Abstract
Purpose
NDM-1-producing Citrobacter portucalensis and Citrobacter freundii simultaneously occurred in a hospital. This study aims to characterize the blaNDM-1-carrying plasmids in these Citrobacter strains.
Methods
Cf7303, Cf7308, and Cf7313 were recovered from three patients in a teaching hospital from September 24 to October 1, 2021. Bacteria were identified by MALDI-TOF mass spectrometry, and antibiotics susceptibility tests were determined by VITEK® 2 compact system. Whole-genome sequencing (WGS) was performed using the HiSeq Illumina and QNome platform to characterize the genomes.
Results
Cf7303 was identified as C. portucalensis Sequence Type 328 by WGS, and harbored two plasmids, namely pCf7303 and a novel IncFIB pNDM-Cf7303 on which antibiotic-resistant genes (blaTEM-1, blaCTX-M-14, blaNDM-1, aac (3)-IId, aadA2, fosA3, sul1, sul2, catA2, tetD, dfrA12, qacEdelta1, mph(A), and bleMBL) are located. C. freundii strain Cf7308 and Cf7313 belonged to the same Sequence Type 98. Cf7308 contained two plasmids, pCf7308, and an IncN1 pNDM-Cf7308 with homology to pNDM-BTR in E. coli and pNDM-CWH001 in C. freundii.
Conclusion
We characterized a putatively novel IncFIB plasmid carrying blaNDM-1 in C. portucalensis. In addition, the closely related blaNDM-1-carrying IncN1 plasmids in E. coli and C. freundii suggest that interspecies or intraspecies horizontal transfer occurs in China.
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) have spread widely and emerged as a health concern worldwide. Since carbapenemase genes located on mobile genetic elements (MGEs) are easily transmissible within and across bacterial cells of the same or different species, carbapenemase production has become the predominant mechanism of CRE.
Carbapenemases include Ambler class A Klebsiella pneumoniae carbapenemase (KPC), class B metallo-β-lactamase (MBL), and some class D β-lactamases (OXA-48). Recently, a novel, non-β-lactam β-lactamase inhibitor avibactam performs excellent activity against KPC- and OXA-48-producing CRE,Citation1 albeit it cannot target MBL-producing strains, which remain worrisome to clinical practice. New Delhi metallo-β-lactamase-1 (NDM-1) is a kind of MBLs commonly reported in Enterobacteriaceae. Since blaNDM-1-carrying Escherichia coli and Klebsiella pneumoniae were firstly described in 2009,Citation2 NDM has been reported in a wide variety of species, such as Providencia rettgeri, Citrobacter freundii, Klebsiella oxytoca, Salmonella enterica, and Enterobacter cloacae.Citation3–5
Here we report the simultaneous occurrence of NDM-1-producing C. portucalensis and C. freundii clinical strains in a tertiary hospital in Beijing, China and the characteristics of blaNDM-1-carrying IncFIB and IncN1 plasmids.
Materials and Methods
Bacterial Isolation and Identification
Three carbapenem-resistant C. freundii strains Cf7303, Cf7308, and Cf7313 were isolated from postoperative intraabdominal drainage cultures in a teaching hospital from September 24 to October 1 in 2021. Cf7303 was from a surgical ward, while Cf7308 and Cf7313 were from an intensive care unit. These strains were identified as C. freundii using MALDI-TOF MS (Bruker Dalton GmbH, Leipzig, Germany).
Antimicrobial Susceptibility Testing
The minimal inhibitory concentrations (MICs) of piperacillin/tazobactam (TZP); ceftazidime (CAZ), cefepime (FEP), aztreonam (ATM), imipenem (IPM), meropenem (MEM), amikacin (AMK), ciprofloxacin (CIP), tobramycin (TOB), doxycycline (DOC), tigecycline (TGC), and sulfamethoxazole/trimethoprim (SXT) were determined by VITEK® 2 compact system (BioMérieux, France). The results were interpreted following the Clinical and Laboratory Standards Institute (CLSI) M100-Ed 31 in 2021. E. coli ATCC 25922 was used as quality control in antimicrobial susceptibility tests.
Carbapenemase enzymes, including KPC, NDM, OXA-48, imipenemase (IMP), and Verona integron-encoded metallo-β-lactamase (VIM), were detected by a lateral flow immunoassay NG-test CARBA5 (NG Biotech, France).
