Abstract
Background
Bloodstream infection (BSI) is a serious medical issue causing non-relapsed mortality in patients receiving hematopoietic stem cell transplantations (HSCT).
Methods
The characteristics of all patients receiving HSCT (autologous and allogeneic HSCT) in our hospital from 2013 to 2019 were studied. Ratios, medians, and ranges were calculated to describe categorical variables. Chi-square tests were performed to compare the difference between ratios.
Results
A total of 741 patients receiving 746 HSCT procedures—including 376 allogeneic, 370 autologous, and four of both types—were included in the study. The overall incidence of BSI in post-transplantation patients was 8.8% (N = 65). Gram-negative bacteria were the most common strains each year (33.3–81.3%), and E. coli was the most frequently isolated (33.3%). Enterobacterales represented 64.9% of multidrug-resistant (MDR) bacteria, and the ratio of MDR rebounded from 25% to 100% within a year. A total of 27 patients died from BSI after HSCT, and the seven-day and 30-day death tolls were 12 and 18, respectively. MDR caused 63% of deaths among patients with BSI and the mortality rate caused by tigecycline-resistance was as high as 100%.
Conclusion
Our results reveal the changing epidemiology of BSI and antibiotic resistance in patients receiving HSCT in Southwest China, as well as showing that MDR and tigecycline-resistant microorganisms should be given more attention. Thus, long-term routine microorganism epidemiological and resistance monitoring in patients undergoing HSCT should be a vital practice in future.
Introduction
Infection is one of the most common complications causing transplantation failure and non-relapse mortality (NRM) in hematological malignancy patients after hematopoietic stem cell transplants (HSCT).Citation1 Bloodstream infection (BSI) is a high risk and is related to incidence and NRM rates, with BSI rates ranging from 13% to 55.8%, and NRM ranging from 24% to 43.6%.Citation2–8 Therefore, an in-depth understanding of the characteristics of BSI in patients receiving HSCT is necessary. Since the 1960s, the major pathogen type implicated in BSI has shifted from gram-negative bacteria (GNB) to gram-positive bacteria (GPB) and back again.Citation6,Citation9–11 This trend has continued into the 21st century, to the extent, that GNB has again become the main pathogen of BSI.Citation4,Citation12,Citation13
Furthermore, multidrug-resistant (MDR) bacteria and extensively drug-resistant (XDR) bacteria have become an increasing global concern,Citation14 representing significant challenges to the medical profession. The problem is more prominent in patients undergoing HSCT due to the extended use of antibiotic prophylaxis and broad-spectrum antibiotics.Citation4,Citation15 According to available—but incomplete statistics—the quinolone resistance rate has increased to 86% in some countries, while the incidence of carbapenem resistance increases to 25% from the initial zero-resistance state in post-transplantation patients on average every year.Citation4,Citation6,Citation16,Citation17 The overall resistance rate of tigecycline—one of the last possible treatment options of antibiotics—has not been reported in China or abroad to date. Understanding the resistance rate is important when choosing antibiotics, as trends in resistance profiles change annually and have a significant bearing on treatment outcomes.Citation12
To date, the characteristics of post-HSCT BSI have been retrospectively investigated in the United States and various European countries at different times, but data about BSI after HSCT in China is scarce, especially in Southwest China. Therefore, our study was designed to describe the frequency of BSI and antimicrobial susceptibility in patients receiving HSCT in our hospital from 2013 to 2019, with the aim of clarifying the epidemiology of BSI and the rates of resistance to antibiotics demonstrated after HSCT in Southwest China.
Materials and Methods
Patient Population
The study was designed to analyze BSI in patients during HSCT retrospectively at West China Hospital from 2013 to 2019. All patients older than 14 who underwent HSCT were included. In our study, each year was analyzed as a separate period, and BSI data for different years were compared. Certain characteristics of all patients were noted, including demographics, underlying disease, transplant type, BSI episodes, infection sites, antimicrobial susceptibility, and survival. Incidence was calculated according to the positive rate of blood culture episodes per the annual number of transplants, excluding positive results related to the catheter and occurring before HSCT. As the study was performed with retrospective resources but did not involve active human participants or tissue, the Institutional Review Board of West China Hospital waived the need to obtain ethics approval and patient consent. This study complies with the Declaration of Helsinki. All methods were implemented following the relevant guidelines and regulations.
