Abstract
The management of infections caused by multidrug-resistant Gram-negative bacteria, particularly Pseudomonas aeruginosa, continues to be a significant challenge to clinicians. Ceftolozane/tazobactam is a novel antibacterial and β-lactamase-inhibitor combination that has shown appreciable activity against wild-type Enterobacteriaceae and potent activity against P. aeruginosa. Moreover, ceftolozane/tazobactam has not demonstrated cross-resistance to other antimicrobial classes, particularly those affected by extended-spectrum β-lactamases, AmpC β-lactamase, a loss in porin channels, or the overexpression of efflux pumps in P. aeruginosa. Ceftolozane/tazobactam has completed two Phase II clinical trials in complicated intra-abdominal and complicated urinary tract infections. A Phase III, multicenter, prospective, randomized, open-label study has been initiated to evaluate the safety and efficacy of ceftolozane/tazobactam versus piperacillin/tazobactam for the treatment of ventilator-associated pneumonia. A Medline search of articles from inception to May 2013 and references for selected citations was conducted. Data from abstracts presented at conferences were also appraised. This article reviews the antimicrobial, pharmacokinetic, and pharmacodynamic profile of ceftolozane/tazobactam, and discusses its potential role in therapy.
Introduction
In the past decade, significant attention has been given to research on multidrug-resistant Gram-positive organisms, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and associated treatment modalities.Citation1–Citation3 However, infections caused by resistant Gram-negative bacilli continue to cause significant morbidity and mortality without decline.Citation4–Citation6 A report from the Centers for Disease Control and Prevention estimated 1.7 million health care-associated infections in the US in 2002, with approximately 99,000 associated deaths.Citation7 Emergence and spread of drug-resistant Gram-negative bacteria are particularly concerning.Citation8–Citation11 Health care-associated infections caused by resistant Gram-negative bacteria can lead to additional attributable hospital cost and length of stay of 29.3% and 23.8%, respectively, as reported in a single-center study.Citation12
Pseudomonas aeruginosa is a leading nosocomial Gram-negative pathogen well known for its intrinsic as well as extraordinary ability to develop resistance to various antimicrobial agents. It can cause a wide array of infections, due to its multitude of cell-associated (eg, quorum sensing) and secreted virulence factors (eg, type III secretion system).Citation13–Citation17 Infections caused by P. aeruginosa remain a significant challenge to clinicians, given that therapeutic options are limited to a handful of agents in three major classes: antipseudomonal β-lactams, antipseudomonal fluoroquinolones, and aminoglycosides.Citation18 Further complicating the matter is the rapid emergence of antibiotic-resistant strains of P. aeruginosa. Data from the 2004 National Nosocomial Infections Surveillance System Report summary showed increasing resistance rates to imipenem, quinolones, and ceftazidime.Citation19 More specifically, the Centers for Disease Control and Prevention reported the prevalence of resistance to carbapenems, fluoroquinolones, and antipseudomonal extended-spectrum cephalosporins at 16.0%, 29.6%, and 23.3%, respectively, in a collection of strains that caused health care-associated infections.Citation20 Of greater concern is the spread of multidrug-resistant strains of P. aeruginosa due to combinations of resistance mechanisms, including enzymatic degradation, decreased outer-membrane permeability, and efflux pumps,Citation21,Citation22 as the observed proportion of strains with resistance to three classes of antimicrobial agents was 10%.Citation23 Infections caused by such strains are associated with severe outcomes, including increased mortality, increased length of hospital stay, and poorer functional capacity at discharge.Citation24,Citation25
Ceftolozane/tazobactam (previously referred to as CXA-201) is a novel antibacterial and β-lactamase-inhibitor combination with the potential to meet the challenges of infections caused by multidrug-resistant strains of P. aeruginosa and other resistant Gram-negative bacteria. Ceftolozane has demonstrated increased stability to AmpC β-lactamases,Citation26–Citation28 and is less affected by changes in porin permeability and efflux pumps due to enhanced binding of select penicillin-binding proteins (PBPs).Citation27 The addition of tazobactam in a 2:1 ratio broadens its spectrum of activity against β-lactamase-producing Enterobacteriaceae, including those producing extended-spectrum β-lactamases (ESBLs).Citation26,Citation29 The primary aim of this review is to provide an overview of this novel cephalosporin and β-lactamase-inhibitor combination, and evaluate its place in therapy.
