Abstract
Background
Fungi are rich source of biologically active metabolites aimed for the improvement of human health through the prevention of various diseases, including infections and inflammatory disorders.
Aim
We aimed to in vitro examine the anti-SARS CoV-2 activity of the aqueous extract of each Pleurotus (P.) ostreatus, Lentinula (L.) edodes and Agaricus (A.) bisporus edible mushroom followed by docking analysis of certain metabolites against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-main protease (protease Mpro).
Methods
Antiviral and cytotoxic effects were tested on hCoV-19/Egypt/NRC-3/2020/Vero-E6 cells and analyzed via (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide Assay (MTT) assay. Ligand-protein and protein-protein docking studies were performed to explore the interaction of different mushroom extracts at the binding site of protease Mpro. Molecular dynamics (MD) simulations were performed on the most promising ligand-target complexes to investigate their dynamic properties and confirm docking results.
Results
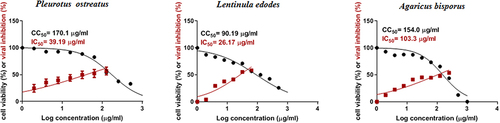
Substantial antiviral activities with an IC50 of 39.19, 26.17, and 10.3.3 µg/mL and a selectivity index (SI) of 4.34, 3.44, and 1.5 for P. ostreatus, L. edodes and A. bisporus, were observed, respectively. Docking analysis revealed that, catechin from three mushroom isolates, chlorogenic acid from A. bisporus, kamperferol of P. ostreatus and quercetin from L. edodes, with a C-DOCKER interaction energy in the range of 22.8–37.61 (Kcal/mol) with protease compared to boceprevir ligand of 41.6 (Kcal/mol). Docking of superoxide dismutase, catalase from the three mushrooms, tyrosinase from A. bisporus showed ligand contact surface area with the protein as 252.74 Å2 while receptor contact surface area was 267.23 Å2.
Conclusion
P. ostreatus, L. edodes and A. bisporus have potential and remarkable in vitro antiviral activities against SARS-CoV-2. Quercetin from L. edodes, Kaempferol from P. ostreatus, chlorogenic acid and ascorbic acid, catechin, superoxide dismutase and catalase of the three mushrooms extracts were effectively bounded to Mpro of SARS-CoV-2 as conferred by docking analysis.
Introduction
Coronaviruses are single-strand RNA viruses that can spread between humans and animals, and they have been linked to a variety of infections.Citation1 These infections can manifest as symptoms ranging from a simple cold to far more serious illnesses and complications.Citation1 The Severe Acute Respiratory Syndrome (SARS-CoV, or SARS) and the Middle East Respiratory Syndrome (MERs-CoV, or MERs) are the highly pathogenic and have resulted in regional and pandemic outbreaks.Citation2,Citation3
Regardless, the fact that a lot of effort is being put towards vaccine development and drug repurposing, the majority of the pharmaceuticals used to treat COVID-19 symptoms are based mainly on clinical symptoms.Citation4 Many efforts have been conducted for testing various natural products for their potential anti SARS-CoV-2 activities.Citation5 To avert SARS-CoV-2 infection, many agents are being investigated in laboratory or clinical studies.Citation6 However, with these drugs, there is constantly high probability of drug non-susceptibility, particularly with viral enzyme inhibitors.Citation7 Accordingly, there is a strong demand to develop and explore novel and economic antiviral agents.Citation8 Apart from their nutritional properties, mushrooms are rich in biologically active components that exhibit merit of pharmacological actions.Citation9 Mushrooms represent an important source of natural products with high medical relevance. Polyphenols, as flavonoids, are widespread compounds in the plant kingdom. In this study, the genus Pleurotus, Lentinula and Agaricus are a common member of Basidiomycete family, which is made up of versatile group of mushrooms that are valued for their nutritious and health benefits.Citation10,Citation11 These mushroom species (known as edible mushrooms) are cholesterol free, low-fat and low sodium food products.Citation12 Besides, they are valued for their vital source of water- and fat-soluble vitamins, chitin, minerals, glucans, and proteins. Also, such mushrooms contain crucial compounds with biological activity such as phenolic acids, ergosterol, antioxidant amino acid and lovastatin.Citation13 Accordingly, the fruiting bodies and mycelia of Pleurotus species possess various therapeutic activities, such as antioxidant, anti-inflammatory, antimicrobial, antitumor and immune-regulating activities.Citation14,Citation15 Pleuran polysaccharide is used as a food supplement to enhance immunity among infants and adults.Citation16 The structural characterization of lentinan (LNT-1) was determined from L. edodes mycelia (shiitake).Citation16 Besides, LNT-1 proved to regulate the innate immune response and possess antiviral activity. The natural immunity is a vital factor for controlling the severity and prognosis of COVID-19 cases.Citation16,Citation17 Moreover, the L. edodes, P. ostreatus and A. bisporus mushrooms are some of the major dietary suppliers of Beta-glucans (β-glucans).Citation18 β-glucans, naturally occurring polysaccharides, are present as constituents of the cell wall of cereal grains, mushrooms, algae, or microbes including bacteria, fungi, and yeast. β-glucans has important role on the immune system relative to cancer treatment, infection immunity, and restoration of damaged bone marrow. McCarty and DiNicolantonio recently described the potential role of β-glucan for enhancing type 1 interferon activity against influenza and coronavirus.Citation19 As an immunomodulating agent, β-glucan acts through the activation of innate immune cells such as macrophages, dendritic cells, granulocytes, and natural killer cells; resulting in β-glucan exert multiple effects against various ailments. In a previous research conducted in our Lab, the phenolic, flavonoid and vitamin contents followed by untargeted LC/MS- TripleTOF based proteomics analysis of L. edodes, P. ostreatus and A. bisporus mushrooms were carried out.Citation20 This analysis led to identification and characterization of quercetin, kaempferol, apigenin, ascorbic acid and catechin which are vital bioactive compounds. Accordingly, in this study we aimed to in vitro investigate the anti SARS CoV-2 activities of P. ostreatus, L. edodes and A. bisporus mushroom extracts and test its anti-viral potential followed by docking studies of certain abundant small and macromolecules against SARS-CoV-2 protease (protease Mpro), the main enzyme involved in the viral replication.
