Abstract
Background
The world is currently facing a pandemic of Coronavirus Disease 2019 (Covid-19). It has caused significant morbidity and mortality. So far little is known about recovery time (prolonged hospital stay) from Covid-19 and its determinants in Ethiopia as well as in the study area. Therefore, the aim of this study was to determine time to recovery from Covid-19, and identify predictors of time to recovery among patients admitted to treatment centers of Southern Nations Nationalities and Peoples Region (SNNPR).
Methods and Materials
A facility-based retrospective cohort study was conducted among Covid-19 patients admitted to care centers of SNNPR from May 30, 2020 to October 15, 2021. A sample of 845 patients was included in the study. Summarization of the data was done using mean (standard deviation) and median (inter quartile range). Kaplan–Meier Survival Curve was used to estimate recovery time from Covid-19 and the independent effects of covariates on recovery time was analyzed using multivariable Cox-proportional hazard model.
Results
The incidence density of recovery was 8.24 per 100 person-days (95% CI: 7.67, 8.85). The overall median recovery time was 10 days (IQR: 8–16 days). Critical stage of Covid-19 (aHR = 0.19, 95% CI: 0.12, 0.29), severe stage of Covid-19 (aHR = 0.40, 95% CI: 0.29, 0.56), mechanical ventilation (aHR = 0.20, 95% CI: 0.073, 0.56) and treatment center (aHR = 0.68, 95% CI: 0.51, 0.90) were significant predictors of recovery rate among Covid-19 patients.
Conclusion
The median time to recovery from Covid-19 was relatively short. The incidence density of recovery was 8.24 per 100 person-days. The hazard of recovery was lower for patients at higher levels of Covid-19 severity and for patients in need of mechanical ventilation. Early identification of severity levels of the patients is required at the time of admission. Special attention, critical follow–up and management is warranted for patients at higher levels of Covid-19 severity.
Introduction
The world is currently facing a pandemic of Coronavirus Disease 2019 (Covid-19). Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2) was identified as the cause of Covid-19 in December 2019 in Wuhan, the Hubei province of China.Citation1,Citation2 The coronavirus infection primarily targets the human respiratory system.Citation3 Patients may be asymptomatic, have atypical symptoms such as hyposmia, nasal congestion, rhinorrhea, sputum, abdominal discomfort, vomiting, and diarrhea, or have classic symptoms suc as fever, headache, dry cough, and dyspnea.Citation4 Based on severity, Covid-19 is classified as mild, moderate, severe and critical illness.Citation5 Despite the measures established to control the outbreak,Citation6 SARS-CoV-2 has spread globally, and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020.Citation7 As of May 20, 2022, there had been 521,920,560 confirmed Covid-19 cases and 6,274,323 deaths all over the world since the first case was reported. The continent with the most cases (219,393,358) was Europe followed by America (155,496,306). Africa has the sixth highest number of confirmed cases (8,935,659) and deaths (172,260) according to WHO regions as of May 20, 2022.Citation8
The outcome of disease varies from uneventful recovery to multi-organ dysfunction (including respiratory failure, septic shock, acute cardiac damage, or acute renal failure), psychological trauma as well as death depending on characteristics of individual patients.Citation9–11 It kills 5 out of every 100 patients.Citation8 Covid-19 has the potential to have catastrophic economic implications. On average, hospitals lose thousands of dollars per Covid-19 patient due to length of stay and resource intensity.Citation12 In individuals hospitalized with severe COVID-19, prolonged symptom duration and impairment are prevalent.Citation13
There is no specific Covid-19 treatment other than infection management and supportive care. Aside from case fatality, prolonged hospitalization is a well-known predictor of disease severity care.Citation1,Citation2
Older age, the existence of pre-existing comorbidities, gender, smoking history, body temperature, respiratory rate, and oxygen saturation at admission, body mass index (BMI), and aberrant significant laboratory indicators were all found as possible predictors for recovery.Citation1,Citation9,Citation14 There is evidence that the effects of the aforementioned factors vary from location to location, implying that there is still no well-established fact about the disease. Furthermore, our country’s and Africa’s underlying population demographics, behaviors, economic status, healthcare system, and endemic disease patterns differ from other parts of the world, making it challenging to forecast and generalize conclusions based on others’ setup.Citation3