Pharmacology
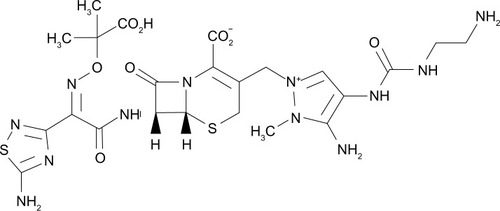
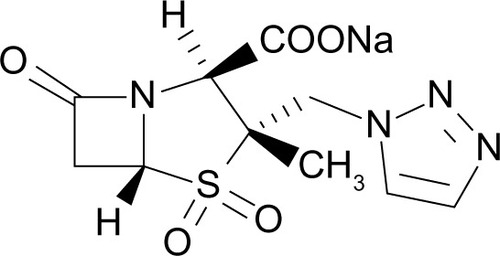
Chemical structure
Ceftolozane (previously referred to as FR264205 or CXA-101) is a novel oxyimino-aminothiazolyl cephalosporin that was developed via the introduction of amino groups to the 4-position of a 3-amino-2-methylpyrazole cephalosporin (). The addition of amino groups to the 4-position of a 3-amino-2-methylpyrazole cephalosporin improved the minimum inhibitory concentration (MIC) values against AmpC β-lactamases.Citation30 Additionally, a conformational restriction of the 4-position substituent on the pyrazolium ring of FR264205 decreased the potential for convulsion-inducing effects.Citation27,Citation30 The addition of tazobactam sodium, empiric formula C10H11N4NaO5S, broadens its activity to include select ESBL-producing organisms ().Citation27
Mechanism of action
Ceftolozane/tazobactam is an intravenous cephalosporin combined with a β-lactamase inhibitor in a fixed 2:1 ratio.Citation31,Citation32 Similar to other cephalosporins, ceftolozane inhibits cell-wall synthesis via binding of PBPs. More specifically, ceftolozane exhibits greater affinity for all essential PBPs (1b, 1c, 2, and 3), in comparison to ceftazidime and imipenem.Citation33 Tazobactam is a β-lactam sulfone that inhibits most class A β-lactamases and some class C β-lactamases.Citation34
In vitro activity
Ceftolozane has favorable intrinsic activity against wild-type Enterobacteriaceae, and potent activity against P. aeruginosa, with MICs at four- to 16-fold dilutions below the comparative MICs for ceftazidime. However, similar to other extended-spectrum cephalosporins, ceftolozane is susceptible to enzymatic degradation by ESBLs and carbapenemases.Citation35 The addition of the β-lactamase inhibitor tazobactam potentiates the activity of ceftolozane against select organisms producing degrading enzymes, particularly those exhibiting the ESBL phenotype.Citation26
P. aeruginosa
Data from the Program to Assess Ceftolozane/Tazobactam Susceptibility (PACTS) surveillance study have shown greater ceftolozane/tazobactam potency (two- to eightfold) for 973 clinical isolates of P. aeruginosa in comparison with ceftazidime and cefepime. At an MIC of ≤8 mg/L, ceftolozane/tazobactam inhibited 97.7% of the isolates. Ceftazidime and cefepime inhibited 80.9% and 80.7% of P. aeruginosa isolates using the Clinical and Laboratory Standards Institute (CLSI) breakpoint criteria of 8 mg/L.Citation29 It should also be noted that ceftolozane/tazobactam retained activity against ceftazidime nonsusceptible strains (88.2% had an MIC of ≤8 mg/L), meropenem nonsusceptible strains (89.6% had an MIC of ≤8 mg/L), and strains with concomitant ceftazidime and meropenem nonsusceptibility (78.8% at an MIC of ≤8 mg/L).Citation29 In a study examining the effects of various known resistance mechanisms on ceftolozane/tazobactam, the agent appears to be unaffected by upregulation of efflux pumps or loss of porin channels.Citation27P. aeruginosa strains overexpressing multidrug efflux (Mex)-CD–opioid receptor (Opr)-J and MexEF-OprN resulted in a 16-fold increase in MIC to ciprofloxacin. However, overexpression of these efflux pumps, as well as MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY, in P. aeruginosa did not appear to affect the MIC of ceftolozane in this study.Citation27 Similarly, a 16-fold increase in MIC was observed for imipenem due to membrane impermeability (loss of OprD), while ceftolozane retained its activity against such strains.Citation27 Stability of ceftolozane against porin OprD loss was also reported in other studies.Citation36,Citation37
The activity of ceftolozane/tazobactam against 100 isolates of P. aeruginosa (first and last available isolates) from 50 patients with cystic fibrosis was also evaluated.Citation38 The MIC50 and MIC90 were 0.5 and 2 mg/L, respectively, with 95% of the isolates inhibited at MIC ≤ 8 mg/L. Ceftolozane/tazobactam maintained activity against a high distribution of isolates resistant to select antipseudomonal agents tested (ceftazidime, cefepime, imipenem, meropenem, levofloxacin, tobramycin, and piperacillin/tazobactam). In addition, ceftolozane/tazobactam retained some activity for multidrug resistant strains with MIC50 and MIC90 of 2 and 16 mg/L, respectively. It also conserved activity against 96% of the last isolates from each patient with MIC ≤ 8 mg/L. Cross-resistance from other antipseudomonal agents was not observed.