Materials and Methods
Mushroom Materials
The fruiting bodies of the strain P. ostreatus, L. edodes and A. bisporus were collected from the Orman botanical garden, Cairo, Egypt. Identification was done through sequencing of the internal transcribed (ITS) region of the ribosomal DNA in our previous study with accession number MK603976, MN622787 and MZ642282, respectively.Citation20 The phylogenetic analysis was carried out as previously reported.Citation21 The mushroom fruiting bodies (500 g) were air dried and powdered and stored in aerated area at room temperature until further use. For mycelia isolation, sterile surgical blades were used to cut the fruit to expose the inner mycelia, the mycelia were then inoculated on sterile Rose Bengal Chloramphenicol agar (RBA; Sigma-Aldrich, Milan, Italy) plates and incubated at 25 °C for 7 days. The mycelia were then sub-cultured on sterile Potato Dextrose agar (PDA; Merck, Darmstadt, Germany) media every month for preservation.Citation22
Preparation of Aqueous Extracts
Aliquots (500 g) of each of the powdered mushroom isolates were macerated in distilled water for three successive days at room temperature, ultra-sonicated for one hour then filtered. Ethanol (80%) was used for yield preservation and before use a rotary evaporator was exploited to evaporate ethanol at a water bath below 50 °C. P. ostreatus, L. edodes and A. bisporus aqueous extract (500 mL) was freeze dried to yield dry extract (20 g).Citation23
Phenolic and Flavonoid Determination
High performance liquid chromatography (HPLC) Analysis was carried out for the identification and quantification of selected phenolic and flavonoid compounds according to the method of Singh et al.Citation24 In a previous research conducted in our Lab, the phenolic and flavonoid including, catechin was found in all three mushroom extracts (retention time 26.48 min), chlorogenic acid was detected in A. bisporus extract at retention time 29.61 min, and quercetin was found in L. edodes extract at retention time 56.86 min. Kaempferol and apigenin were only detected in P. ostreatus at 59.13 and 59.56 min, respectively.Citation20 There results were confirmed by including a mixture of standard phenolic and flavonoid that has shown the same retention times as those of the detected in the mushroom extract.Citation20
Vitamin Analysis
Identification and quantification the water-soluble vitamins and fat-soluble vitamin content of the mushroom extract was done according to Duffy et al.Citation25 Water- and fat-soluble vitamin contents were detected via HPLC.Citation20 Mushroom species are rich in vitamin C, riboflavin was only observed in A. bisporus. Vitamin D was encountered in the three mushroom species. Determined amounts of vitamins B3, B6 were noticed.Citation20
Untargeted LC/MS – Triple TOF-Based Proteomics Analysis
Analysis of proteomics was handled in the proteomics and metabolomics unit at Children’s Cancer Hospital 57357, Cairo, Egypt. Protein extraction and denaturation of P. ostreatus, L. edodes and A. bisporus samples were done as previously reported.Citation20 Analysis of Proteome was conducted using LC-Triple TOF-MS analysis with a “95% confidence of identification” and a false discovery rate (FDR) of < 5% and. P. ostreatus, a total of 753 proteins were identified. A total of 433 proteins were detected in L. edodes and a total of 489 proteins in A. bisporus. The MS-Triple TOF data were analyzed by ProteinPilot with the Paragon Algorithm and the spectrum of the mushroom extract analyzed by Analyst TF 1.7.1 (Sciex software) as previously conducted in our Lab.Citation20
Anti SARS CoV-2 Assay
Virus and Cell Culture
hCoV-19/Egypt/NRC-3/2020 (Accession Number on GSAID: EPI_ISL_430820) and Vero-E6 cells were obtained from Nawah Scientific, Cairo, Egypt. Vero-E6 cells were maintained on Dulbecco’s Modified Eagle’s medium (DMEM) containing Fetal Bovine Serum (FBS; 10%) (Invitrogen, Germany) and Penicillin/Streptomycin (1% pen/strep) antibiotic mixture, at 37 °C, 5% CO2 to generate monolayers 24 hours prior to infection.Citation26
Propagation of the SARS-CoV-2 Virus
One day before infection, Vero cells were seeded in cell culture flasks. Infection was done at a multiplicity of infection (MOI) and propagation was performed as previously reported.Citation26 The supernatants were gathered and centrifuged at 2500 rpm for 5 min for small particulate cell debris removal.