The first incidence of Covid-19 in Ethiopia was verified on March 13, 2020. The Ethiopian government has taken several public health measures to combat the Covid-19 threat, including raising awareness, closing schools, restricting large gatherings and movement of people, and preparing and equipping treatment centers to treat Covid-19 patients and isolate contacts with confirmed cases.Citation8
Despite the aforementioned safeguards, the unexpected rapid spread of the Covid-19 pandemic in Ethiopia, as well as around the world, is placing a significant overload on hospitals, intensive care units (ICUs), and medical resources. The number of new cases, the severity of the disease, and the mortality rate are all on the rise. The pattern may be worsening as a result of our society’s vulnerability in the face of a triple burden of diseases, including communicable diseases, noncommunicable diseases, and injuries, as well as a strained healthcare system.Citation12 As of May 20, 2022, there had been 471,145 confirmed cases of Covid-19 with 7512 deaths since the first case was reported on March 13, 2020. As of August 25, 2020, there were 958 cases and 9 deaths in the South Nations Nationalities and Peoples Region (SNNPR).Citation8
As research progresses, more information regarding the disease’s clinical, epidemiologic, laboratory, and radiologic aspects becomes available. The symptoms appear to vary from location to location, as well as from person to person, depending on sex, age, and other factors, indicating the role of patients’ background characteristics in the clinical presentation, severity, and fate of the disease.Citation3 The duration of viral shedding could differ from individual to individual.Citation15 When am I going to get better? It is a question that many admitted patients have. Increased hospitalization length places a tremendous strain on the hospitalized patient, his or her family, and the healthcare system.Citation16,Citation17 Identifying risk indicators at the time of presentation that predict illness development would aid clinicians in determining which patients may be successfully maintained in district hospitals and which require early transfer to tertiary centers.
The quality of the offered health services at the health institution is reflected by the recovery time. Understanding the factors that influence the length of a hospital stay can help patients and their families make informed decisions about how long they will be in the hospital.
As a result, this research will fill in the gaps in knowledge. So far, nothing is known regarding Covid-19 recovery time (extended hospital stay) and its determinants in the research area. The goal of this study is to use the Cox regression model to determine time to recovery and its predictors among Covid-19 patients admitted to SNNPR treatment centers.
Methods and Materials
Study Setting and Period
The research was carried out at Covid-19 treatment centers of SNNPR, Ethiopia. Southern Nations Nationalities and Peoples Region is the country’s third-largest administrative region and the most diversified in terms of language, culture, and ethnic origin, covering more than 10% of the country’s land area. More than 80 ethnic groups live in the region. The SNNPR’s capital city is Hawassa. It is 273 kilometers south of Addis Ababa. The SNNPR is bordered on the south by Kenya, on the west by South Sudan, on the northwest by Gambela, and on the north and east by Oromia (). The research took place between May 30, 2020, and October 15, 2021. There are 17 administrative zones and 6 special woredas in the region. In the region, there are 17 Covid-19 care centers. Over 45 indigenous ethnic groups are represented in the SNNPR, which is made up of the main homelands of several ethnic groups. In 2018, the population was expected to be 20,768,000.Citation18,Citation19
Figure 1 Map of Southern Nations Nationalities and Peoples Regional State (SNNPRS) displaying administrative zones and special woredas covered by the study (retrieved at: www.rippleethiopia.org/page/snnpr).
Study design: Facility-based retrospective cohort study was conducted.
Study population: All inpatients who tested positive on nasopharyngeal swab for Covid-19 using real-time reverse-transcriptase polymerase-chain-reaction (rRT-PCR) and admitted to Covid-19 care centers of SNNPR from May 30, 2020 to October 15, 2021. Charts of patients with incomplete data for major variables (date of admission, date of discharge and discharge status) were excluded.
Sample Size Determination
Sample size was determined using STATA software Version 15. A hazard ratio of covariates was predetermined to obtain the maximum sample size. A hazard ratio of 0.31 for age when other covariates are held constant was found to be a covariate of interest that maximizes the sample size.Citation20 Other parameters were standard deviation (0.11), probability of success (recovery) observed (0.66), 5% probability of type I error, 80% power and 0.152 probability of loss to follow up. The total sample size required was 845 with the number of events required to be observed in the study, E = 473.