Enterobacteriaceae
The activity of ceftolozane/tazobactam against selected Gram-negative bacilli is outlined in and .Citation29,Citation39 Ceftolozane/tazobactam was tested against 1,244 US clinical isolates of Escherichia coli in the PACTS surveillance study, with 99.3% of E. coli strains inhibited by ceftolozane/tazobactam at the proposed MIC breakpoint of ≤8 mg/L. Comparatively, ceftriaxone and ceftazidime inhibited 87.7% and 91.5% of the isolates using the CLSI breakpoint criteria, respectively.Citation29 Among the 840 strains of Klebsiella spp. tested, 92.1% of strains were inhibited at a ceftolozane/tazobactam MIC of ≤8 mg/L. Using the established CLSI breakpoint, 84.4%, 86.2%, and 90.0% of the strains were inhibited by ceftriaxone, ceftazidime, and cefepime, respectively.
Table 1 Comparative activity of ceftolozane/tazobactam against selected U.S. clinical Gram-negative bacilli
Table 2 Comparative activity of ceftolozane/tazobactam against selected European clinical Gram-negative bacilli
β-lactamase-producing organisms
Ceftolozane appears to be minimally affected by primitive β-lactamases, such as TEM-1, TEM-2, SHV-1, and OXA-1.Citation27,Citation36 However, its activity against ESBL-producing organisms is reduced. The addition of tazobactam enhanced the activity of ceftolozane against select ESBL-producing organisms in a concentration-dependent manner.Citation26 In a checkerboard titration study of ceftolozane and tazobactam versus 57 ESBL-producing Enterobacteriaceae isolates, the addition of tazobactam at a concentration of 8 mg/L restored the MIC of ceftolozane to ≤4 and ≤8 mg/L in 76% and 93% of the isolates, respectively.Citation26 In a larger collection of clinical isolates, data from the PACTS surveillance study suggest that the combination of ceftolozane and tazobactam retains activity against 91.1%, 60.3%, and 100.0% of E. coli, K. pneumoniae, and Proteus mirabilis with the ESBL phenotype at an MIC of 4 mg/L, and 94.6%, 68.0%, and 100% at an MIC of 8 mg/L, respectively.Citation29
While ceftolozane is also susceptible to hydrolysis by the AmpC enzyme, the efficiency of hydrolysis may be dependent on the genus of the organism. In a checkerboard titration study of ceftolozane and tazobactam against 20 AmpC hyperproducing isolates, the MIC of ceftolozane was restored to ≤8 mg/L in 95% of the isolates at a tazobactam concentration of 8 mg/L.Citation26 However, it should be noted that seven (35%) of the strains exhibited lower MICs to ceftolozane at baseline (MIC 1–2 mg/L) without the addition of tazobactam. This finding is consistent with a previous study that described the relative stability of ceftolozane to some AmpC enzymes, with fourfold-greater activity in comparison to ceftazidime.Citation27,Citation28
The activity of ceftolozane is compromised in the presence of carbapenemases, such as metallo-β-lactamases and K. pneumoniae carbapenemases. The addition of tazobactam does not appear to enhance the activity of ceftolozane against carbapenemase-producing organisms.Citation26,Citation27
Pharmacokinetics
Ceftolozane is parenterally administered and exhibits linear pharmacokinetics after single doses of 500/250 mg, 1,000/500 mg, and 2,000/1,000 mg, and multiple doses of 1,000/500 mg every 8 hours and 1,500/750 mg every 12 hours of ceftolozane/tazobactam.Citation31,Citation40 In healthy adults, the maximum plasma concentration (Cmax) and plasma half-life (t½) for ceftolozane/tazobactam 1,000/500 mg and 2,000/1000 mg infused over 60 minutes given every 8 hours were 74.4 mg/L, 3.12 hours, and 117 mg/L, 2.67 hours, respectively.Citation40,Citation41 Accumulation of ceftolozane after multiple dosing was negligible.Citation31,Citation40,Citation42 Ceftolozane is eliminated unchanged primarily through the urine (>90%).Citation31,Citation40,Citation42 Pharmacokinetic parameters such as clearance, t1/2, area under the curve (AUC), steady-state volume, and Cmax are similar when tazobactam is coadministered with ceftolozane compared to ceftolozane alone.Citation40 Minimal increases in AUC and t1/2 were observed in patients with mild renal impairment (creatinine clearance 60–89 mL/minute). In patients with moderate renal impairment (creatinine clearance 30–59 mL/minute), the observed increases in AUC and t1/2 were 2.6- and 2.1-fold for ceftolozane, and 2.0- and 1.6-fold for tazobactam, respectively. As such, no dosage adjustment is required in patients with mild renal impairment. However, patients with moderate renal impairment may require a 50% dose reduction.Citation43 The data describing the pharmacokinetic profile of ceftolozane/tazobactam in patients with severe renal insufficiency and hemodialysis have been submitted for presentation at a conference at the time of writing.