MTT (3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide Assay
The cytotoxic activity of the extracts was tested on VERO-E6 cells by using the MTT method with minor modifications. Briefly, in 96 well-plates, the cells were seeded (100 µL/well at a density of 3×105 cells/mL) and incubated in 5% CO2 for 24 hours at 37 °C. After 24 hours, cells were treated with the tested extract at various concentrations in triplicates (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90 and 1.95 µg/mL). The supernatants were discarded 24 hours later, and the cytotoxicity was calculated as previously reported.Citation26
Determination of Inhibitory Concentration 50 (IC50)
A 2.4×104 Vero-E6 cells inoculum, in 96-well tissue culture plates were distributed in each well and incubated at 37 °C overnight under 5% CO2 condition. The cell monolayers were washed and exposed to virus adsorption (hCoV-19/Egypt/NRC-03/2020 for 60 min at room temperature. The cell monolayers were overlaid with DMEM (100 μL) including various concentrations (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90 and 1.95 µg/mL) of the extract. The Inhibitory concentration 50 (IC50) and Selectivity index (SI) were calculated as previously determined.Citation26
Molecular Docking Analysis
Molecular docking is an essential tool in drug design and discovery used to identify interactions between the key amino acids of the target protein and different ligands.Citation27,Citation28 Hereby, in this study, Discovery Studio 4.0 Software (Waltham, MA, USA); [https://discover.3ds.com/discovery-studio-visualizer-download.com] was used to perform molecular docking simulations to estimate the binding affinity of different ligands of either small molecules or macromolecules interacting with the identified receptor. The crystal structure of SARS-CoV-2 main protease Mpro in complex with boceprevir (PDB ID: 6XQU) was downloaded from Protein Data Bank (www.rcsb.org). Target Protein was cleaned and hydrogen atoms was added to amino acid residues, to complete the missing residues. Also, water molecules was removed, and Force Field using CHARMm and MMFF94 partial charge was applied. Protease protein was prepared and minimized. The active site was defined and Boceprevir ligand was removed to be ready for docking with the new ligands.
Molecular Docking of Small Molecules
Small molecule ligands were prepared for molecular modeling simulation study where docking of kaempferol, quercetin, ascorbic acid, catechin and caffeic acid into the binding site of protease using C-DOCKER algorithm was performed in addition to Boceprevir ligand docking. The new ligands were previously prepared via “Prepare Ligand” protocol. Force Field using CHARMm and MMFF94 partial charge was applied. The resulted binding modes compared to that of Boceprevir ligand was studied to determine the biological activity and to analyze the binding affinity to the target.
Molecular Docking of Macromolecules
The Crystal Structure of Agaricus Bisporus Mushroom Tyrosinase (PDB ID: 2Y9X), ubiquitin-like protein, Rub1 (PDB ID: 1BT0), Human Nuclear Valosin containing protein Like (NVL), C-terminal AAA- ATPase domain (PDB ID: 2X8A), Human Erythrocyte Catalase Cyanide Complex (PDB ID: 1DGG), Estrogen Sulfotransferase With Inactive Cofactor Pap And Vanadate (PDB ID: 1BO6) were all downloaded from protein data bank and prepared for docking. Where clean protein was applied followed by simulation with CHARMm forcefield and MMFF94 partial charge. Preparation of protein was performed via prepare protein for ligand proteins before docking and co-crystallized ligands were removed. ZDOCK algorithm was applied on the prepared SARS-CoV-2 main protease (PDB ID: 6XQU), the Angular Step Size was set at 6 for finer conformational sampling to produce more accurate predictions. Rerank initial-stage ZDOCK predictions with detailed electrostatics, Van der Waals, and desolvation energy terms (ZRANK) was set true, number of top scoring poses to be rescored with the ZRANK algorithm was set to be 20 and finally number of top-scoring poses to cluster was set at 20.
Molecular Dynamics Simulation
Molecular Dynamics Simulation (MD simulation) is considered a valuable approach to validate the stability of the docked ligands.Citation29 The study provides information about the dynamic behavior of both the ligand and the target protein and evaluate the ligand’s key binding interactions within the docked complex.Citation29 Hereby, the ligand-protein and protein-protein complexes showing the most promising molecular docking results, were selected and enrolled within 100 ns all-atom MD simulation using Discovery Studio 4.0. CHARMm force field parameters for the investigated ligands was generated automatically. Each ligand protein complex was solvated allowing about 10 Å marginal distance. Protein residues were first adjusted to standard ionization states at normal physiological conditions (pH 7.0), and the selected complexes were then neutralized. Finally, the MD simulations were run for 100 ns under constant pressure (NPT ensemble). Data calculation including calculation of the binding free energy, root-mean-square deviation (RMSD) and root-mean-square fluctuation (RMSF) in order to investigate the possible interactions of ligands with the important protein residues and to predict stability of the docked complexes at the binding site.Citation29
Statistical Analysis
All experiments were conducted in triplicates. Statistical tests and graphical data presentation were performed using GraphPad Prism 5.01 software.
