CRPA isolates to the tested antimicrobial drugs were relatively higher compared to CSPA isolates, the rankings of the top five resistant rates were imipenem (87.7%), meropenem (70.3%), ciprofloxacin (51.0%), levofloxacin (48.4%), cefoperazone (43.2%).
Risk Factors for Elderly Patients Infected with CRPA
All 155 patients with CRPA infection were set to case group. The control group, patients with CSPA infection, was randomly selected from the remaining 445 CSPA infection patients as the ratio 1:1. Finally, the medical records of the 310 index patients were reviewed. Patients’ characteristics and the result of univariate logistic analysis for CRPA and CSPA were illustrated in . There was no significant difference in sex distribution between the both groups of patients, whereas age showed a statistically significant difference (P<0.05), the median ages were 83.0 (74.0–89.0) years for the CRPA group and 80.0 (71.0–86.0) years for the CSPA group, respectively.
Table 2 Univariate and Multivariate Analysis of Risk Factors for Inpatients with CRPA Infection
Univariate analysis of patients between CRPA group and CSPA group () revealed age (75–84 years old, ≥ 85 years old), comorbidities (cerebrovascular disease, hypertension, diabetes mellitus, coronary artery disease), admitted to ICU, invasive operation (nasogastric tube, foley catheter, mechanical ventilation), length of hospital stay (7–13 days, ≥ 14 days), laboratory findings (CRP > 30 mg/L, mild anemia (90–120 g/L), ALB < 35g/L), co-carriage (Klebsiella pneumoniae), previous antibiotic exposure within 30 days (combination of antibiotics, carbapenems, third- or fourth-generation cephalosporins) were risk factors for patients with CRPA infection (all P<0.05).
In a multivariate conditional logistic regression analysis, significant risk factors related to CRPA infection included age 75–84 years (odds ratio [OR]=0.458, 95% confidence interval [CI]: 0.263–0.800, P=0.006), cerebrovascular disease (OR=3.517, 95% CI: 2.054–6.021, P<0.001), foley catheter (OR=2.073, 95% CI: 1.135–3.784, P=0.018), length of hospital stay ≥ 14 days (OR=1.980, 95% CI: 1.154–3.399, P=0.013), Albumin < 35 g/L (OR=2.049, 95% CI: 1.121–3.746, P=0.020), previous antibiotic exposure to carbapenems (OR=7.022, 95% CI:1.861–26.493, P=0.004), previous antibiotic exposure to third- or fourth-generation cephalosporins (OR=12.649; 95% CI: 2.473–64.690, P=0.002), .
Clinical Outcomes and Risk Factors for Mortality Among Patients with CRPA Infection
Of the 155 patients with CRPA infections, 26 (16.8%) patients were no-survived. By univariate analysis (), patients with renal dysfunction (P=0.012), foley catheter (P=0.007), mechanical ventilation (P=0.002), LOS 10–19 days (P=0.005), LOS ≥ 20 days (P=0.012), CRP ≥ 10 mg/L (P=0.007), WBC > 9.5×109 /L (P=0.003), neutrophil percentage ≥ 80% (P<0.001) were associated with a higher mortality. Multivariate logistic regression analysis showed that patients with receiving mechanical ventilation (OR=3.671, 95% CI: 1.424–9.467, P=0.007) and neutrophil percentage > 80% (OR=2.908, 95% CI: 1.151–7.343, P=0.024) remained independent risk factors which associated with worse clinical outcomes ().
Table 3 Risk Factors for Mortality in Inpatients with CRPA Infection
Discussion
The emerging resistance of P. aeruginosa poses a global threat, as carbapenems antibiotics are widely used in clinics, the growing carbapenem resistance among P. aeruginosa has been reported in many large-scale surveillance data,Citation2,Citation17 but its prevalence varies across different geographic regions, specimen source, patient age, patient setting, and selective pressure from broad spectrum antibiotics.Citation6,Citation18 In the present study, we investigated the antimicrobial resistance profiles of P. aeruginosa among elderly patients in a tertiary hospital for 3 years. We found the rates of CRPA and MDR PA (25.8% and 22.3%, respectively) among overall P. aeruginosa isolates were higher than in Australia (6.5% and 7.4%, respectively), but lower than in India (29.3% and 28.2%, respectively) from the antimicrobial testing leadership and surveillance (ATLAS) program from 2015 to 2019 report.Citation6 Among the carbapenems, resistance rates to imipenem (22.7%) and meropenem (18.2%) were close to a report from CHINET in 2020 with national rates of 23.2% for imipenem and 19.3% for meropenem, but much lower than in a general hospital in Henan, reported with 56.7% for imipenem and 47.3% for meropenem.Citation19 These varied results implied the prevalence of CRPA was significantly different between geographic and patient population. Our study results reckoned the overall resistances to antipseudomonal carbapenems and fluoroquinolones of P. aeruginosa were relative higher compared to aminoglycosides (amikacin, tobramycin, gentamicin), cephalosporins (cefepime, ceftazidime, piperacillin-tazobactam), which still demonstrated excellent susceptibility rates being 92.3%, 84.7%, 80.7%, 82.3%, 79.8%, and 79.3%, respectively. CRPA showed more resistant to all tested antimicrobial agents compared to CSPA, but the susceptible rates of CRPA were still high toward amikacin, cefepime, gentamicin, and tobramycin, which were 80.0%, 67.1%, 66.5%, and 65.2%, respectively. The data was important for empiric antimicrobial therapy in clinical practice.