Ceftolozane/tazobactam exhibits rapid tissue distribution, including excellent lung penetration, and low protein binding.Citation31,Citation41,Citation44 The probability of achieving 40% time of unbound drug above the MIC of the organism (T > MIC) in plasma and epithelial lining fluid was observed in >90% of the simulated ventilator-associated pneumonia (VAP) population for important Gram-negative pathogens, such as P. aeruginosa, E. coli, and K. pneumoniae.Citation41,Citation44
Pharmacodynamics
The bactericidal potential of ceftolozane was characterized by evaluating the in vitro killing kinetics of P. aeruginosa strain PAO1. A greater than 3-log reduction in bacterial load was observed after 8 hours at 1 × MIC (0.5 mg/L).Citation33 In comparison to ceftazidime, ceftolozane initiated killing at two- to fourfold-lower multiples of MIC. Similarly, with the addition of tazobactam to ceftolozane, concentration-independent rapid bactericidal activity was observed against four isogenic strains of E. coli (wild type, β-lactamase-producing AmpC, CMY10, and ESBL CTX-M 15 strains), with 99.9% kill within 8 hours.Citation45 In vivo infection models in neutropenic mice also revealed bactericidal activity against most organisms (four non-ESBL-producing E. coli and K. pneumonia strains, and four P. aeruginosa strains), with greater than 2-log reduction in bacterial load at 24 hours.Citation46 The addition of tazobactam enhances the killing potential of ceftolozane against various β-lactamase-producing organisms. However, this observation is dependent on the dosing schedule of tazobactam. Bacterial reduction of greater than 2-log10 colony-forming units at 24 hours was observed when tazobactam was administered every 6 or every 8 hours. Administration of tazobactam every 12 or 24 hours resulted in much less bacterial killing (≤2-log reduction) at 24 hours in comparison to administration every 6 or 8 hours.Citation47
Similar to other cephalosporins, the pharmacodynamic parameter predicting bacteriological efficacy is the T > MIC of the organism. Ceftolozane/tazobactam concentrations remain above MIC approximately 40%–50% of the time between dosage administrations, similar to other cephalosporins.Citation32 However, the percentage of T > MIC required for ceftolozane against Enterobacteriaceae and P. aeruginosa in an infected murine thigh model is much less than observed with other cephalosporins.Citation46 The mean (±standard deviation) percentages of T > MIC required for stasis and 1-log kill of wild-type Enterobacteriaceae, Enterobacteriaceae-producing ESBLs, and P. aeruginosa were 26.3 ± 2.1 and 31.6 ± 1.6; 31.1 ± 4.9 and 34.8 ± 4.4; and 24.0 ± 3.3 and 31.5 ± 3.9, respectively.Citation46
The probability of pharmacokinetic and pharmacodynamic target attainment was determined using Monte Carlo modeling. The simulated target attainment revealed that 50% T > MIC of 8 mg/L was achieved in 90% of the subjects with a 1,500 mg dose infused over 60 minutes, every 8 hours. Based on observed MIC distribution of select organisms in the 2008 surveillance data, a high probability of target attainment was observed for E. coli (MIC90 = 0.25), K. pneumoniae (MIC90 = 2), and P. aeruginosa (MIC90 = 2). Thus, ceftolozane/tazobactam demonstrated a high probability of target attainment using 50% T > MIC, supporting a proposed breakpoint of 8 mg/L.Citation48
Resistance
Ceftolozane is predominantly active against Gram-negative bacteria. Takeda et al reported a lower frequency of spontaneous resistance of P. aeruginosa to ceftolozane compared to ceftazidime at concentrations of 4 ×, 8 ×, and 16 × MIC, as well as compared to imipenem and ciprofloxacin at 4 × MIC.Citation27 More specifically, after five serial passages, a fourfold reduction in susceptibility was observed for ceftolozane. Comparatively, 32-, 16-, and 16-fold reductions in susceptibility were observed for ceftazidime, imipenem, and ciprofloxacin, respectively.Citation34 The most significant mechanism is the production of β-lactamases with hydrolysis of ceftolozane. While ceftolozane has demonstrated stability against AmpC β-lactamases,Citation28 the addition of tazobactam is necessary to improve its stability and activity against organisms producing ESBLs.Citation26 However, significant hydrolysis is observed in organisms producing carbapenemases (metallo-β-lactamase and K. pneumoniae carbapenemase), despite the combination of ceftolozane and tazobactam.Citation26