Results
Molecular Identification of the Mushroom Strain
Alignment of partial ITS spacers 1 (ITS1) and ITS spacers 4 (ITS4) ribosomal RNA gene sequence with standard reference taxa in the Blast GenBank® database with highest percentage of identity resulted in identification of the mushroom species as P. ostreatus, L. edodes and A. bisporus with accession number MK603976, MN622787 and MZ642282, respectively. The phylogenetic trees of the respected three mushrooms are displayed in Figures S1–S3 (Supplementary Materials).
Anti-SARS-CoV-2 Activities
The antiviral screening revealed that, the tested mushroom samples exhibited a promising in vitro activity against SARS-CoV-2 and has declared antiviral activities with a high SI as 4.34 and 3.44 for P. ostreatus and L. edodes while low SI of 1.5 was observed with A. bisporus for antiviral activity relative to cellular toxicity. , the recorded half cytotoxic concentration (CC50) was 170.1, 90.19 and 154 µg/mL, while the half inhibitory concentration (IC50) was 39.19, 26.17 and 103.3 µg/mL for P. ostreatus, L. edodes and A. bisporus, respectively.
Molecular Docking of Small Molecules
The 2D-Diagram of the docking study revealed that all the extracted compounds gave a C-DOCKER interaction energy in the range of 22.8–37.61 (Kcal/mol) with protease compared to boceprevir ligand of 41.6 (Kcal/mol). With similar binding mode with the essential amino acids forming hydrogen acceptor with GLU166, HIS164 and Pi interaction with MET165, LEU141 and HIS41 compared to ligand . Catechin formed a more stable complex with protease binding site.
Table 1 The CDOKER Interaction Energy of the Small Molecule Ligands
Molecular Docking of Macromolecules
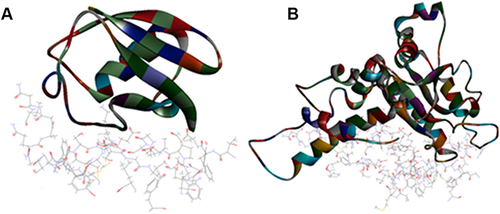
Results of the ZDOCK were visualized where top poses of the largest clusters were browsed and analyze protein interface was performed for interaction analysis of all the extracted proteins. Results showed that A. bisporus mushroom tyrosinase (PDB ID: 2Y9X) showed best interaction with protease target. P. ostreatus mushroom superoxide dismutase 1BO6 and catalase 1DGG showed best interaction with protease target. represents best z-ranked pose in best clustering (Pose 1, cluster 1) of 2Y9X, 1BO6 and 1DGG, respectively with the Mpro protein 6XQU. , showed 15 and 26 hydrogen bond interactions and 1 electrostatic interaction between amino acids chain in target and in ligand residues with 6XQU, respectively.
Table 2 Main H-Bond & Electrostatic Interactions Between Mushroom Tyrosinase (2Y9X) and Mpro of COVID-19 (6XQU) Using ZDOCK Algorithm
Table 3 Main H-Bond Interactions Between Superoxide Dismutase (1BO6) and Mpro of COVID-19 (6XQU)
Table 4 Main H-Bond Interactions Between Catalase (1DGG) and Mpro of COVID-19 (6XQU)
Figure 2 Top pose in largest z-ranked clustering of mushroom tyrosinase (ribbon shaped) (2Y9X) from A. bisporus with Mpro of COVID-19 (6XQU) (line shaped).
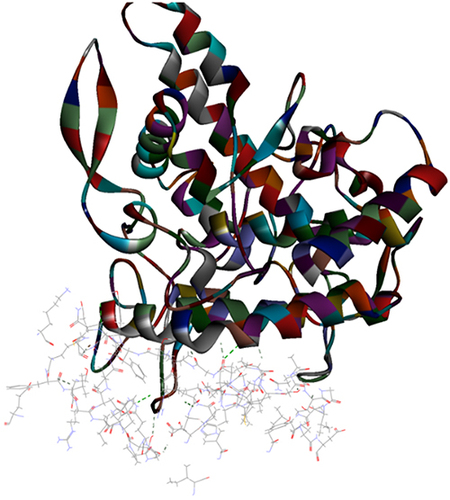
Figure 3 Top pose in largest z-ranked clustering of superoxide dismutase (ribbon shaped) (1BO6) from P. ostreatus, L. edodes and A. bisporus with Mpro of COVID-19 (6XQU) (line shaped).
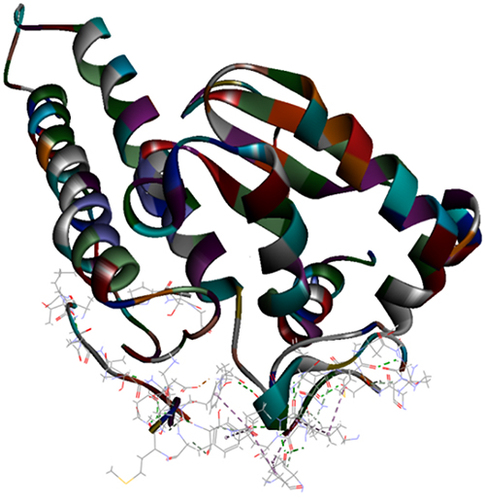
Figure 4 Top pose in largest z-ranked clustering of catalase (1DGG) from P. ostreatus, L. edodes and A. bisporus with Mpro of COVID-19 (6XQU) (line shaped).