The study identified several independent predictors of elderly inpatients of infection with CRPA, including prior antimicrobial use, cerebrovascular disease, receiving foley catheter, albumin < 35 g/L and LOS ≥ 14 days. An important predisposing factor for CRPA infection having the history of using carbapenems and third- or fourth-generation cephalosporins within 30 days increased the risk about seven and thirteen times higher, which concurs with other previous studies.Citation13,Citation20–Citation21 Resistance acquisition driven by antipseudomonal agents exposure can be reached by either selecting mutants in patients previously colonized or infected by susceptible phenotypes or promoting selection of an already resistant strain.Citation22–Citation23 Long-term and high frequency usage of such antibiotics causes P. aeruginosa to undergo gene mutation or produce new drug resistance genes for survival under the pressure of antibiotics, resulting in changes in the drug resistance characteristics of bacteria.Citation24–Citation25 Carbapenems had the shortest duration of prior antibiotic exposure for carbapenem-resistant P. aeruginosa (3.5 days),Citation26 whereas piperacillin-tazobactam for piperacillin-tazobactam-resistant P. aeruginosa (3 days).Citation27 Therefore, physicians may predict resistance development in accordance with previous carbapenems and third- or fourth-generation cephalosporins exposure and duration. More attention must be paid to these kinds of antibiotic agents’ stewardship in order to delay the emergence of resistance.
Furthermore, cerebrovascular disease was significantly linked to CRPA infection.Citation13 The elderly with cerebrovascular disease are often subjected to repeated silent aspiration pneumonia, bacteria colonized in the oral cavity and oropharynx easily enter the lung during sleep causing an infection. A previous study revealed high prevalence (38.5%) and high average bacterial number (2.00×108 CFU/mL) of P. aeruginosa were found in oropharyngeal microflora with cerebrovascular disease and dysphagia.Citation28 This was consistent with our results 77.4% of enrolled patients with CRPA infection suffered from pulmonary infection and 80.0% of CRPA isolates were cultured from lower respiratory tract. Moreover, invasive operations such as tracheal intubation, tracheotomy and central venous catheterization are generally required in the rescue of patients with cerebrovascular diseases, which makes the incidence of nosocomial infection 5–10 times higher than that in other general patients.Citation29
Another one attributed to CRPA infection was indwelling devices in the patient’s body, similar to previous studies by Leihof RFCitation30 and Kang JS,Citation13 patients undergone foley catheter had approximately twice as high rate of CRPA infection occurrence as those without foley catheter. Indwelling catheter is easy to destroy patients with urethral or bladder mucosa, the mucosal barrier, during invasive operation and weaken its defensive effect, increasing the chance of bacterial invasion and resulting in increased chance of infection with P. aeruginosa.Citation13,Citation31 Therefore, it is vital to emphasize the importance of avoiding or limiting the use of this type of invasive device and improving the aseptic conditions during these procedures, especially in elderly patients.