Ceftolozane/tazobactam has demonstrated minimal cross-resistance with other antimicrobials, as well as maintained susceptibility in some organisms that exhibit resistance to other antipseudomonal agents.Citation49 More specifically, ceftolozane/tazobactam is unaffected by upregulation of efflux pumps or loss of porin channels that may affect selected antipseudomonal β-lactams, fluoroquinolones, and aminoglycosides.Citation27 Overexpression of MexAB-OprM, MexCD-OprJ, MexEF-OprN, or MexXY in clinical isolates of P. aeruginosa did not increase the MIC of ceftolozane.Citation27 Similarly, ceftolozane maintained its activity against imipenem-resistant clinical isolates of P. aeruginosa demonstrating loss of OprD.Citation27
Clinical trials
A Phase II clinical trial evaluating the safety and efficacy of ceftolozane/tazobactam in the setting of complicated intra-abdominal infections and a Phase II trial evaluating the safety and efficacy of ceftolozane alone in complicated urinary tract infections have been completed. In the Phase II, randomized, double-blind, multicenter study of ceftolozane/tazobactam (1.5 g intravenously every 8 hours) in combination with metronidazole (500 mg intravenously every 8 hours) against meropenem (1 g intravenously every 8 hours) for the management of complicated intra-abdominal infections, comparable cure rates of 91% versus 94% were reported, respectively. The cure rates for the microbiological intent-to-treat population and microbiologically evaluable population were 84% versus 96% and 89% versus 96%, for ceftolozane/tazobactam and meropenem, respectively (Cubist Pharmaceuticals, correspondence, March, 2013).
In the Phase II, randomized, double-blind studies comparing the safety and efficacy of ceftolozane and ceftazidime in complicated urinary tract infections, no difference in microbiologic cure rates at the test-of-cure visit was reported. More specifically, in the setting of complicated urinary tract, complicated lower urinary tract, and pyelonephritis, the observed microbiologic cure rates were 83% versus 76%, 82% versus 73%, and 86% versus 83% for ceftolozane and ceftazidime, respectively.Citation31,Citation50,Citation51 The current formulation in clinical trials includes the addition of tazobactam to provide coverage for selected β-lactamase-producing organisms. However, the clinical implications of this combination need to be evaluated.
Currently, a Phase III, multicenter, prospective, randomized, open-label study has been initiated to evaluate the safety and efficacy of ceftolozane/tazobactam 3 g intravenously every 8 hours versus piperacillin/tazobactam 4.5 g intravenously every 6 hours for the treatment of VAP.Citation52 Eligibility criteria include adult patients age 18 years or older, receipt of mechanical ventilation for greater than 48 hours, Acute Physiology and Chronic Health Evaluation score of 11–35, presence of new or progressive infiltrate on chest X-ray, and presence of clinical criteria consistent with VAP. Exclusion criteria include history of moderate or severe hypersensitivity to β-lactam antibiotics and known end-stage renal disease or requirement for dialysis.Citation52 The rationale for a higher dosing regimen of ceftolozane partially stems from the results of a rabbit pneumonia experimental model that demonstrated significantly greater reduction in pulmonary bacterial load with ceftolozane human-equivalent dose of 2 g every 8 hours compared to ceftolozane human-equivalent dose of 1 g every 8 hours.Citation53 In addition, the probability of target attainment (T > MIC 40%) of ceftolozane/tazobactam when patients with renal hyperclearance are modeled (estimated creatinine clearance 180–250 mL/minute) was 98%–100% with a 3 g every 8 hour dose compared to 71%–93% with a 1.5 g every 8 hour dose at an MIC of 8 mg/L. The primary objective measure for this Phase III VAP study is clinical response at the end-of-therapy visit in the modified intention-to-treat population.Citation52 The estimated completion date for this study is January 2016.Citation52 A randomized, double-blind study comparing ceftolozane/tazobactam to imipenem/cilastatin is also planned.