Definitions
HSCT-associated BSI was defined as isolates of bacteria or fungi from blood culture occurring during transplantation. This included commensal flora—such as coagulase-negative staphylococci (CoNS) and some fungi on the skin, which are harmful to patients with severe neutropenia—and excluded catheter-related sample contamination. BSI-associated mortality was defined as death occurring after a positive blood culture during the same hospitalization period. If two or more pathogens were found in a single blood culture, it was defined as polymicrobial. An unknown or endogenous infection source was recorded when no other positive examination results or physical signs for infection presented except blood culture. All patients received prophylactic antibiotic treatment before transplantation. Blood culture was conducted when body temperature surpassed 38 degrees or shivering occurred, and empirical antibiotic treatment protocols were developed immediately according to the international febrile neutropenia guidelines.Citation18,Citation19 Furthermore, antibiotics were adjusted according to the results of antimicrobial susceptibility testing if the infection symptoms were not alleviated; otherwise, the antibiotics were used continuously. MDR, XDR, and pan- drug-resistant (PDR) bacteria were classified based on the standardized international terminology created by the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention.Citation20 The incidence of drug resistance was calculated by resistance strains/all isolates. Outcomes for patients were not statistically analyzed in our study; we simply assessed the mortality at seven days and 30 days after BSI was recorded.
Statistical Analysis
Ratios, medians, and ranges were calculated to describe categorical variables. Chi-square or Fisher’s exact tests were performed to compare the difference between ratios. All p values in our study were two-sided, and p < 0.05 was considered significant. All analyses were conducted with SPSS (version 24.0, SPSS, Inc, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) software.
Results
Characteristics of Patients
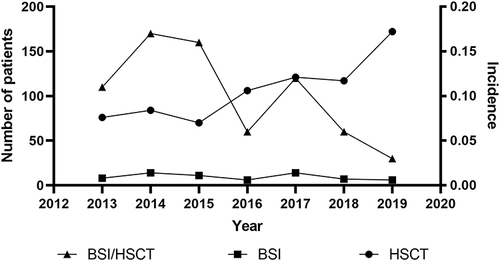
From January 2013 to December 2019, a total of 741 patients received 746 HSCT procedures, including 376 allogeneic (50.4%), 370 autologous (49.6%), and four of both types (0.5%), with an increasing number reported every year. The median patient age was 40 (range: 14–66), and 426 of the patients were male (57.1%). Acute myeloid leukemia (AML) and non-Hodgkin’s lymphoma (NHL) were the most common underlying diseases in allogeneic HSCT (allo-HSCT) and autologous HSCT (auto-HSCT), respectively (). Over the seven-year study period, 75 BSI episodes in 65 patients (8.8%) were recorded with isolated pathogenic microorganisms in blood culture. Out of the 65 patients, 52 were in allo-HSCT (80.0%), 12 in auto-HSCT (18.5%), and one was both (1.5%). The median age was 38 (range:14–58) and 34 were male (52.3%). The most frequent baseline diseases remained AML in allo-HSCT and NHL in auto-HSCT. The period in which BSI occurred after HSCT ranged from 0 to 125 days (median: 13 days). showed the number of transplantations performed and BSIs recorded every year. The overall trend in the incidence of BSI shows a decrease, despite increasing HSCT. The main site of infection was pulmonary. Other information concerning patients with BSI is shown in detail in .
Table 1 Characteristics of Patients After HSCT
Microbiology and Resistance to Antibiotics
Information on microorganisms from 75 episodes and their progression are shown in . GPB, including CoNS, was isolated in 18 episodes (24.0%), GNB in 49 (65.3%) episodes, and fungi in 8 (10.7%) episodes. No difference could be observed in the incidence of any kind of microorganisms across the years analyzed (p = 0.669). GNB was the main pathogen causing BSI per year, and in this category, E. coli was the most frequently isolated, followed by P. aeruginosa. Among GPB infections, Enterococcus spp. was the most frequently detected, followed by staphylococcus spp. The eight fungi isolates included Candida tropicalis (N = 3), Candida krusei (N = 2), Trichosporon japonicum (N = 1), Fusarium (N = 1), and an untyped strain (N = 1).