Whole-Genome Sequencing and Molecular Analysis
Genomic DNA was extracted from bacteria culture using Wizard® Genomic DNA Purification Kit (Promega). Sequencing was conducted using an Illumina HiSeq X Ten platform with a 500 bp insert size at Shanghai Majorbio Bio-pharm Technology Company (Shanghai, China). The genome was assembled de novo using SOAPdenovo2 and analyzed using the I-Sanger Cloud Platform (www.i-sanger.com) from Shanghai Majorbio. Assemblies were annotated using Prokka (https://github.com/tseemann/prokka).
Cf7303 and Cf7308 were selected for additional long-read sequencing. Libraries were prepared using a Qiagen-8 sequencing kit. Sequencing was performed using a Qcell-3841 sequencing chip on a QNome platform (QitanTech, China). Fast5 files were base-called using NiuTouGeng V3 (QitanTech, China). The raw reads were filtered using NanoPlot v1.38.1 and NanoFilt v2.8.0.Citation6 Hybrid assemblies were conducted using Unicycler v0.4.8 and Flye v2.8.Citation7,Citation8 Furthermore, we used Pilon V1.24Citation9 to carry out genomes polishing and manually checking by remapping raw reads against the plasmids. Genomic sequences were annotated using Prokka V1.14.6.Citation10
Multilocus sequence typing was determined by using the genomic sequence to query the multilocus sequence typing (MLST) database of Citrobacter spp. on the website of https://pubmlst.org/. Antimicrobial resistance genes and plasmid replicons were also predicted using the ResFinder tool (98%, minimum threshold for identity; 80%, minimum coverage) and the PlasmidFinder tool (90%, minimum threshold for identity; 80%, minimum coverage) from the Center for Genomic Epidemiology http://genomicepidemiology.org/, respectively.
Phylogenetic Analysis
Genome sequences of 34 available C. freundii isolates were downloaded from the NCBI database for phylogenetic analysis (accessed November 1, 2021). C. freundii strain B38 (GenBank accession number CP016762) was used as the reference genome for comparison. We used Snippy software (https://github.com/tseemann/snippy) to call SNPs for queried C. freundii isolates from the reference in order to produce an alignment of “core SNPs,” and then the SNPs were concatenated and aligned to construct the Maximum-Likelihood phylogenetic tree using RAxML (v8.2.4). iTOL (https://itol.embl.de/) was used to graph the RAxML best-trees output.
Comparative Genomic Analysis of blaNDM-Carrying Plasmids
In order to elucidate the evolution of blaNDM-1-encoding plasmids, the similarity of sequences was analyzed using BLSATn and Minimap2 (95%, minimum threshold for identity). MCscan pipeline for synteny inference of JCVI utility librariesCitation11 was used for comparative analysis.
Nucleotide Sequence Accession Number
The genome sequences have been deposited to GenBank under the BioProject PRJNA792258. Sequences accession numbers: Cf7303 (CP092466-CP092468), Cf7308 (CP092463-CP092465), and Cf7313 (JAJUBD000000000).
Results
Antibiotic Resistance Profile
Strain Cf7303 was extensively drug-resistant (), resistant to piperacillin/tazobactam, ceftazidime, cefepime, imipenem, meropenem, ciprofloxacin, doxycycline, sulfamethoxazole/trimethoprim, but it was susceptible to aztreonam, amikacin, and tigecycline.
Table 1 Antimicrobial Susceptibility Profiles for Citrobacter spp. Strains
Meanwhile, strains Cf7308 and Cf7313 had identical antibiotic-resistant profiles, resistant to piperacillin/tazobactam, ceftazidime, cefepime, imipenem, meropenem, ciprofloxacin, but it was susceptible to aztreonam, amikacin, tobramycin, doxycycline, tigecycline, and sulfamethoxazole/trimethoprim.
NG-test CARBA5 results displayed that the three isolates produced the New Delhi metallo-β-lactamase.
Whole-Genome Sequencing and Molecular Analysis
Strain Cf7303 was identified as ST328 C. portucalensis by WGS analysis, consisting of a 5,120,606-bp chromosome and a 4187-bp plasmid pCf7303, and a 233,864-bp plasmid pNDM-Cf7303. Cf7303 contains multiple resistance determinants (). The resistance genes blaCMY-77 and qnrB6 were located on the chromosome, while other determinants including sul2, catA2, aac (3)-IId, blaTEM-1, tetD, dfrA12, aadA2, qacEdelta1, blaNDM-1, bleMBL, sul1, mph(A), fosA3, and blaCTX-M-14 located on pNDM-Cf7303 whose backbone genes were separated by multiple IS26 insertion sequences and other IS elements such as IS15, ISEc63. ().