The formula for manual calculation of the sample size is as follows.Citation21
E = the number of events required to be observed
= standard normal percentile of confident coefficient
= standard normal percentile for the power to be achieved
= variance
lnHR = the natural logarithm of the hazard ratio.
Then, the total sample size needed in order to achieve the calculated number of events was calculated using the following formula.
n = total sample size needed
E = the number of events to be observed (calculated above)
Pr (E) = the probability that the event of interest (which is recovery in this context) will occur.
Sampling Technique
Cluster sampling technique was used. There are 17 treatment centers (TC) in SNNPR. In first stage, 4 TC such as Otona comprehensive specialized Hospital, Agana primary hospital, Worabe comprehensive specialized hospital and Nigist Elleni Mohammed memorial comprehensive specialized hospital were selected randomly. We included all patients hospitalized in these treatment centers consecutively.
Data Collection Tool and Procedure
A data extraction tool was developed using the selected variables from the patient registration, follow up and discharge forms, and literatures. The tool has socio-demographic and clinical sub-sections. Trained health professionals who have been working in the treatment center extracted the data.
Data Quality Assurance
Data collectors and supervisors were trained on sampling technique, content of data extraction tool, data extraction procedures and risks of poor data quality. Supervision was carried out for receiving completed check lists, dealing with errors and updating data collectors. Timely feedback was communicated to the data collectors. Continuous variables were checked for outliers by box plot.
Study Variables
Outcome variables: Time to Recovery from Covid-19.
Independent variables: Age, sex, place of residency, TC, presence of co-morbidity, severity levels and oxygen supplement.
Operational Definition
Recovery: Recovery from Covid-19 infection as evidenced by two negative PCR tests done at least 24 hours apart and improvement in symptoms.
Time to Recovery: The time from confirmation of infection with a laboratory test to when the patient is discharged from the center after two negative PCR tests done at least 24 hours apart and improvement in symptoms calculated by number of days (hospital stay).
Median time of recovery: is the time when 50% of the patients recovered.
Event: Recovery from Covid-19 infection.
Censoring: patients lost to follow-up, transferred out, died or completed the follow-up period without achieving recovery.
Incidence density of recovery: number of recovered cases during follow-up period per sum of the lengths of time each study participant was observed and at risk of recovery.
Asymptomatic patient: any patient who has tested positive for Covid-19 but does not have any symptoms.
Mild cases: Symptomatic patients meeting the case definition for Covid-19 without evidence of hypoxia or pneumonia.
Common symptoms include fever, cough, fatigue, anorexia, dyspnea, and myalgia. Other non-specific symptoms include sore throat, nasal congestion, conjunctivitis, runny nose, headache, diarrhea, nausea/vomiting, and loss of smell/taste.
Moderate Cases
Adolescent or adult: clinical signs of pneumonia (i.e., fever, cough, dyspnea, fast breathing) but no signs of severe pneumonia, including blood oxygen saturation levels (SpO2) ≥90% on room air.
Children: clinical signs of non-severe pneumonia (i.e., cough or difficulty breathing plus fast breathing and/or chest indrawing) and no signs of severe pneumonia. Fast breathing is defined as:
<2 months of age: ≥60 breaths/minute
2–11 months of age: ≥50 breaths/minute
1–5 years of age: ≥40 breaths/minute.
Severe Cases
Adolescent or adult: clinical signs of pneumonia (i.e., fever, cough, dyspnea, fast breathing) plus one of the following:
Respiratory rate >30 breaths/minute
Severe respiratory distress
SpO2 <90% on room air.
Children: clinical signs of pneumonia (i.e., cough or difficulty in breathing) plus at least one of the following:
Central cyanosis or SpO2 <90%.
Severe respiratory distress (e.g., fast breathing, grunting, very severe chest in drawing).
General danger sign: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions.
While the diagnosis can be made on clinical grounds, chest imaging may assist in diagnosis and identify or exclude pulmonary complications.