Consistent with the previous study by Koichi Kitagawa,Citation32 we found patients with lower albumin levels (< 35g/L) have a strong tendency to become CRPA infection compared to patients with normal albumin level. Hypoalbuminemia indicates protein-energy malnutrition and impaired immunological response.Citation33 Elderly patients usually have more critical underlying diseases and low immune function, once they suffered infection it is difficult to treat and control, leading to further prolonged treatment and recovery time.Citation34 Tsao LH reported the risk of CRPA infection increases 1% with each hospitalization day,Citation35 which was in line with another independent risk factor LOS > 14 days.Citation36–Citation37 With the extension of hospital stay, they were more likely to infect P. aeruginosa, including endogenous infection by colonization in nasopharynx, respiratory tract, skin and intestinal tract, and exogenous infection by the prolonged survival of P. aeruginosa in hospital surroundings.Citation36
To explore the possible influence of CRPA pathogen on the outcome of elderly patients, patients’ characteristics were systematically evaluated. The overall mortality rate among patients with CRPA infection was 16.8% (26/155). Contrary to other studies’ results that higher mortality rates of P. aeruginosa infection were related to patients’ comorbidities, the site of primary infection, disease severity, multidrug resistance, and inappropriateness of empirical therapy,Citation20,Citation38 in this study, we found mechanical ventilation (OR=3.671, P=0.007) and neutrophil percentage ≥80% (OR=2.908, P=0.024) were the strongest risk factors associated with all-cause in hospital mortality. Mechanical ventilation is an important tool for critically ill patients, those patients who frequently require invasive medical devices are more susceptible to subsequent, recurrent infections, and to death due to infectious complications.Citation39 Neutrophils are essential phagocytes in the defense system against P. aeruginosa infection in the lower respiratory tract, and alveolar macrophages play an important role in neutrophil accumulation by release of neutrophil chemotactic factor after the P. aeruginosa phagocytosis.Citation40 A previous study reported by Kamoshida,Citation41 found Acinetobacter baumannii exploits human neutrophils by adhering to and inducing interleukin-8 release for bacterial portage. We speculated that neutrophils initially play an important role against P. aeruginosa infection, which also leads to the dissemination of P. aeruginosa in the body, leading the patient to worse clinical outcome.
Our study has several limitations. Firstly, this study focused on elderly patients was a single-center study conducted at a tertiary university hospital in the eastern region of China, and the number of CRPA samples was relatively small. Then, our results might not be extrapolated to other hospitals and regions of the country. Secondly, in our study, the detection of P. aeruginosa still mainly comes from sputum samples accounting for 80.8%, which is more difficult to identify whether the pathogen is a colonized or an infected strain and easily leads to overestimation of the detection rate of CRPA. Additionally, our study could not identify specific mechanisms associated with resistance given the retrospective study design.
Conclusion
Rates of CRPA, MDR PA were high in elderly patients. Cerebrovascular disease, foley catheter, length of hospital stay ≥ 14 days, Albumin < 35 g/L, previous antibiotic exposure to carbapenems, previous antibiotic exposure to third- or fourth-generation cephalosporins were significant risk predictors for acquisition of CRPA infections. Mechanical ventilation and neutrophils percentage ≥ 80% were two factors independently associated with mortality of CRPA infection. The analysis of antimicrobial susceptibility emphasizes the need for antimicrobial stewardship and infection control in hospitals. These findings of risk factors for patients with CRPA infection and mortality are useful in identifying patients at high risk for CRPA infection that may benefit from alternate empiric treatment.
Data Sharing Statement
All data included in this study are availability upon request by contact with the corresponding author.
Ethics Approval
This study was reviewed and approved by the research ethics committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (approval number (2021)060). This study is a retrospective non-interventional study, which does not interfere with routine diagnosis and treatment, does not affect any medical rights of patients, and does not increase the risk of patients. Consent was waived because most of the patients could not be found and the research project did not involve personal privacy or commercial interests. In order to fully protect personal privacy, the names of the included patients were coded, and the medical records were stored in the special computer of the Department of Clinical Laboratory, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, for researchers’ access only. We declare that this study is in accordance with the Helsinki Declaration and the information of all patients included in the study was confidential.
Consent to Participate
Informed consent was not needed due to the retrospective nature of the study.
Author Contributions
Jie Qin contributed to study conception, data acquisition, data analysis, and manuscript drafting. Haiying Wang contributed to study conception, study design, manuscript drafting, and critical manuscript revision. All authors contributed to data collection, data analysis, critical revising the paper, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this article.
Additional information
Funding
References
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi:10.1128/cmr.00040-09
- HuF, Guo Y, Zhu D, et al. CHINET surveillance of bacterial resistance: results of 2020. Chin J Infect Chemother. 2021;21(4):377–387. doi:10.16718/j.1009-7708.2021.04.001. Chinese.