Table 2 Overall Features of the Citrobacter spp. Genomes
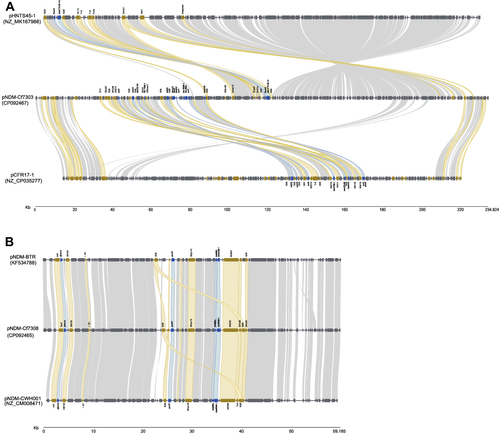
Figure 1 Comparison of blaNDM-1-carrying plasmids. (A) Comparison of pNDM-Cf7303 (CP092467) with its maximum identity of sequences of pCFR17-1 (NC_ CP035277) and pHNTS45-1(NZ_MK167988). (B) Comparison of pNDM-Cf7308 (CP092465) with pNDM-BTR (KF534788) and pNDM-CWH001 (NZ_CM008471). Open reading frames are denoted by block arrows and colored based on gene function. Insertion sequence (IS) elements are shown in yellow, antibiotics resistant genes in blue, and others in grey. Shading regions denote regions of homology (> 95% nucleotide identity).
The draft genome sequencing displayed C. freundii Cf7308 was identical to Cf7313, belonging to ST98 sequence typing. The representative Cf7308 consists of a 5,108,147-bp chromosome, a 319,832-bp plasmid pCf7308, and a 59,165-bp plasmid pNDM-Cf7308. Cf7308 contained six antibiotic resistance determinants (). The resistance genes blaCMY-109 and qnrB38 are located on the chromosome, while dfrA14, blaNDM-1, bleMBL, and qnrS1 are located on pNDM-Cf7308.
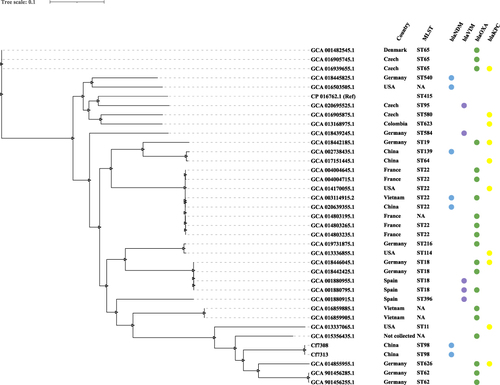
To examine the phylogenetic relationship of Cf7308 and Cf7313 to other C. freundii strains producing less common beta-lactamase such as OXA beta-lactamases, KPC, VIM, and NDM, 34 available genomes of C. freundii were downloaded from the NCBI database to construct a whole-genome sequence phylogenetic tree (). These multidrug-resistant isolates belonged to three lineages and diverse STs.
Figure 2 A whole-genome sequence phylogenetic tree calculated from Cf7308, Cf7313, and 34 available genomes of C. freundii producing less common beta-lactamase such as OXA beta-lactamases, KPC, VIM, and NDM. C. freundii strain B38 (GenBank accession number CP016762) was used as the reference genome for comparison. NA of MLST indicates not be assigned a matched ST when querying the multilocus sequence typing (MLST) database of Citrobacter spp.
Characterization of blaNDM-1-Carrying Plasmid pNDM-Cf7303
The complete nucleotide sequence of pNDM-Cf7303 was 233,864 bp in length, constituting a circular DNA with an average G + C content of 52.4%. 282 open reading frames were annotated. pNDM-Cf7303 was assigned to the IncFIB group due to containing an IncFIB-type repA (plasmid replication initiation) gene. Antibiotic-resistant genes of sul2, catA2, aac(3)-IId, blaTEM-1, tetD, dfrA12, aadA2, qacEdelta1, sul1, blaNDM-1, bleMBL, sul1, mph(A) were clustered as the main 50,000 bp multi-drug resistance (MDR) region on pNDM-Cf7303, coupled with an additional 7,000 bp MDR region of IS26-fosA3-blaCTX-M-14-IS26. IS26 frequently flanked the antibiotic-resistant genes. The genetic context of blaNDM-1 was IS26-dfrA12-qacEdelta1-aadA2-sul1-blaNDM-1-bleMBL-sul1- mph(A)-IS26.