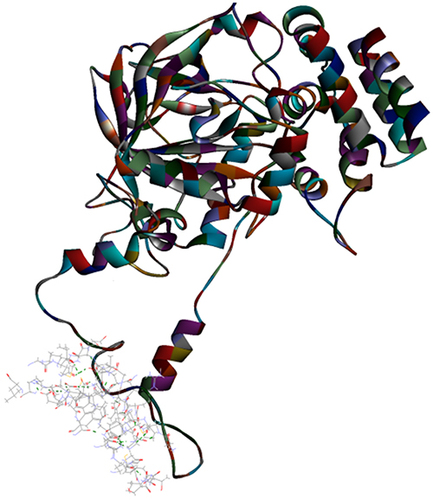
The Ligand Contact Surface Area with the protein was 252.74 Å2 while Receptor Contact Surface Area was 267.23 Å2, which confirms good, and predicted Protein-Protein interaction. However, results showed no interaction of ubiquitin-like protein such as Rub1 (PDB ID: 1BT0) and valosin (PDB ID: 2X8A). and . ZDOCK results showed Z score and Z Rank of the different protein ligands and explained the different interaction of the different ligand proteins to the target. Where 2Y9X showed the highest results and 1BT0 showed the lowest results .
Table 5 ZDOCK Results for the Different Ligand Proteins
Molecular Dynamics Simulation
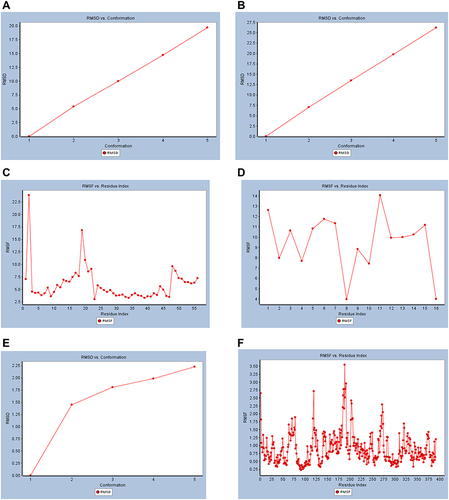
In the presented MD simulation, the best small molecule Catechin and the most promising macromolecule mushroom tyrosinase (PDB ID: 2Y9X) were subjected to MD simulation study to determine their dynamic properties and confirm the docking results. Screenshot samples of the uncropped results are depicted in the supplementary material for further review (Figure S4, Supplementary File). Total energy versus time dynamics Plots of the docked ligand catechin was presented and compared to that of free 6XQU and undocked catechin. Also, the best macromolecule 2Y9X total energy versus time dynamics Plot was presented in . Where the docked catechin results showed stability after docking by −31.35 and −31.45 kcal/mol at time intervals 22 and 24, respectively. Compared to free undocked energy of 7.400, 7.550 kcal/mol and −1712, −1413 kcal/mol for catechin and 6XQU at time intervals 22 and 24, respectively. While 2Y9X showed −12,720 and −12,730 at time intervals 22 and 24, respectively (). RMSD results of 6XQU before and after docking showed great similarity over the five different conformations. While RMSF versus Residue Index results indicated the stability of the complex at the binding site showing stability of 6QXU complex after docking with catechin (14.0) compared to the free 6QXU before docking (22.5). While 2Y9X showed comparable RMSD results with RMSF value of (3.5) showing good stability results ().
Figure 6 Total energy versus time dynamics plot for (A) Mpro of COVID-19 (6XQU), (B) Mushroom tyrosinase (2Y9X), (C) undocked catechin and (D) Catechin complexed with 6XQU after docking.
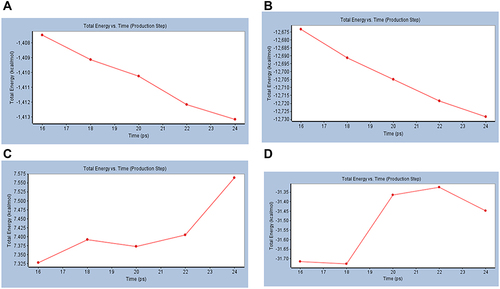
Figure 7 Dynamic simulation of (A) RMSD plot of Mpro of COVID-19 (6XQU) before docking, (B) RMSD plot of (6XQU) after catechin docking, (C) RMSF plot of 6QXU before docking, (D) RMSF plot of 6QXU after catechin docking, (E) RMSD plot of mushroom tyrosinase (2Y9X), and (F) RMSF plot of mushroom tyrosinase (2Y9X).
