Abstract
Cephalosporins and azithromycin are clinical antibiotics used to treat infections. Co-resistance to cephalosporins and azithromycin has been observed in some Enterobacterales, but it has only rarely been reported in Vibrio species. In this study, we isolated a cephalosporin- and azithromycin-resistant V. furnissii strain, VFN3, from hospital sewage. Whole-genome sequencing results showed that the strain VFN3 possesses an IncA/C2 plasmid, pVFN3-blaOXA-193K. This conjugative plasmid carries several clinically relevant drug resistance genes, including mph(A) and blaOXA-1. We also found that in the strain VFN3, mph(A) and blaOXA-1 are surrounded by insertion sequences and class I integrons, respectively. These data suggest that mobile elements mediate the transfer of mph(A) and blaOXA-1. This is the first reported Vibrio species that possesses an mph(A)- and blaOXA-1-bearing conjugative plasmid. The emergence of this conjugative multi-drug-resistance plasmid is of great concern to public health.
Short Report
Cephalosporins and azithromycin are antimicrobial drugs commonly used to fight diseases caused by Gram-positive and Gram-negative pathogens, such as respiratory illness, skin infections, and diarrhea.Citation1,Citation2 However, a few bacteria have evolved to withstand both cephalosporin and azithromycin. For example, Aeromonas sp., Escherichia coli, and Salmonella enterica serotype Typhimurium strains encoding both mph(A) and blaOXA-1 have been reported.Citation3–6 These two drug resistance genes have also been observed in clinical Vibrio fluvialis isolates. These isolates also possess other drug resistance genes, such as blaOXA-7, blaCTX-M-3, and blaNDM-1, but the genetic backgrounds of these antibiotic resistance genes are unclear.Citation7 V. furnissii is an emerging human pathogen. This marine bacterium can lead to acute gastroenteritis, bacteremia, cellulitis, and skin lesion.Citation8–10 Hospital sewage is generally regarded as a reservoir of antibiotic-resistant bacteria, and the concentration of azithromycin in hospital sewage could be high.Citation11 However, little information about the prevalence of azithromycin-resistant Vibrio species from hospital sewage is available. In this study, we report one V. furnissii isolate recovered from hospital sewage in Zhuhai, a coastal city in China, carrying both mph(A) and blaOXA-1, which are azithromycin and cephalosporin resistance genes, respectively.
Zhuhai People’s hospital has one main hospital and three branch hospitals. In order to find out the distribution of azithromycin-resistant bacterial species in each hospital, 8 hospital sewage samples (2 samples/site) were collected from the main hospital area and its three branch hospitals within Zhuhai in April 2021. Then, 5 mL of each sample was added to 95 mL Luria-Bertani (LB) broth in a 250-mL flask with 100 mg/L azithromycin and incubated at 37°C overnight (X). Individual 100 μL aliquots of bacterial culture from each flask were spread on LB agar plates with 100 mg/L azithromycin. We used 100 mg/L azithromycin was due to the fact that a minimal inhibitory concentration (MIC) ≥32 mg/L has been proposed as the azithromycin resistance breakpoints, and a previous study showed that some diarrhoeagenic E. coli strains can withstand high concentration azithromycin (MIC >100 mg/L).Citation12 After incubation at 37°C overnight, colonies grown on the plates were obtained. To identify the azithromycin-resistant bacterial species, 16S rRNA genes were amplified and sequenced. Most of the azithromycin-resistant isolates were identified as Enterobacterales. However, one strain, VFN3, was identified as Vibrio species. It was recovered from the southern branch of Zhuhai People’s Hospital sewage, which is less than 1.0 km from the coast. Given that azithromycin-resistant Enterobacterales species have been reportedCitation3,Citation4,Citation6,Citation12 and azithromycin-resistant Vibrio species are rare, we focused on this strain in the following analysis. First, the broth microdilution method was used for antimicrobial susceptibility testing, and the results were interpreted based on the CLSI guidelines (for other antibiotics)Citation13 and a published paper (for azithromycin).Citation12 The MIC data showed the strain VFN3 to be resistant to ampicillin, azithromycin, ceftiofur, ceftazidime, ciprofloxacin, chloramphenicol, ceftriaxone, gentamicin, kanamycin, streptomycin, and tetracycline but susceptible to tigecycline, meropenem, aztreonam and colistin (). Next, to investigate the mechanism by which strain VFN3 is resistant to azithromycin, its genome was sequenced. The short-read and long-read sequencing data were obtained using Illumina HiSeq 2500 and an Oxford Nanopore MinION sequencer. To obtain the complete genome sequences of strain VNF3, both short-read and long-read data were combined to perform hybrid de novo assembly with Unicycler v0.4.4.Citation14 RAST server (https://rast.nmpdr.org) was used for genome annotation. ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/) was used to identify potential antibiotic resistance genes. PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/) was utilized to screen the plasmid replicon type. BLAST Ring Image Generator (BRIG) and Easyfig were used for sequence comparisons and visualizations.Citation15,Citation16
Table 1 Minimal Inhibitory Concentrations (MICs) of Strains Against Different Antimicrobials Used in This Study
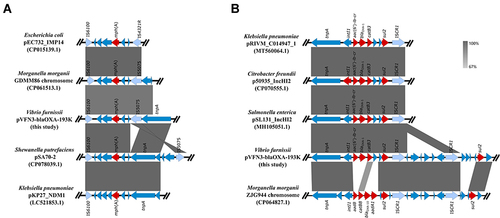
Strain VFN3 harbors two chromosomes and one plasmid. Based on Ribosomal MLST (https://pubmlst.org/species-id) with whole-genome sequences, strain VFN3 was identified as V. furnissii. In strain VFN3, chromosome I is 3,404,211 bp with 50.50% GC content and chromosome II is 1,588,410 bp with 50.90% GC content. No drug resistance gene was detected in the chromosomes. However, we found an IncA/C2 replicon-type plasmid, pVFN3-blaOXA-193K (193,896 bp and 52.79%GC content), carrying several antibiotic resistance genes, including tet(D), floR, aac(3), sul2, sul1, blaOXA-1, mph(A), aac(6’)lb-cr, and catB3 (). To the best of our knowledge, pVFN3-blaOXA-193K is the first reported plasmid that carries both mph(A) and blaOXA-1 in Vibrio species. BLAST analysis showed that pVFN3-blaOXA-193K shares 99% identity with pEC-13-49-NDM-1 at 80% coverage, pKP-16-57-NDM-1 at 80% coverage, pPV835TEM24 at 77% coverage, and pVC1447 at 77% coverage, hosted by E. coli, Klebsiella pneumoniae, Proteus vulgaris and V. cholerae, respectively (). Unlike those of pVFN3-blaOXA-193K, pEC-13-49-NDM-1 and pKP-16-57-NDM-1, neither pPV835TEM24 nor pVC1447 encodes mph(A). Further analysis showed that mph(A) in pVFN3-blaOXA-193K is surrounded by insertion sequences, IS6100 and IS5075. Similar genetic structures were observed in pEC732_IMP14, pSA70-2, pKP27_NDM1 and the chromosome of Morganella morganii strain GDMM86 (). These data indicated that the insertion sequences could mediate mph(A) transfer. We also found that, in pVFN3-blaOXA-193K, blaOXA-1 is located in a class I integron with the structure tnpA-intI1-aac(6’)lb-cr-blaOXA-1-catB3-arr3-qacEΔ1-sul1-ISCR1. Similar genetic structures were observed in pRIVM_C014947_1, p50935_IncHI2, and pSL131_IncHI2, hosted by K. pneumoniae, Citrobacter freundii, and S. enterica, respectively. In the chromosome of M. morganii strain ZJG944, blaOXA-1 was also detected, and a similar genetic structure with that of pVFN3-blaOXA-193K was observed (). These results suggest that class I integron facilitates the spread of blaOXA-1.
Figure 1 Circular comparisons of pVFN3-blaOXA-193K and related plasmids, pEC-13-49-NDM-1, pKP-16-57-NDM-1, pPV835TEM24, and pVC1447, available in the NCBI database (https://www.ncbi.nlm.nih.gov/). The outermost circle denotes pVFN3-blaOXA-193K, with arrows indicating coding genes.
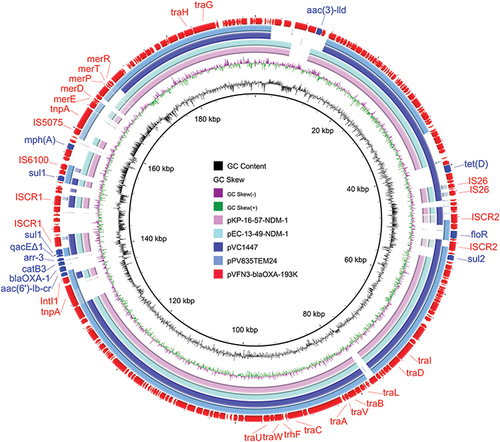
Figure 2 Sequence comparisons of mph(A) and blaOXA-1 genetic environments. (A) Linear comparisons of the mph(A) genetic environment in pVFN3-blaOXA-193K with other related mph(A) encoding bacteria. (B) Linear comparisons of the blaOXA-1 genetic environment in pVFN3-blaOXA-193K with other related blaOXA-1 encoding bacteria. Red arrows indicate the resistance genes.
A conjugation assay was performed to test the transferability of pVFN3-blaOXA-193K. Briefly, strain VFN3 and E. coli C600 (rifampin resistance) were incubated in LB broth at 37°C overnight. Next, 100 μL of strain VFN3 and 500 μL of E. coli C600 were mixed. After centrifugation for 2 min at 12,000 rpm, cells were resuspended in 100 μL LB broth, and co-cultures were incubated at 37°C for 18 h. Transconjugants were selected using Eosin-Methylene Blue agar plates with 100 mg/L azithromycin plus 200 mg/L rifampicin. The transconjugant was confirmed by PCR. Conjugation assay revealed that pVFN3-blaOXA-193K can transfer to E. coli C600, rendering the transconjugant resistant to many drugs ().