Table 2 Changing Prevalence of BSI in Patients After HSCT
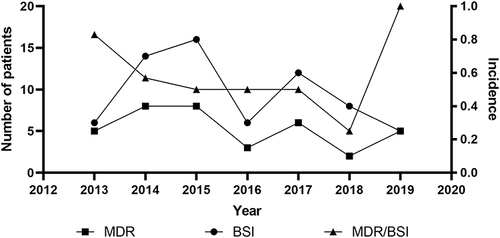
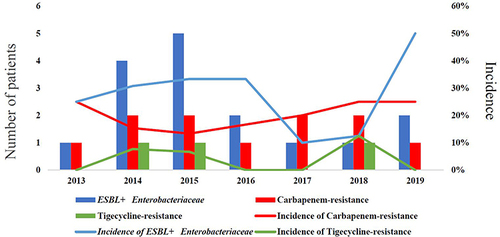
Of the 75 episodes, 67—including 61 bacteria and 6 fungi—were tested for antimicrobial susceptibility (another eight patients died before blood culture results became available). A total of 37 episodes were MDR (55.2%) and 11 were XDR (16.4%); no PDR cases were recorded. The ratios of MDR for each year are shown in . Notably, the incidence of MDR tended to rebound despite decreasing BSI, especially in 2019, where all BSI causes were identified as MDR or XDR bacteria. Enterobacterales and Enterococcus were reported as the highest frequency infections for MDR or XDR cases among GNB and GPB, respectively. The resistant isolates are shown in . Additionally, the incidence of extended-spectrum β-Lactamase (ESBL)-producing Enterobacterales, carbapenem resistance, and tigecycline resistance was also significant, with the trends for these three types of resistance illustrated in . The incidence of carbapenem resistance and tigecycline resistance per year, excluding natural resistance, ranged from 13.3% to 25.0% and from 0% to 12.5%, respectively, which included E. coli (N = 4), K. pneumoniae (N = 3), P. aeruginosa (N = 2), E. Faecium (N = 2), and A. baumannii (N = 1) for carbapenem resistance, and E. coli (N = 2) and K. pneumoniae (N = 1) for tigecycline resistance ().
Table 3 The Overall Distribution of MDR or XDR Microorganism
Table 4 Carbapenem- or Tigecycline-Resistance in Different Strains
Outcomes for Post-Transplantation Patients with BSI
Despite a lack of detailed survival analysis to date, only 57 patients with BSI after HSCT were followed. The median time between BSI and death was nine days (range: 1–55 days). Of the 57 patients, 27 died from BSI, including 24 allogeneic and three autologous HSCT patients, 17 of whom were MDR or XDR. All tigecycline-resistant patients died, while 8 of 11 carbapenem-resistant patients died. Twelve patients died within seven days of BSI detection, and 18 patients died within 30 days of BSI detection (including the twelve patients died within seven days). MDR or XDR (N = 9) was the main cause of seven-day mortality. Three patients died of fungal BSI within 30 days. Seventeen patients with MDR or XDR remained alive. The resistance to antibiotics and outcomes is shown in .
Table 5 Seven- and Thirty-Day Mortality of Different Antibiotics-Resistance
Discussion
In our study, 8.8% (N = 65) of patients undergoing HSCT had isolated bacteria or fungi in blood cultures from 2013 to 2019. GNB was the common pathogen in BSI every year, and E. coli was most frequently isolated in post-transplantation patients. A total of 10.7% (N = 8) of patients with BSI had isolated fungus in blood samples, while 55.2% (N = 37) of patients with antimicrobial susceptibility tests were identified as MDR or XDR, with a decreasing trend over the study period. A total of 27 patients died of BSI, 12 of whom (including nine patients with MDR or XDR) died within seven days of BSI detection. A total of three patients with fungal BSI died within 30 days.