Critical Cases
• Presence of acute respiratory distress syndrome (ARDS), sepsis, or septic shock.
• Other complications include acute pulmonary embolism, acute coronary syndrome, acute stroke, and delirium.
Data Analysis
Data entry and cleaning was done in EpiData. Stata software version 15 was used to analyze the data. Percentages and frequencies were used to summarize categorical variables. The results were presented by tables, texts and graphs based on the nature of the variable. Mean with standard deviation and median with interquartile range were used to summarize normally and non-normally distributed continuous variables, respectively.
Kaplan–Meier Survival Curve was used to describe proportion of hospital stays over time and to compare groups. The log rank test was used to test the null hypothesis of no difference in the distribution of survival times.
Cox proportional hazards regression modeling was used to analyze the data. All covariates with p-value <0.25 were entered into the multivariable model. Adjusted hazard ratios with their 95% Confidence Interval (CI) was estimated and p-value less than 0.05 was used to declare presence of significant association between recovery and covariates.
Assessing Model Assumption
The Proportional hazard assumption was checked on STATA by a global test using estat phtest. The global test tests the null hypothesis that the effect of covariate is the same over time. Overall model adequacy of proportional hazard model was assessed by using Cox Snell residual graph.
Results
We included 845 patients, two-thirds (66.98%) were male. The median age of the participants was 30 years (IQR: 40–65 years) with 36.69% of the participants aged ≥40 years. Majority of the participants (46.27%) were from OCSH TC ().
Table 1 Socio-Demographic Characteristics of Covid-19 Cases Admitted to Treatment Centers of Southern Ethiopia, 2021
Baseline Clinical Characteristic of Patients
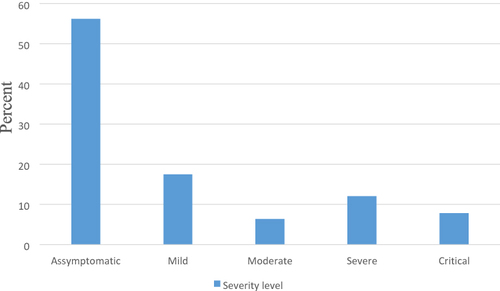
More than one-fifth (22.72%) of the patients had comorbidity. Nearly 10% (9.82%) of the patients had received oxygen supplementation, of them 2.72% were on mechanical ventilation, and 5.44% were on nasal oxygen. More than half (56.21%) of the patients were asymptomatic ().
Table 2 Clinical Characteristics of Covid-19 Cases Admitted to Treatment Centers of Southern Ethiopia from May 30, 2020–October 15, 2021
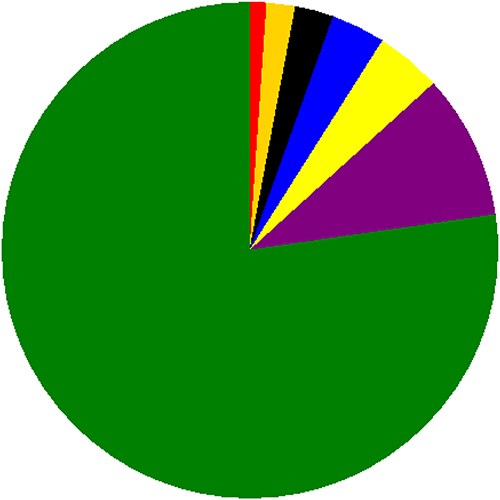
Hypertension was the commonest comorbidity (4.26%) followed by diabetes mellitus (3.55%) (). Concerning severity of the disease, 17.51% had mild disease and 12.07% had severe disease ().
Figure 2 Frequency distribution of types of comorbidity among Covid-19 cases admitted to treatment centers of Southern Ethiopia from May 30, 2020–October 15, 2021.
Incidence of Recovery and Treatment Outcome
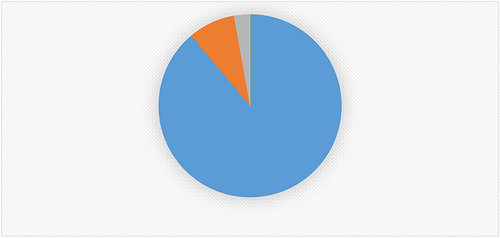
Out of 845 patients, 751 (88.88%) of them recovered with improvement and 70 (8.28%) died (). The total person days contributed by the study participants were 9108 days. The overall incidence density of recovery obtained was 8.24 per 100 person-days (95% CI: 7.67, 8.85).