Adverse effects
The safety of ceftolozane/tazobactam has been evaluated in Phase I and II studies, which found the drug to be generally well tolerated. In 64 healthy volunteers who received single and multiple ascending infusions of ceftolozane/tazobactam, no serious adverse events requiring discontinuation of drug therapy or deaths were observed. The most common adverse event was peripheral infusion-site reactions, while two subjects experienced diarrhea and one experienced flushing; no dose-limiting toxicity was reported.Citation42 In the evaluation of intrapulmonary penetration of ceftolozane/tazobactam in 51 healthy adults who received ceftolozane/tazobactam or piperacillin/tazobactam, all reported adverse events were of mild severity, and event incidence was similar between the two groups.Citation44 Adverse events experienced in the ceftolozane/tazobactam group included diarrhea, viral upper respiratory tract infection, musculoskeletal chest pain, somnolence, hematuria, and cough; none of the reported adverse events were serious or included death.Citation44
Safety data derived from Phase II, randomized, double-blind, controlled clinical trials comparing ceftolozane and ceftazidime in complicated urinary tract infection have shown comparable adverse-effect profiles. The most common treatment-emergent adverse events were constipation (9.4%), sleep disorder (7.1%), headache (5.9%), and nausea (5.9%).Citation51
Discussion
The increasing prevalence of multidrug-resistant Gram-negative pathogens presents significant challenges to clinicians. The Antimicrobial Availability Task Force of the Infectious Diseases Society of America released a report titled “Bad Bugs, No Drugs: As Antibiotic R&D Stagnates, a Public Health Crisis Brews” to address the lack of antibiotic development in an era of increasing resistance.Citation9 More specifically, infections caused by P. aeruginosa result in significant morbidity and mortality, particularly in strains demonstrating cross-resistance to the handful of agents with clinical utility against P. aeruginosa.Citation4,Citation54,Citation55 Currently, several β-lactam/β-lactamase-inhibitor combinations are in clinical development (ceftazidime/avibactam, ceftaroline/avibactam, and imipenem/cilastatin).Citation30
Ceftolozane/tazobactam is a novel antibacterial and β-lactamase-inhibitor combination consisting of ceftolozane, a novel antipseudomonal cephalosporin, and tazobactam, a well-established β-lactamase inhibitor. It has demonstrated potent bactericidal activity against select Gram-negative isolates, mainly wild-type Enterobacteriaceae and P. aeruginosa. The addition of tazobactam has enhanced the spectrum of ceftolozane to Enterobacteriaceae-producing select ESBLs. Moreover, ceftolozane/tazobactam has not demonstrated cross-resistance to other antimicrobial classes, particularly those affected by ESBLs, AmpC β-lactamase, a loss in porin channels, or the overexpression of efflux pumps in P. aeruginosa.
It is in this context that the utility of ceftolozane/tazobactam appears particularly attractive. The selection of antimicrobial agents in infectious settings where P. aeruginosa may be a likely pathogen requires careful review of the institution-specific antimicrobial susceptibility profile, an antibiogram. Specifically, in institutions lacking antipseudomonal agents with reliable susceptibility, a combination of agents (including agents with narrow therapeutic indices and high risk of toxicity) may be needed. Ceftolozane/tazobactam carries the potential to be a first-line agent in this setting, given its in vitro/in vivo data and favorable adverse-effects profile. In contrast, in institutions with favorable antipseudomonal susceptibility profiles, ceftolozane/tazobactam nonetheless requires serious consideration for addition to the formulary, for the management of challenging cases caused by strains with resistant phenotypes. However, given its early stage of development, the role of ceftolozane/tazobactam will be determined by the results of Phase III clinical data.
Acknowledgments
We would like to thank Philip Tam, PhD for building the chemical structures of ceftolozane and tazobactam. Chemical structures were developed using BKChem software (http://bkchem.zirael.org). We would also like to thank John Mohr, PharmD for provision of data.
Disclosure
The authors report no conflicts of interest in this work. Donald Hsu is a speaker for Astellas, Inc, Optimer Pharmaceuticals, and Merck Inc. The views expressed in this article are solely those of the authors and do not necessarily reflect the views of the Western University of Health Sciences, St Joseph Hospital Orange, Veterans Affairs San Diego Healthcare System, nor University of California San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences.