No significant sequence homology to pNDM-Cf7303 was founded using BLASTn search and minimap2. According to the maximum identity of sequences, a pairwise comparison was conducted with pCFR17_1 (NC_ CP035277) and pHNTS45-1(NZ_MK167988). showed that the backbone of pNDM-Cf7303 was probably reconstituted by pCFR17_1 and pHNTS45-1 from C. freundii, and the blaNDM-1 gene could be acquired by IS26 elements.
Characterization of blaNDM-1-Carrying Plasmid pNDM-Cf7308
The complete nucleotide sequence of pNDM-Cf7308 was 59,165 bp in length, constituting a circular DNA with an average G + C content of 52.1%. 75 open reading frames were annotated. pNDM-Cf7308 was assigned to the IncN1 group due to containing an IncN1-type repA (plasmid replication initiation) gene. The genetic context of blaNDM-1 was IS26-qnrS1-bleMBL-blaNDM-1-IS3000. A pairwise comparison showed it was homology to pNDM-BTR (KF534788) and pNDM-CWH001 (NZ_CM008471) which were harbored by E. coli BTRCitation12 and C. freundii CWH,Citation13 respectively. ().
Discussion
Being a member of the Enterobacteriaceae family, Citrobacter spp. are regarded as opportunistic pathogens owing to exiting ubiquitously in the environment, food, and intestine of humans or animals.Citation14 Carbapenem-resistance Citrobacter strains have been frequently collected from salad, urine, bloodstream, and rectal swabs.Citation14,Citation15 Here, we reported three clinical NDM-1-producing Citrobacter strains, including C. portucalensis strain Cf7303 and C. freundii strain Cf7308, Cf7313.
C. portucalensis was proposed as a novel species within the genus Citrobacter in 2017.Citation16 In 2019, a multidrug-resistant C. portucalensis strain NR-12 was isolated from poultry droppings.Citation17 Soon later, a carbapenem-resistant C. portucalensis 3839 ST165 was first isolated from the sputum of a patient with type 2 diabetes mellitus in China.Citation18 In this study, we isolated another NDM-producing C. portucalensis strain Cf7303 ST328 from a postoperative patient. In Cf7303, the blaNDM-1 gene was carried by a novel IncFIB plasmid different from an IncX3 plasmid in C. portucalensis 3839.Citation19 IncF plasmids are the most prevalent narrow-host-range plasmids accounting for dissemination of about 40% of plasmid-borne carbapenemases.Citation5 For example, IncF plasmids, including pCRCB-101_1, pCB1_SE1_NDM, pKPX-1, are responsible for disseminating blaNDM-1 in C. freundii, Citrobacter werkmanii, and K. pneumoniae.Citation20,Citation21 As IncF plasmids have been mostly found to carry multiple antibiotic-resistant genes (ARGs), particularly blaCTX-M-15, pNDM-Cf7303 harbored 14 ARGs for β-latam resistance (blaTEM-1, blaCTX-M-14, blaNDM-1), aminoglycoside resistance (aac(3)-IId, aadA2), tetracycline resistance (tetD), macrolide resistance (mphA), fosfomycin resistance (fosA3), chloramphenicol resistance (catA2), glycopeptide resistance (bleMBL), sulfonamide resistance (sul1, sul2), and trimethoprim resistance (dfrA12), resistance to quaternary ammonium (qacEdelta1). Given no sequence homology to pNDM-Cf7303, blaNDM-1 was carried by a novel IncF plasmid in Cf7303. We thus suppose C. portucalensis has the potential to disseminate multiple antibiotic resistance. Given the close relation to C. freundii, many C. portucalensis strains were previously misidentified as C. freundii in GenBank.Citation18 Thereby, the occurrence of carbapenem-resistant C. portucalensis may have been underestimated in the clinic.