To determine whether other V. furnissii strains possess azithromycin resistance gene, we downloaded all genome sequences of V. furnissii strains deposited in NCBI in April 2022. Using ResFinder, we found that no V. furnissii strain encodes azithromycin resistance gene except strain VFN3.
In conclusion, this study isolated the azithromycin- and cephalosporin-resistant V. furnissii strain VFN3 from hospital sewage. This strain harbors an ≈193kb conjugative plasmid, pVFN3-blaOXA-193K. In this IncA/C2 plasmid, mph(A), blaOXA-1, and other antibiotic resistance genes were identified. This multi-drug resistant pathogen may pose a threat to public health. Further research is needed to investigate the prevalence of pathogens carrying multi-drug-resistance plasmids in hospital sewage.
Data Sharing Statement
The complete sequences of strain VFN3 were submitted to the NCBI database with accession numbers CP089603-CP08960.
Disclosure
The authors declare no competing interests in this work.
Acknowledgments
We would like to thank Dr. Cong Hu in Zhuhai People’s Hospital for bacteria isolation. This work was supported by the National Natural Science Foundation of China (81650003).
References
- Gomes C, Martinez-Puchol S, Palma N, et al. Macrolide resistance mechanisms in Enterobacteriaceae: focus on azithromycin. Crit Rev Microbiol. 2017;43:1–30. doi:10.3109/1040841X.2015.1136261
- Arizpe A, Reveles KR, Patel SD, Aitken SL. Updates in the management of cephalosporin-resistant gram-negative bacteria. Curr Infect Dis Rep. 2016;18(12):39. doi:10.1007/s11908-016-0552-7
- Woodford N, Carattoli A, Karisik E, et al. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25: H4-ST131clone. Antimicrob Agent Chemother. 2009;53:4472–4482. doi:10.1128/AAC.00688-09
- Leao C, Clemente L, Moura L, et al. Emergence and clonal spread of CTX-M-65-Producing Escherichia coli from retail meat in Portugal. Front Microbiol. 2021;12:653585. doi:10.3389/fmicb.2021.653595
- Marti E, Balcazar JL. Multidrug resistance-encoding plasmid from Aeromonas sp. strain P2GI. Clin Microbiol Infect. 2012;18:e366–e368. doi:10.1111/j.1469-0691.2012.03935.x
- Dong N, Li Y, Zhao J, et al. The phenotypic and molecular characteristics of antimicrobial resistance of Salmonella enterica subsp. enterica serovar Typhimurium in Henan Province, China. BMC Infect Dis. 2020;20:511. doi:10.1186/s12879-020-05203-3
- Chowdhury G, Ramamurthy T, Ghosh A, Dutta S, Takahashi E, Mukhopadhyay A. Emergence of azithromycin resistance mediated by phosphotransferase-encoding mph(A) in diarrheagenic Vibrio fluvialis. mSphere. 2019;4:e00215–e00219. doi:10.1128/mSphere.00215-19
- Brenner D, Hickman-Brenner F, Lee J, et al. Vibrio furnissii (formerly aerogenic biogroup of Vibrio fluvialis), a new species isolated from human feces and the environment. J Clin Microbiol. 1983;18:816–824. doi:10.1128/jcm.18.4.816-824.1983
- Derber C, Coudron P, Tarr C, et al. Vibrio furnissii: an unusual cause of bacteremia and skin lesions after ingestion of seafood. J Clin Microbiol. 2011;49:2348–2349. doi:10.1128/JCM.00092-11
- Hashimoto T, Takaya S, Kutsuna S, et al. A case report of Vibrio furnissii bacteremia and cellulitis in a malnourished patient without an apparent site of entry. J Infect Chemother. 2018;24:65–67. doi:10.1016/j.jiac.2017.08.016
- Omuferen L, Maseko B, Olowoyo J. Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: current knowledge between 2010 and 2019. Environ Monit Assess. 2022;194:306. doi:10.1007/s10661-022-09846-4
- Gomes C, Ruiz-Roldán L, Mateu J, Ochoa TJ, Ruiz J. Azithromycin resistance levels and mechanisms in Escherichia coli. Sci Rep. 2019;9:6089. doi:10.1038/s41598-019-42423-3
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 31th ed. Wayne, PA: CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021.
- Wick R, Judd M, Gorrie L, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi:10.1371/journal.pcbi.1005595
- Alikhan F, Petty K, BenZakour L, Beatson A. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom. 2011;12:402. doi:10.1186/1471-2164-12-402
- Sullivan J, Petty K, Beatson A. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi:10.1093/bioinformatics/btr039