References
- CarmeliYEliopoulosGMozaffariESamoreMHealth and economic outcomes of vancomycin-resistant enterococciArch Intern Med2002162192223222812390066
- CosgroveSESakoulasGPerencevichENSchwaberMJKarchmerAWCarmeliYComparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysisClin Infect Dis2003361535912491202
- HidayatLKHsuDIQuistRShrinerKAWong-BeringerAHigh-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicityArch Intern Med2006166192138214417060545
- BoucherHWTalbotGHBradleyJSBad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of AmericaClin Infect Dis200948111219035777
- GiskeCGGeJNordmannPActivity of cephalosporin CXA-101 (FR264205) and comparators against extended-spectrum-{beta}-lactamase-producing Pseudomonas aeruginosaJ Antimicrob Chemother200964243043119474066
- RiceLBFederal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPEJ Infect Dis200819781079108118419525
- KlevensRMEdwardsJRRichardsCLJrEstimating health care-associated infections and deaths in US hospitals, 2002Public Health Rep2007122216016617357358
- GaynesREdwardsJROverview of nosocomial infections caused by gram-negative bacilliClin Infect Dis200541684885416107985
- TalbotGHBradleyJEdwardsJEJrGilbertDScheldMBartlettJGBad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of AmericaClin Infect Dis200642565766816447111
- Centers for Disease Control and PreventionGram-negative bacteria infections in healthcare settings2011 Available from: http://www.cdc.gov/hai/organisms/gram-negative-bacteria.htmlAccessed April 29, 2013
- SteinGEAntimicrobial resistance in the hospital setting: impact, trends, and infection control measuresPharmacotherapy20052510 Pt 244S54S16178675
- MauldinPDSalgadoCDHansenISDurupDTBossoJAAttributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteriaAntimicrob Agents Chemother201054110911519841152
- AgnelloMWong-BeringerADifferentiation in quinolone resistance by virulence genotype in Pseudomonas aeruginosaPloS One201278e4297322905192
- CossonPZulianelloLJoin-LambertOPseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host systemJ Bacteriol2002184113027303312003944
- ShaverCMHauserARRelative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lungInfect Immun200472126969697715557619
- Van DeldenCIglewskiBHCell-to-cell signaling and Pseudomonas aeruginosa infectionsEmerg Infect Dis1998445515609866731
- VeesenmeyerJLHauserARLisboaTRelloJPseudomonas aeruginosa virulence and therapy: evolving translational strategiesCrit Care Med20093751777178619325463
- GiamarellouHAntoniadouAAntipseudomonal antibioticsMed Clin North Am2001851194211190351
- National Nosocomial Infections Surveillance SystemNational Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004Am J Infect Control200432847048515573054
- SievertDMRicksPEdwardsJRAntimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010Infect Control Hosp Epidemiol201334111423221186
- LivermoreDMMultiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare?Clin Infect Dis31200234563464011823954
- RossoliniGMMantengoliETreatment and control of severe infections caused by multiresistant Pseudomonas aeruginosaClin Microbiol Infect200511Suppl 4173215953020
- KallenAJHidronAIPatelJSrinivasanAMultidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008Infect Control Hosp Epidemiol201031552853120334552
- AloushVNavon-VeneziaSSeigman-IgraYCabiliSCarmeliYMultidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impactAntimicrob Agents Chemother2006501434816377665
- CaoBWangHSunHZhuYChenMRisk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infectionsJ Hosp Infect200457211211815183240
- LivermoreDMMushtaqSGeYChequerboard titration of cephalosporin CXA-101 (FR264205) and tazobactam versus beta-lactamase-producing EnterobacteriaceaeJ Antimicrob Chemother20106591972197420595207
- TakedaSNakaiTWakaiYIkedaFHatanoKIn vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosaAntimicrob Agents Chemother200751382683017145788
- TakedaSIshiiYHatanoKTatedaKYamaguchiKStability of FR264205 against AmpC beta-lactamase of Pseudomonas aeruginosaInt J Antimicrob Agents200730544344517644319
- SaderHSFlammRKStreitJMJonesRNActivity of novel antimicrobial ceftolozane/tazobactam tested against contemporary clinical strains from USA hospitals (2011)Abstracts: 52nd Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2012
- TodaAOhkiHYamanakaTSynthesis and SAR of novel parenteral anti-pseudomonal cephalosporins: discovery of FR264205Bioorg Med Chem Lett200818174849485218701284
- Cubist PharmaceuticalsCeftolozane/tazobactam – overview Available from: http://www.cubist.com/sup/img/drugs/Ceft-Taz-overview_880.pngAccessed January 10, 2013
- Cubist PharmaceuticalsGram-negative: ceftolozane/tazobactam Available from: http://www.cubist.com/products/cxa_201Accessed January 10, 2013