- Strateva T, Yordanov D.Pseudomonas aeruginosa - a phenomenon of bacterial resistance. J Med Microbiol. 2009;58(9):1133–1148. doi:10.1099/jmm.0.009142-0
- Sader HS, Castanheira M, Duncan LR, et al. Antimicrobial susceptibility of Enterobacteriaceae and Pseudomonas aeruginosa isolates from United States Medical Centers stratified by infection type: results from the International Network for Optimal Resistance Monitoring (INFORM) surveillance program, 2015–2016. Diagn Microbiol Infect Dis. 2018;92(1):69–74. doi:10.1016/j.diagmicrobio.2018.04.012
- Walkty A, Lagace-Wiens P, Adam H, et al. Antimicrobial susceptibility of 2906 Pseudomonas aeruginosa clinical isolates obtained from patients in Canadian hospitals over a period of 8 years: results of the Canadian Ward Surveillance Study (CANWARD), 2008–2015. Diagn Microbiol Infect Dis. 2017;87(1):60–63. doi:10.1016/j.diagmicrobio.2016.10.003
- Lee YL, Ko WC, Hsueh PR. Geographic patterns of carbapenem-resistant Pseudomonas aeruginosa in the Asia-Pacific Region: results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) program, 2015–2019. Antimicrob Agents CH. 2021;AAC0200021. doi:10.1177/1747493018778713
- Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:1–13. doi:10.3389/fmicb.2011.00065
- Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/s1473-3099(17)30753-3
- Lodise TP, Bassetti M, Ferrer R, et al. All-cause mortality rates in adults with carbapenem-resistant Gram-negative bacterial infections: a comprehensive review of pathogen-focused, prospective, randomized, interventional clinical studies. Expert Rev Anti Infect Ther. 2021;12:1–13. doi:10.1080/14787210.2022.2020099
- Hu YY, Cao JM, Yang Q, et al. Risk factors for carbapenem-resistant Pseudomonas aeruginosa, Zhejiang Province, China. Emerg Infect Dis. 2019;25(10):1861–1867. doi:10.3201/eid2510.181699
- Chen SZ, Xu JJ, Xiao TT, et al. Clinical characteristics and prognostic risk factors analysis of carbapenem-resistant organism in the department of hematology. Chin J Hematol. 2021;42(7):563–569. doi:10.3760/cma.j.issn.0253-2727.2021.07.006. Chinese.
- Freire MP, Camargo CH, Yamada AY, et al. Critical points and potential pitfalls of outbreak of IMP-1-producing carbapenem-resistant Pseudomonas aeruginosa among kidney transplant recipients: a case-control study. J Hosp Infect. 2021;115:83–92. doi:10.1016/j.jhin.2021.05.006
- Kang JS, Moon C, SJ Mun, et al. Antimicrobial susceptibility trends and risk factors for antimicrobial resistance in Pseudomonas aeruginosa bacteremia: 12-year experience in a tertiary hospital in Korea. J Korean Med Sci. 2021;36(43):e273. doi:10.3346/jkms.2021.36.e273
- Ministry of Health of the People’s Republic of China. Diagnostic criteria for nosocomial infection (for trial implementation). Natl Med J China. 2001;5:61–67.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne: Clinical and Laboratory Standards Institute; 2020.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–271. doi:10.1111/j.1469-0691.2011.03570.x
- Jean SS, Lee YL, Liu PY, et al. Multicenter surveillance of antimicrobial susceptibilities and resistance mechanisms among Enterobacterales species and non-fermenting Gram-negative bacteria from different infection sources in Taiwan from 2016 to 2018. J Microbiol Immunol Infect. 2021;3:1684–1782. doi:10.1016/j.jmii.2021.07.015
- Morrow BJ, Pillar CM, Deane J, et al. Activities of carbapenem and comparator agents against contemporary US Pseudomonas aeruginosa isolates from the CAPITAL surveillance program. Diagn Microbiol Infect Dis. 2013;75(4):412–416. doi:10.1016/j.diagmicrobio.2012.12.012
- Zhang Y, Li Y, Zeng J, et al. Risk Factors for Mortality of Inpatients with Pseudomonas aeruginosa Bacteremia in China: Impact ofResistance Profile in the Mortality. Infect Drug Resist. 2020;12(13):4115–4123. doi:10.2147/IDR.S268744
- Lin KY, Lauderdale TL, Wang JT, et al. Carbapenem-resistant Pseudomonas aeruginosa in Taiwan: prevalence, risk factors, and impact on outcome of infections. J Microbiol Immunol Infect. 2016;49(1):52–59. doi:10.1016/j.jmii.2014.01.005