Since 2000, isolates of GPB have declined rapidly due to fewer catheter-related bacteria, and GNB has increased parallel to the rising rate of quinolone resistance,Citation21,Citation22 to the extent that GNB has gradually replaced GPB as the main isolate in blood culture.Citation23 The incidence of BSI decreased between 2013 and 2019 and GNB was the most common infection type in our study; these trends were consistent with the data reported in other countries and regions.Citation4,Citation24 E. coli and Enterococcus, both normal commensal bacteria in the human intestine, were the most frequently found GNB and GPB types in our study, respectively (the top pathogen in BSI after HSCT or high-dose chemotherapy is almost always reported to be E. coli or Enterococcus).Citation12,Citation15,Citation24–26 Some researchers have reported that prolonged neutropenia and marked gastrointestinal mucositis caused by various types of conditioning regimens damage the phagocyte and the defensive barrier, which facilitate normal commensal bacteria entering the blood.Citation27,Citation28 Furthermore, it was significant that fungi were isolated relatively often in our study: A total of eight patients (12.3%) were diagnosed with fungal BSI while 33 (2.8%) and two (2.25%) patients with fungal BSI were confirmed in Spain and East China, respectively.Citation12,Citation24 The warm, humid climate in Southwest China is beneficial to the growth and reproduction of fungi, which could cause a higher incidence of fungal infection in post-transplantation patients. Thus, antifungal therapy for patients after transplantation is an important consideration in similar climates.
MDR has already become a major medical challenge worldwide and it is particularly prominent in patients undergoing HSCT.Citation14,Citation24 However, studies have shown that the rate of MDR is declining.Citation29,Citation30 The rate declined in our study from 2013 to 2018, but the incidence of MDR in 2019 was substantially elevated. Furthermore, despite fewer BSI diagnoses, all patients were MDR, a finding which indicates that MDR microorganism monitoring cannot be relaxed. Since carbapenem is often used in patients with neutropenia and tigecycline is the last treatment option for anti-infective therapy in clinics, resistance to these two drugs is of particular concern. We found that E. coli and K. pneumoniae were the most prevalent causes of bacteria resistance to carbapenem or tigecycline. Additionally, they are reported to be the most common isolates in patients with repeated BSI.Citation31–33 These two microorganisms evolve into MDR mainly owing to hydrolase caused by plasmid mutations during the long-term use of antibiotics.Citation34,Citation35 One or two episodes with carbapenem- or tigecycline-resistant BSI were recorded per year in our study, and the overall incidence of resistance to carbapenem and tigecycline were 18.3% and 5.0%, respectively. Published articles regarding carbapenem-resistance in transplant patients reported overall resistance rates of 15.8–25.0% in Central and East China and 17.0–44.0% in Western countries.Citation4,Citation15,Citation16,Citation24,Citation36 Therefore, our data is consistent with local and international findings. To date, no studies have reported data on the incidence of tigecycline resistance in patients undergoing HSCT. Despite a resistance rate of only 5.0% and very low absolute quantity, tigecycline resistance should be studied further due to the very high mortality rate (100%) and the reports of cases each year. Another noteworthy risk is the incidence of ESBL+ Enterobacterales, which has been demonstrated an obvious upward trend over the last years, reminding doctors that antibiotics with β-Lactamase enzyme inhibitors are preferred in the treatment of BSI.
As this is a retrospective study, outcome data were analyzed simply. In line with the 30–50% mortality rates shown for patients with BSI in other studies,Citation15,Citation37,Citation38 our study indicated a mortality rate of 47.4%. Since prolonged or severe neutropenia has been shown to be an independent risk factor for BSI mortality, we focused on the mortality rates at seven days and 30 days, which is when severe or prolonged neutropenia occurred in our study. The results showed rates of 17.9% and 26.9% at seven-day and 30-day periods, respectively, which was higher than the rates in other developed areas, both domestically and internationally.Citation4,Citation24 Advances in supportive care built on strong personal and collective economic circumstances have been shown to improve post-HSCT survival.Citation39 Hence, our short-term mortality rate was likely suboptimal, in part due to unfavorable personal economic status and national conditions. Additionally, it is acknowledged that BSI is associated with a poor prognosis for HSCTCitation37,Citation40,Citation41 and that MDR or XDR bacteria are of key importance in determining a prognosis for BSI,Citation15,Citation42 especially carbapenem- and tigecycline-resistant pathogens. Notably, two patients died from fungal BSI in our study. One of these patients died on the day of transplant, with the antimicrobial susceptibility test revealing that no antibiotics were effective except amphotericin B. This indicated that fungal BSI could be fatal to transplant recipients.Citation4,Citation42
Our study reported the BSI of patients after HSCT in Southwest China and reflected some regional characteristics. More importantly, this was the first reporting of data on tigecycline resistance in this area. However, our study had some limitations. First, the sample size was small, because all patients were from a single center where donor unavailability and economic difficulties were common for most patients. Second, this was a retrospective study. We reported observations based on historical data and were thus unable to perform a detailed analysis. Further, in-depth exploration of treatment factors, such as examination of the mechanisms of antibiotics resistance, could not be carried out. Third, the conditioning regimen, graft versus host disease (GVHD), comorbidities, and treatment for BSI were not included in the analysis. Therefore, it was difficult to perform a detailed survival analysis with any accuracy. Data from multiple hospitals could be included in future studies, including analysis of the factors outlined above.