Until recently, carbapenemase-producing C. freundii has been reported worldwide. The KPC-producing C. freundii isolates emerged early in 2005 in a hospital in China.Citation22 In addition to the sporadic spread, outbreaks of KPC-2, NDM-1, and IMP-4 producing C. freundii have been reported.Citation23–25 Here, a clone of ST98 C. freundii Cf7308 and Cf7313 was recovered from two patients within a week in an ICU, so blaNDM-1-carrying C. freundii was spreading in this ward. To our knowledge, ST98 C. freundii has rarely been detected (www.pubMLST.org/cfreundii). Phylogenetic analysis of the WGS data showed that the OXA beta-lactamases, KPC, VIM, and NDM producing C. freundii strains belong to diverse STs.
In C. freundii strains, blaNDM-1 was found to be carried by diverse plasmids, including IncX3, IncA/C, IncHI1, IncL/M, IncFII, and IncN1.Citation5,Citation13,Citation19,Citation26–29 In Cf7308, blaNDM-1 was carried on an IncN1 plasmid identical to pNDM-BTR from a local E. coli isolate and pNDM-CWH001 from a C. freundii isolate in Wuhan city. The closely related blaNDM-1-carrying IncN1 plasmids in E. coli and C. freundii suggested interspecies or intraspecies horizontal transfer has occurred in China. Since the IncN plasmid is a broad-host-range type with high transmission efficiency, more intensive infection control measures should be employed.
Conclusions
Our study first disclosed a simultaneous occurrence of NDM-1-producing C. portucalensis and C. freundii in a hospital. In addition, our findings describe IncF and IncN1 plasmids are associated with the dissemination of blaNDM-1 in Citrobacter spp. With the increasing number of carbapenem-resistant Citrobacter spp., we should underpin the active screening of multiple-drug resistance organisms.
Ethical Approval Statements
This retrospective study was approved by the Evaluation Committee and the Biomedical Ethics Committee of Beijing Tsinghua Changgung Hospital (22031-0-01). Because of the retrospective and anonymous nature of the study, the Ethics Committee did not require written informed consent provided by participants.
Disclosure
Yu He is affiliated with Qitan Technology Ltd. All authors declare no other conflicts of interest in this work.
References
- Shirley M. Ceftazidime-Avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675–692. doi:10.1007/s40265-018-0902-x
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054. doi:10.1128/aac.00774-09
- Mataseje LF, Peirano G, Church DL, Conly J, Mulvey M, Pitout JD. Colistin-nonsusceptible Pseudomonas aeruginosa sequence type 654 with blaNDM-1 arrives in North America. Antimicrob Agents Chemother. 2016;60(3):1794–1800. doi:10.1128/aac.02591-15
- Li Z, Lin Y, Lu L, et al. Genetic characterisation of a complex class 1 integron in an NDM-1-producing Citrobacter freundii ST396 clinical strain isolated from a urine sample. J Glob Antimicrob Resist. 2020;23:64–66. doi:10.1016/j.jgar.2020.08.002
- Kopotsa K, Osei Sekyere J, Mbelle NM. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: a review. Ann N Y Acad Sci. 2019;1457(1):61–91. doi:10.1111/nyas.14223
- De Coster W, D’Hert S, Schultz DT, Cruts M, Van Broeckhoven C. NanoPack: visualizing and processing long-read sequencing data. Bioinformatics. 2018;34(15):2666–2669. doi:10.1093/bioinformatics/bty149
- Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi:10.1371/journal.pcbi.1005595
- Lin Y, Yuan J, Kolmogorov M, Shen MW, Chaisson M, Pevzner PA. Assembly of long error-prone reads using de Bruijn graphs. Proc Natl Acad Sci U S A. 2016;113(52):E8396–E8405. doi:10.1073/pnas.1604560113
- Walker BJ, Abeel T, Shea T, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9(11):e112963. doi:10.1371/journal.pone.0112963
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi:10.1093/bioinformatics/btu153
- Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. Synteny and collinearity in plant genomes. Science. 2008;320(5875):486–488. doi:10.1126/science.1153917