Discussion
In the current circumstances, with a pandemic that appears to be incurable and candidate medications still in the testing stage, other prophylactic and therapeutic principles must be considered. COVID-19 impose a life-threatening hazard to public health, in the light of absence of clinically approved therapy.Citation26 Furthermore, currently used treatment protocols are too expensive, sometimes unavailable; therefore, low-cost alternatives are urgently required. One potential candidate is prophylaxis and/or therapy using the medicinal mushrooms since they are rich in various biologically active metabolites and at lower cost.Citation30
The Agaricomycotina among the Basidiomycetes mushrooms, P. ostreatus is a well-known medicinal mushroom that has been used worldwide for a range of diseases in traditional medicine.Citation31 The biologically active metabolites obtained from mushrooms have been proved to have potential antiviral activities.Citation32 It was previously reported that mushroom compounds diminish viral infection by several mechanisms.Citation9 They are rich in Bioactive compounds with potential antiviral activities including, polysaccharides, carbohydrate-binding proteins, peptides, enzymes (laccase and tyrosinase), several other compounds.Citation33 It was previously confirmed that mushrooms can suppress viral entry, replication, in addition to their interfering properties in the protein expression.Citation8 It was previously reported that certain mushroom proteins can enhance immunity against many viruses particularly those of relevant medical importance.Citation8 Interestingly, polysaccharide, lectin, lentin, and laccase from certain mushrooms showed IC50 in the range of 0.1–2.2 µM against HIV-1.Citation34,Citation35
The current study investigated the antiviral activity of P. ostreatus, and L. edodes showed a promising cytotoxic activity against COVID −19 with IC50 39.19 µg/mL and 26.17 µg/mL, respectively. However, A. bisporus showed a moderate activity with IC50 103.3 µg/mL. Evaluation of the antiviral related activity to the biologically active chemicals was evidenced by in silico molecular modeling. Three small molecules; catechin, kaempferol and ascorbic acid, were found to bind to the Mpro of SARS-CoV-2 which is essential for viral replication. Another highly bioactive catechin, Epigallocatechin gallate (EGCG) is known to prevent influenza A and B virus infections in Madin-Darby canine kidney cells.Citation36 Furushima et al also reported that tea catechins repressed influenza viral infections by many mechanisms.Citation31 It was also previously documented, that catechins showed promising activities against some cold virusesCitation31 as well as immunomodulatory activities against viral infection.Citation36
Kaempferol was also found to interact with the binding site Mpro of SARS-CoV-2. Huang et al 2020Citation34 also reported the anti-SARS-CoV-2 activity of kaempferol based on the inactivation of protein kinase B, phosphorylation of protein kinase and blocking the 3a channel in SARS-CoV infected cells. In addition to, its anti-inflammatory and immunomodulatory effects.Citation37
As it was reported in our findings that ascorbic acid was the only vitamin found in high amount relative to other vitamins in the mushroom extract.Citation36 There is a prove that ascorbic acid and quercetin co-administration employs a synergistic antiviral activity.Citation32 The use of vitamin C and quercetin combination was recently reported for prophylaxis and management of COVID-19 patients in addition to remdesivir or convalescent plasma.Citation38 During infection, vitamin C is concentrated within macrophages, is essential for neutrophil killing, is responsible for T cell maturation, in addition to promoting phagocytosis and apoptosis of spent neutrophils.Citation39
The obtained molecular docking result revealed that SARS-CoV-2 M pro is considered a key enzyme with a vital role in viral replication. This makes it an appealing target for drug invention of new COVID-19 candidates. The extracted compounds from mushroom showed antiviral activity with promising anti COVID results after molecular simulation study via docking on 6XQU. C-DOCKER protocol was applied on small molecules while ZDOCK was used for protein- protein docking on the extracted proteins. The findings indicated the highest binding interaction for quercetin on protease with (E= −39.66 Kcal/mol) showing interaction with the key amino acids via 2 HBA with GLU166, and 1 HBA with each of GLY143 & CYS145. In addition to Pi-alkyl interaction with MET165 compared to ligand (E= −41.6 Kcal/mol). While catechin (E= −37.61 Kcal/mol) showed an additional HBA interaction with GLU166 compared to ligand and an additional Pi- alkyl interaction with MET165 compared to ligand. Caffeic acid (E= −24.9 Kcal/mol) showed 1 HBA with GLY143 & CYS145 and an additional Pi- alkyl interaction with MET165. Ascorbic acid (E= −22.8 Kcal/mol) showed 2 HBA with GLU166 and 1HBA with HIS164. Other studies reported that aqueous solvent was employed to prepare raw extracts of V. vinifera leaves and its chemical profile was analyzed through HPLC-MS in negative ionization mode. This analysis led to the identification of 35 flavonoids, most of which were derivatives of quercetin. Others included derivatives of luteolin, kaempferol, apigenin, isorhamnetin, myricetin, chrysoeriol, biochanin, isookanin, and scutellarein. Furthermore, the antiviral potential of the extract was tested against HSV-1 and SARS-CoV-2 with very interesting results, showing the capability of flavonoids to inhibit SARS-CoV-2 for the first time. Considering the current pandemic emergency, our results represent a promising resource for pharmaceutical industrial applications.