- MoyáBZamoranoLJuanCGeYOliverAAffinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosaAntimicrob Agents Chemother20105493933393720547785
- ShlaesDMNew β-lactam-β-lactamase inhibitor combinations in clinical developmentAnn N Y Acad Sci2013127710511423346860
- MushtaqSWarnerMLivermoreDMActivity of cephalosporin CXA-101 (FR264205) with β-lactamase inhibitors vs EnterobacteriaceaeAbstracts: 48th Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2009
- LivermoreDMMushtaqSGeYWarnerMActivity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolatesInt J Antimicrob Agents200934540240619428220
- MoyaBZamoranoLJuanCPérezJLGeYOliverAActivity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patientsAntimicrob Agents Chemother20105431213121720086158
- ZamoranoLJuanCFernández-OlmosAGeYCantónROliverAActivity of the new cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa isolates from chronically-infected cystic fibrosis patientsClin Microbiol Infect20101691482148720002107
- SaderHFarrellDJonesRNActivity of the novel antimicrobial CXA-201 tested against contemporary clinical strains from European hospitals (2012)Presented at: 22nd Annual Meeting of the European Congress of Clinical Microbiology (ECCMID)March 31–April 3, 2012London, UK
- MillerBHershbergerEBenzigerDTrinhMFriedlandIPharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending dosesAntimicrob Agents Chemother20125663086309122450972
- MillerBChandorkarGUmehOSafety and PK of IV ceftolozane/tazobactam 3 g Q8h and cumulative fraction of response in plasma and epithelial lining fluid in a simulated VAP populationAbstracts: 52nd Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2012
- GeYWhitehouseMJFriedlandITalbotGHPharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusionsAntimicrob Agents Chemother20105483427343120457817
- MillerBHershbergerEBenzigerDPharmacokinetics of CXA-101/tazobactam in subjects with mild or moderate renal impairmentPresented at: 21st Annual Meeting of the European Congress of Clinical MicrobiologyMay 7–10, 2011Milan, Italy
- ChandorkarGHuntingtonJAGotfriedMHRodvoldKAUmehOIntrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjectsJ Antimicrob Chemother201267102463246922773741
- SoonRLForrestAHoldenPNIn vitro pharmacodynamics of ceftolozane/tazobactam against β-lactamase producing Escherichia coliAbstracts: 52nd Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2012
- CraigWAAndesDRIn vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic miceAntimicrob Agents Chemother20135741577158223274659
- VanscoyBMendesRENicasioAMPharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection modelAntimicrob Agents Chemother20135762809281423629705
- HershbergerEMouksassiMSteenbergenJNCXA-101/tazobactam probability of target attainment using population pharmacokinetic analysisPresented at: 21st European Congress of Clinical Microbiology and Infectious Diseases and Twenty-seventh International Congress of ChemotherapyMay 7–10, 2011Milan, Italy
- CabotGMuletXMoyaBDynamics and mechanisms of resistance development to ceftazidime, meropenem and ceftolozane/tazobactam in wild-type and mutator P. aeruginosa strainsAbstracts: 52nd Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2012
- ClinicalTrials.gov [website on the Internet]Safety and efficacy of IV CXA-101 and IV ceftazidime in patients with complicated urinary tract infections2010 Available from: http://clinicaltrials.gov/ct2/show/NCT0921024?term=CXA±and±ceftazidime&rank=1Accessed June 7, 2013
- UmehOCebrikDFriedlandIA double-blind, randomized, phase 2 study to compare the safety and efficacy of intravenous CXA-101 (CXA) and intravenous ceftazidime (CTZ) in complicated urinary tract infection (cUTI)Abstracts: 50th Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2010
- ClinicalTrials.gov [website on the Internet]Study of intravenous ceftolozane/tazobactam vs piperacillin/tazobactam in ventilator associated pneumonia2013 Available from: http://clinicaltrials.gov/ct2/show/NCT1853982?term=ceftolozane&rank=2Accessed May 17, 2013
- JacquelineCBretonniereCDesessardCIn vivo activity of CXA-101 against Pseudomonas aeruginosa(PA) in a rabbit experimental model of pneumonia: comparison with ceftazidime (CAZ), piperacillin/tazobactam (TZP), and imipenem (IMP)Abstracts: 51st Interscience Conference on Antimicrobial Agents and ChemotherapyWashingtonAmerican Society for Microbiology2011
- HsuDIOkamotoMPMurthyRWong-BeringerAFluoroquinolone-resistant Pseudomonas aeruginosa: risk factors for acquisition and impact on outcomesJ Antimicrob Chemother200555453554115728150
- NguyenLHHsuDIGanapathyVShrinerKWong-BeringerAReducing empirical use of fluoroquinolones for Pseudomonas aeruginosa infections improves outcomeJ Antimicrob Chemother200861371472018222951