Conclusion
To summarize, our study concluded that the epidemiology of BSI and antibiotic resistance in patients receiving HSCT were continually changing in Southwest China between 2013 and 2019. MDR-associated BSI was heavily associated with poor outcomes. Thus, long-term routine epidemiological and resistance monitoring for pathogenic microorganisms, especially MDR microorganisms, will be indispensable in future.
Abbreviations
BSI, bloodstream infection; NRM, non-relapsed mortality; HSCT, hematopoietic stem cell transplantations; MDR, multidrug-resistant; XDR, extensively drug-resistant; PDR, pan drug-resistant; GPB, gram-positive bacteria; GNB, gram-negative bacteria; ECDC, European Centre for Disease Prevention and Control; CDC, Centers for Disease Control and Prevention; AML, acute myeloid leukemia; NHL, non-Hodgkin’s lymphoma; ALL, acute lymphoblastic leukemia; AUL, acute undifferentiated leukemia; HL, Hodgkin lymphoma; CML, chronic myeloid leukemia; AA, aplastic anemia; PNH, paroxysmal nocturnal hemoglobinuria; MDS, myelodysplastic syndromes; MM, multiple myeloma; ESBL, extended-spectrum β-Lactamase; GVHD, graft versus host disease.
Ethics Approval and Consent to Participate
As the study was performed with retrospective resources and not involving human participants and/or tissue, the Institutional Review Board of West China Hospital waived the need to obtain ethics approval and patient consent. This study complies with the Declaration of Helsinki.
Data Sharing Statement
The data and materials analyzed in our study are available from the corresponding author on reasonable requests.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was financially supported by development projects of Science and Technology Department of Sichuan Province (2019YFS0104).
References
- Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22(8):505–514. doi:10.1016/j.jiac.2016.05.006
- Young JH, Logan BR, Wu J, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22(2):359–370. doi:10.1016/j.bbmt.2015.09.013
- Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi:10.1038/sj.bmt.1705690
- Mikulska M, Del Bono V, Raiola AM, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47–53. doi:10.1016/j.bbmt.2008.10.024
- Marena C, Zecca M, Carenini ML, et al. Incidence of, and risk factors for, nosocomial infections among hematopoietic stem cell transplantation recipients, with impact on procedure-related mortality. Infect Control Hosp Epidemiol. 2001;22(8):510–517. doi:10.1086/501942
- Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33(7):947–953. doi:10.1086/322604
- Mossad SB, Longworth DL, Goormastic M, Serkey JM, Keys TF, Bolwell BJ. Early infectious complications in autologous bone marrow transplantation: a review of 219 patients. Bone Marrow Transplant. 1996;18(2):265–271.