- Onguru P, Erbay A, Bodur H, et al. Imipenem-resistant Pseudomonas aeruginosa: risk factors for nosocomial infections. J Korean Med Sci. 2008;23(6):982–987. doi:10.3346/jkms.2008.23.6.982
- Riou M, Carbonnelle S, Avrain L, et al. In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. Int J Antimicrob Agents. 2010;36:513–522. doi:10.1016/j.ijantimicag.2010.08.005
- Lipsitch M, Bergstrom CT, Levin BR. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc Natl Acad Sci. 2000;97(4):1938–1943. doi:10.1073/pnas.97.4.1938
- da Silva NCZ, da Rocha JA, Do Valle FM, et al. The impact of ageing on the incidence and mortality rate of bloodstream infection: a hospital-based case-cohort study in a tertiary public hospital of Brazil. Trop Med Int Health. 2021;26(10):1276–1284. doi:10.1111/tmi.13650
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi:10.1016/j.ccc.2013.03.016
- Zou Y, Lian J, Di Y, et al. The quick loss of carbapenem susceptibility in Pseudomonas aeruginosa at intensive care units. Int J Clin Pharm. 2018;40(1):175–182. doi:10.1007/s11096-017-0524-5
- Hyle EP, Gasink LB, Linkin DR, et al. Use of different thresholds of prior antimicrobial use in defining exposure: impact on the association between antimicrobial use and antimicrobial resistance. J Infect. 2007;55(5):414–418. doi:10.1016/j.jinf.2007.07.005
- Hirota K, Yoneyama T, Sakamoto M, et al. High prevalence of Pseudomonas aeruginosa from oropharyngeal biofilm in patients with cerebrovascular infarction and dysphagia. Chest. 2010;138(1):237–238. doi:10.1378/chest.10-0240
- Okeng’o K, Chillo P, Gray WK, et al. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J Stroke Cerebrovasc Dis. 2016;26(4):871–878. doi:10.1016/j.jstrokecerebrovasdis.2016.10.037
- Leihof RF, Ethelberg S, Nielsen KL, et al. Nosocomial urinary tract infection and risk of bacteraemia in elderly patients: urinary catheter, clinical factors and bacterial species. Infect Dis. 2019;51(7):547–549. doi:10.1080/23744235.2019.1599135
- Tomczyk S, Zanichelli V, Grayson ML, et al. Control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: a Systematic Review and Reanalysis of Quasi-experimental Studies. Clin Infect Dis. 2019;68(5):873–884. doi:10.1093/cid/ciy752
- Kitagawa K, Shigemura K, Yamamichi F, et al. Bacteremia complicating urinary tract infection by Pseudomonas aeruginosa: Mortality risk factors. Int J Urol. 2019;26(3):358–362. doi:10.1111/iju.13872
- Horasan ES, Dağ A, Ersoz G, et al. Surgical site infections and mortality in elderly patients. Med Mal Infect. 2013;43(10):417–422. doi:10.1016/j.medmal.2013.07.009
- Folic MM, Djordjevic Z, Folic N, et al. Epidemiology and risk factors for healthcare-associated infections caused by Pseudomonas aeruginosa. J Chemother. 2020;33(5):294–301. doi:10.1080/1120009x.2020.1823679
- Tsao LH, Hsin CY, Liu HY, et al. Risk factors for healthcare-associated infection caused by carbapenem-resistant. Pseudomonas aeruginosa. J Microbiol Immunol Infect. 2018;51(3):359–366. doi:10.1016/j.jmii.2017.08.015
- Jia H, Li L, Li W, et al. Impact of healthcare-associated infections on length of stay: a Study in 68 Hospitals in China. Biomed Res Int. 2019;2019:2590563. doi:10.1155/2019/2590563
- Bourgi J, Said JM, Yaakoub C, et al. Bacterial infection profile and predictors among patients admitted to a burn care center: a retrospective study. Burns. 2020;46(8):1968–1976. doi:10.1016/j.burns.2020.05.004
- Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. doi:10.1128/aac.49.2.760-766.2005
- Tam VH, Gamez EA, Weston JS, et al. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin Infect Dis. 2008;46(6):862–867. doi:10.1086/528712
- Maeda M, Ozaki T, Yasuoka S, et al. [Role of alveolar macrophages and neutrophils in the defense system against infection of Pseudomonas aeruginosa in the respiratory tract and the effect of derivative of muramyl dipeptide]. Nihon Kyobu Shikkan Gakkai Zasshi. 1990;28(1):135–142. Japanese.
- Kamoshida G, Tansho-Nagakawa S, Kikuchi-Ueda T, et al. A novel bacterialtransport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J Leukoc Biol. 2016;100(6):1405–1412. doi:10.1189/jlb.4AB0116-023RR