ZDOCK algorithm was used to predict protein-protein interaction between the extracted proteins and 6XQU. The “Analyze Protein Interface” Report from discovery studio after ZDOCK showed that Ligand Contact Surface Area with the protein as 252.74 Å2 while Receptor Contact Surface Area was 267.23 Å2. These results confirmed good interaction between the target protease protein and the docked ligand protein. Result visualization indicated that mushroom tyrosinase (2Y9X) revealed the best interaction via hydrogen bonding with the essential amino acids in the binding site (Z Rank= −121.32 kcal/mol). The interaction showed 13 hydrogen bonds and 1 electrostatic interaction between amino acids chain in target and in Ligand residues. While valosin (2X8A) and ubiquitin-like protein, Rub1 (1BT0) showed no binding interaction with protease interface (Z Rank= −115.80, −84.7 kcal/mol, respectively). On the other hand, catalase (PDB ID: 1DGG) and superoxide dismutase (PDB ID: 1BO6) showed good binding interaction at the protein interface (Z Rank= −130.59, −117.84 kcal/mol, respectively). MD simulation study on the two most promising docked ligands (catechin and 2Y9X) showed that low free binding energy versus time results after ligand binding to 6XQU confirming stable docked complexes with the target 6XQU compared to the free undocked form. Generally, RMSD calculates the deviation extent to the respective reference structure. Hereby, the low RMSD values are correlated to significant stability, relative to the conformation of the docked molecule. So, ligands with low RMSD values, in their respective ligand–protein complex, would reveal good ligand fitting within the targeted pocket via the adopted MD simulation time-frames.Citation29 RMSF results versus residue index showed good stability of the complexed binding site. Where it defines the fluctuations contributed to protein individual residues with the ligand/protein complex. RMSF evaluates the time evolution of the mean deviation for every residue relative to its reference position.
Complement Drugs or compounds with special properties on viral protease inhibitors have been believed as potential medications against CoVs.Citation40–42 It was previously reported that, the viral proteases can identify the specific sequences of amino acids in their targets and split the peptide bond.Citation41,Citation42 Fungal metabolites potential activities as protease inhibitors have gained the attention of many recent studies.Citation22–24 In our study, the effects of the extracted edible mushrooms on COVID-19 were conducted in vitro using Vero-E6 cells followed by molecular docking analysis of different small molecules using CDOKER and macromolecules using ZDOCK protocol against Mpro of COVID-19.
Conclusion
Aqueous extracts of P. ostreatus, L. edodes and A. bisporus showed in vitro antiviral activities against SARS-CoV-2 with a high selectivity to the viral infected cells. Small fungal biomolecules including, quercetin from L. edodes, kaempferol from P. ostreatus, chlorogenic acid from A. bisporus, ascorbic acid and catechin of the three mushrooms extracts and macromolecules including, tyrosinase from A. bisporus and superoxide dismutase and catalase of the three fungal extracts were able to effectively bind to the Mpro of SARS-CoV-2 as indicated by in silico docking analysis. Further, in vivo investigations are recommended to estimate the antiviral activities of the respective active metabolites for the potential use in humans.
Data Sharing Statement
All the data supporting the findings are included in the manuscript and Supplementary File.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
Acknowledgments
The authors extend their gratitude to the Microbiology and Immunology Department, Faculty of Pharmacy, Ain Shams University, and Ahram Canadian University (ACU) Cairo, Egypt, for the great help and support in the current study. The authors are grateful for Dr. Asmaa A Mandour, Pharmaceutical Chemistry Department, Faculty of Pharmacy, Future University in Egypt (FUE), Cairo, 11835, Egypt, for docking analysis. The authors express their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the research groups program under grant number R.G.P.2/111/41. The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education, in Saudi Arabia, for funding this research work through the project number: IFP-KKU-2020/10.
References
- Cauchemez S, Van Kerkhove MD, Riley S, Donnelly CA, Fraser C, Ferguson NM. Transmission scenarios for middle east respiratory syndrome coronavirus (MERS-CoV) and how to tell them apart. Euro Surveill. 2013;18(24):20503. doi:10.2807/ese.18.24.20503-en
- Cui J, Shen HM, Lim LHK. The role of autophagy in liver cancer: crosstalk in signaling pathways and potential therapeutic targets. Pharmaceuticals. 2020;13(12):432. doi:10.3390/ph13120432
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
- Xu J, Zhao S, Teng T, et al. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi:10.3390/v12020244
- Lim J, Jeon S, Shin HY, et al. The author’s response: case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(7):e89. doi:10.3346/jkms.2020.35.e89
- Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi:10.1016/S1473-3099(20
- Lin LT, Hsu WC, Lin CC. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24–35. doi:10.4103/2225-4110.124335
- Akram M, Tahir IM, Shah SMA, et al. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res. 2018;32(5):811–822. doi:10.1002/ptr.6024