- Zhao Y, Wang L, Zhang Z, et al. Structural genomics of pNDM-BTR harboring In191 and Tn6360, and other bla (NDM)-carrying IncN1 plasmids. Future Microbiol. 2017;12:1271–1281. doi:10.2217/fmb-2017-0067
- Yang L, Li P, Liang B, et al. Multidrug-resistant Citrobacter freundii ST139 co-producing NDM-1 and CMY-152 from China. Sci Rep. 2018;8(1):10653. doi:10.1038/s41598-018-28879-9
- Liu L, Qin L, Hao S, et al. Lineage, antimicrobial resistance and virulence of citrobacter spp. Pathogens. 2020;9(3):195. doi:10.3390/pathogens9030195
- Rödel J, Mellmann A, Stein C, et al. Use of MALDI-TOF mass spectrometry to detect nosocomial outbreaks of Serratia marcescens and Citrobacter freundii. Eur J Clin Microbiol Infect Dis. 2019;38(3):581–591. doi:10.1007/s10096-018-03462-2
- Ribeiro TG, Gonçalves BR, da Silva MS, et al. Citrobacter portucalensis sp. nov., isolated from an aquatic sample. Int J Syst Evol Microbiol. 2017;67(9):3513–3517. doi:10.1099/ijsem.0.002154
- Hasan MS, Sultana M, Hossain MA. Complete genome arrangement revealed the emergence of a poultry origin superbug Citrobacter portucalensis strain NR-12. J Glob Antimicrob Resist. 2019;18:126–129. doi:10.1016/j.jgar.2019.05.031
- Cao X, Xie H, Huang D, et al. Detection of a clinical carbapenem-resistant Citrobacter portucalensis strain and the dissemination of C. portucalensis in clinical settings. J Glob Antimicrob Resist. 2021;27:79–81. doi:10.1016/j.jgar.2021.04.027
- Zhang T, Lin Y, Li P, et al. Characterization of Plasmid Co-Harboring NDM-1 and SHV-12 from a multidrug-resistant Citrobacter freundii strain ZT01-0079 in China. Infect Drug Resist. 2021;14:947–952. doi:10.2147/idr.S301736
- Huang TW, Chen TL, Chen YT, et al. Copy number change of the NDM-1 sequence in a multidrug-resistant Klebsiella pneumoniae clinical isolate. PLoS One. 2013;8(4):e62774. doi:10.1371/journal.pone.0062774
- Yoon EJ, Kang DY, Yang JW, et al. New Delhi metallo-beta-lactamase-producing Enterobacteriaceae in South Korea between 2010 and 2015. Front Microbiol. 2018;9:571. doi:10.3389/fmicb.2018.00571
- Chen S, Hu F, Liu Y, Zhu D, Wang H, Zhang Y. Detection and spread of carbapenem-resistant Citrobacter freundii in a teaching hospital in China. Am J Infect Control. 2011;39(9):e55–60. doi:10.1016/j.ajic.2011.02.009
- Schweizer C, Bischoff P, Bender J, et al. Plasmid-mediated transmission of KPC-2 carbapenemase in Enterobacteriaceae in critically ill patients. Front Microbiol. 2019;10:276. doi:10.3389/fmicb.2019.00276
- Marmor A, Daveson K, Harley D, Coatsworth N, Kennedy K. Two carbapenemase-producing Enterobacteriaceae outbreaks detected retrospectively by whole-genome sequencing at an Australian tertiary hospital. Infect Dis Health. 2020;25(1):30–33. doi:10.1016/j.idh.2019.08.005
- Hammerum AM, Hansen F, Nielsen HL, et al. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother. 2016;71(11):3117–3124. doi:10.1093/jac/dkw289
- Zhu B, Ying C, Xu H, Ying J. Coexistence of NDM-1-producing Escherichia coli and Citrobacter freundii in the same patient. J Glob Antimicrob Resist. 2018;15:79–81. doi:10.1016/j.jgar.2018.04.013
- Dolejska M, Villa L, Poirel L, Nordmann P, Carattoli A. Complete sequencing of an IncHI1 plasmid encoding the carbapenemase NDM-1, the ArmA 16S RNA methylase and a resistance-nodulation-cell division/multidrug efflux pump. J Antimicrob Chemother. 2013;68(1):34–39. doi:10.1093/jac/dks357
- Wu W, Espedido B, Feng Y, Zong Z. Citrobacter freundii carrying blaKPC-2 and blaNDM-1: characterization by whole genome sequencing. Sci Rep. 2016;6(1):30670. doi:10.1038/srep30670
- Lopez-Diaz M, Ellaby N, Turton J, Woodford N, Tomas M, Ellington MJ. NDM-1 carbapenemase resistance gene vehicles emergent on distinct plasmid backbones from the IncL/M family. J Antimicrob Chemother. 2022;77(3):620–624. doi:10.1093/jac/dkab466
- Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50(4):1355–1361. doi:10.1128/jcm.06094-11
- Carattoli A, Zankari E, García-Fernández A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi:10.1128/aac.02412-14