- Vydra J, Shanley RM, George I, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(6):764–770. doi:10.1093/cid/cis550
- Klastersky J. Science and pragmatism in the treatment and prevention of neutropenic infection. J Antimicrob Chemother. 1998;41(SupplD):13–24. doi:10.1093/jac/41.suppl_4.13
- Ortega M, Rovira M, Almela M, et al. Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann Hematol. 2005;84(1):40–46. doi:10.1007/s00277-004-0909-0
- Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36(9):1103–1110. doi:10.1086/374339
- Puerta-Alcalde P, Cardozo C, Marco F, et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant. 2020;55(3):603–612. doi:10.1038/s41409-019-0701-3
- Weisser M, Theilacker C, Tschudin Sutter S, et al. Secular trends of bloodstream infections during neutropenia in 15 181 haematopoietic stem cell transplants: 13-year results from a European multicentre surveillance study (ONKO-KISS). Clin Microbiol Infect. 2017;23(11):854–859. doi:10.1016/j.cmi.2017.03.020
- Oliveira AL, de Souza M, Carvalho-Dias VM, et al. Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;39(12):775–781. doi:10.1038/sj.bmt.1705677
- Macesic N, Morrissey CO, Cheng AC, Spencer A, Peleg AY. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transpl Infect Dis. 2014;16(6):887–896. doi:10.1111/tid.12298
- Cao W, Guan L, Li X, et al. Clinical analysis of bloodstream infections during agranulocytosis after allogeneic hematopoietic stem cell transplantation. Infect Drug Resist. 2021;14:185–192. doi:10.2147/IDR.S280869
- Bock AM, Cao Q, Ferrieri P, Young JA, Weisdorf DJ. Bacteremia in blood or marrow transplantation patients: clinical risk factors for infection and emerging antibiotic resistance. Biol Blood Marrow Transplant. 2013;19(1):102–108. doi:10.1016/j.bbmt.2012.08.016
- Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica. 2013;98(12):1826–1835. doi:10.3324/haematol.2013.091025
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi:10.1016/j.bbmt.2009.06.019
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
- Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M. Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis. 2014;16(1):106–114. doi:10.1111/tid.12175
- Mitchell AE, Derrington P, Turner P, Hunt LP, Oakhill A, Marks DI. Gram-negative bacteraemia (GNB) after 428 unrelated donor bone marrow transplants (UD-BMT): risk factors, prophylaxis, therapy and outcome. Bone Marrow Transplant. 2004;33(3):303–310. doi:10.1038/sj.bmt.1704338
- Liu CY, Lai YC, Huang LJ, et al. Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46(9):1231–1239. doi:10.1038/bmt.2010.286
- Wang L, Wang Y, Fan X, Tang W, Hu J. Prevalence of resistant gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: a single center retrospective cohort study. Medicine. 2015;94(45):e1931. doi:10.1097/MD.0000000000001931
- Ge J, Yang T, Zhang L, et al. The incidence, risk factors and outcomes of early bloodstream infection in patients with malignant hematologic disease after unrelated cord blood transplantation: a retrospective study. BMC Infect Dis. 2018;18(1):654. doi:10.1186/s12879-018-3575-x
- Cappellano P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A. Epidemiology and risk factors for bloodstream infections after allogeneic hematopoietic stem cell transplantion. New Microbiol. 2007;30(2):89–99.
- Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacterales, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19(6):e12762. doi:10.1111/tid.12762
- Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010;24(2):257–272. doi:10.1016/j.idc.2010.01.010
- Gjaerde LI, Moser C, Sengeløv H. Epidemiology of bloodstream infections after myeloablative and non-myeloablative allogeneic hematopoietic stem cell transplantation: a single-center cohort study. Transpl Infect Dis. 2017;19(5). doi:10.1111/tid.12730
- Girmenia C, Bertaina A, Piciocchi A, et al. Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian prospective multicenter survey. Clin Infect Dis. 2017;65(11):1884–1896. doi:10.1093/cid/cix690
- Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977–987. doi:10.1056/NEJMoa044097
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–93. doi:10.1093/cid/cir073
- Trecarichi EM, Pagano L, Candoni A, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21(4):337–343. doi:10.1016/j.cmi.2014.11.022
- Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacterales in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58(9):1274–1283. doi:10.1093/cid/ciu052
- Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34(5):634–640. doi:10.1086/338782
- Stoma I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51(2):102–106. doi:10.5045/br.2016.51.2.102
- Ustun C, Young JH, Papanicolaou GA, et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54(8):1254–1265. doi:10.1038/s41409-018-0401-4
- Ali N, Adil SN, Shaikh MU. Bloodstream and central line isolates from hematopoietic stem cell transplant recipients: data from a developing country. Transpl Infect Dis. 2014;16(1):98–105. doi:10.1111/tid.12176
- Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17(11):1688–1697. doi:10.1016/j.bbmt.2011.05.001
- Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40(3):271–278. doi:10.1007/s15010-011-0229-y
- Zając-Spychała O, Wachowiak J, Frączkiewicz J, et al. Multidrug-resistant bacterial infections in children undergoing haematopoietic stem cell transplantation over a 6-year period: analysis of the Polish Pediatric Group for hematopoietic stem cell transplantation. J Appl Microbiol. 2020;128(1):292–300. doi:10.1111/jam.14452
- Moreno A, Cervera C, Gavaldá J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant. 2007;7(11):2579–2586. doi:10.1111/j.1600-6143.2007.01964.x