- Bellettini MB, Fiorda FA, Maieves HA, et al. Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci. 2019;26(4):633–646. doi:10.1016/j.sjbs.2016.12.005
- Cardwell G, Bornman JF, James AP, Black LJ. A review of mushrooms as a potential source of dietary vitamin D. Nutrients. 2018;10:10. doi:10.3390/nu10101498
- Koutrotsios G, Tagkouli D, Bekiaris G, et al. Enhancing the nutritional and functional properties of Pleurotus citrinopileatus mushrooms through the exploitation of winery and olive mill wastes. Food Chem. 2022;370:131022. doi:10.1016/j.foodchem.2021.131022
- Tsiantas K, Tsiaka T, Koutrotsios G, et al. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: assets and challenges of different analytical approaches. Molecules. 2021;26(7):1832. doi:10.3390/molecules26071832
- Lo YC, Lin SY, Ulziijargal E, et al. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int J Med Mushrooms. 2012;14(4):357–363. doi:10.1615/intjmedmushr.v14.i4.30
- Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2(3):285–299. doi:10.1093/ecam/neh107
- Majtan J. Pleuran (β-glucan from Pleurotus ostreatus): an effective nutritional supplement against upper respiratory tract infections? Med Sport Sci. 2012;59:57–61. doi:10.1159/000341967
- Ren G, Xu L, Lu T, Yin J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int J Biol Macromol. 2018;115:1202–1210. doi:10.1016/j.ijbiomac.2018.04.132
- Rop O, Mlcek J, Jurikova T. Beta-glucans in higher fungi and their health effects. Nutr Rev. 2009;67(11):624–631. doi:10.1111/j.1753-4887.2009.00230.x
- McCarty MF, DiNicolantonio JJ. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. 2020;63(3):383–385. doi:10.1016/j.pcad.2020.02.007
- Elhusseiny SM, El-Mahdy TS, Awad MF, et al. Proteome Analysis and in vitro antiviral, anticancer and antioxidant capacities of the aqueous extracts of Lentinula edodes and Pleurotus ostreatus edible mushrooms. Molecules. 2021;26(15):4623. doi:10.3390/molecules26154623
- Mostafa A, Kandeil A, Elshaier Y AMM, et al. FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals. 2020;13(12):443. doi:10.3390/ph13120443
- Krupodorova T, Rybalko S, Barshteyn V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol Sin. 2014;29(5):284–290. doi:10.1007/s12250-014-3486-y
- Hetland G, Johnson E, Bernardshaw SV, Grinde B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scand J Immunol. 2021;93(1):e12937. doi:10.1111/sji.12937
- Facchini JM, Alves EP, Aguilera C, et al. Antitumor activity of Pleurotus ostreatus polysaccharide fractions on Ehrlich tumor and sarcoma 180. Int J Biol Macromol. 2014;68:72–77. doi:10.1016/j.ijbiomac.2014.04.033
- Seo D, Choi C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: a review. Viruses. 2021;13:350. doi:10.3390/v13020350
- Li X, Wang Z, Wang L, Walid E, Zhang H. In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int J Mol Sci. 2012;13(5):5801–5817. doi:10.3390/ijms13055801
- Borgio JF, Alsuwat HS, Al Otaibi WM, et al. State-of-The-art tools unveil potent drug targets amongst clinically approved drugs to inhibit helicase in SARS-CoV-2. Arch Med Sci. 2020;16(3):508. doi:10.5114/aoms.2020.94567
- Azeez SA, Alhashim ZG, Al Otaibi WM, et al. State-of-The-art tools to identify druggable protein ligand of SARS-CoV-2. Arch Med Sci. 2020;16(3):497. doi:10.5114/aoms.2020.94046
- Al-Karmalawy AA, Dahab MA, Metwaly AM, et al. Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Front Chem. 2021;9. doi:10.3389/fchem.2021.661230
- Wang HX, Ng TB. Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochem Biophys Res Commun. 2000;276(2):587–593. doi:10.1006/bbrc.2000.3540
- Furushima D, Ide K, Yamada H. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules. 2018;23(7):1795. doi:10.3390/molecules23071795
- Huang YF, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19). Pharmacol Res. 2020;158:104939. doi:10.1016/j.phrs.2020.104939
- Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin c: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020;11:1451. doi:10.3389/fimmu.2020.01451
- Anderson O, Beckett J, Briggs CC, et al. An investigation of the antileishmanial properties of semi-synthetic saponins. RSC Med Chem. 2020;11(7):833–842. doi:10.1039/d0md00123f
- Puente XS, Sánchez LM, Overall CM, López-Otín C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4(7):544–558. doi:10.1038/nrg1111
- Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9(9):690–701. doi:10.1038/nrd3053
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi:10.1093/molbev/msy096
- Giunco AJ, Paz MFD, Fonseca GG. Development and evaluation of low-carb cakes produced from green bocaiuva pulp enriched with Pleurotus ostreatus. J Culinary Sci Techno. 2021;1–13. doi:10.1080/15428052.2021.1929637
- Barbosa JR, Freitas MM, Oliveira LC, et al. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO(2). Food Chem. 2020;330:127173. doi:10.1016/j.foodchem.2020.127173
- Singh A, Sharma S, Singh B. Influence of grain activation conditions on functional characteristics of brown rice flour. Food Sci Technol Int. 2017;23(6):500–512. doi:10.1177/1082013217704327
- Duffy SK, Kelly AK, Rajauria G, et al. The use of synthetic and natural vitamin D sources in pig diets to improve meat quality and vitamin D content. Meat Sci. 2018;143:60–68. doi:10.1016/j.meatsci.2018.04.014
- Rangsinth P, Sillapachaiyaporn C, Nilkhet S, Tencomnao T, Ung AT, Chuchawankul S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: an in silico approach. J Tradit Complement Med. 2021;11(2):158–172. doi:10.1016/j.jtcme.2020.12.002