Figure 4 Percentage distribution of treatment outcome among Covid-19 cases admitted to treatment centers of Southern Ethiopia from May 30, 2020–October 15, 2021.
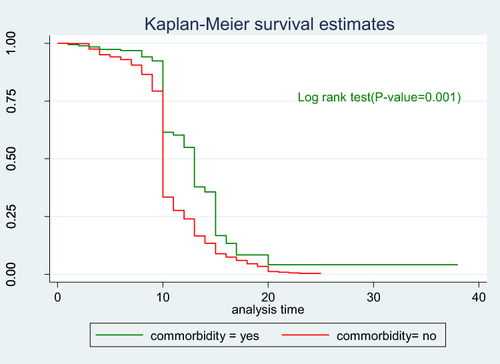
The incidence density of recovery from Covid-19 within first five days, 5–10 days, 10–15 and 15–20 days was 1.04, 12.19, 19.1 and 24.83 per 100 person-days (PD), respectively. The incidence density of recovery from Covid-19 was 80.59 per 100 PD and 86.25 per 100 PD among males and females, respectively. The incidence density of recovery was 63.83 per 100 PD and 88.52 per 100 PD respectively among patients with comorbidity and without comorbidity ().
Table 3 Incidence Density of Recovery by Strata of Categorical Variables Among Covid-19 Cases Admitted to Treatment Centers of Southern Ethiopia from May 30, 2020–October 15, 2021
Median Time to Recovery from Covid-19 (Survival Estimation)
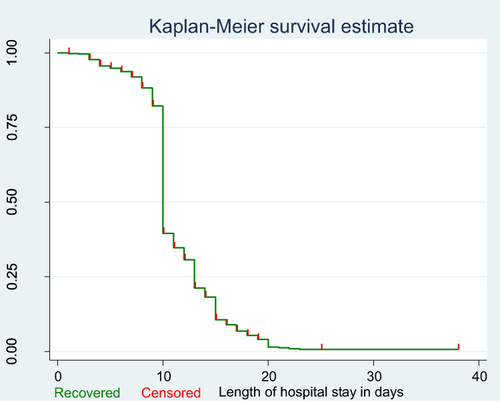
According to the Kaplan–Meier survival estimation the overall median recovery time was 10 days (IQR: 8–16 days). As shown below the graphs decrease rapidly during the first 20 days showing most patients recovered from Covid-19 during this period ().
Figure 5 Cumulative survival distribution among Covid-19 patients admitted to treatment centers of Southern Ethiopia from May 30, 2020–October 15, 2021.
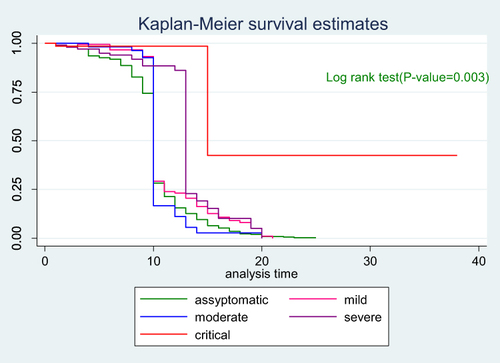
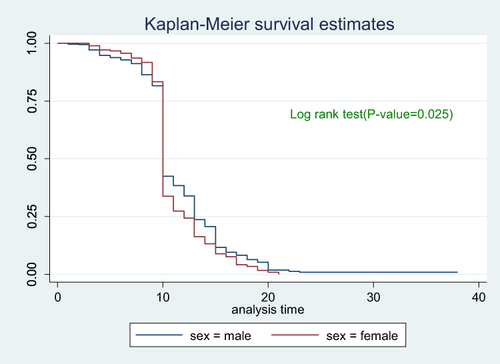
To see the difference in recovery rate between strata of categorical variables a separate Kaplan–Meier survivor functions curve was constructed. As shown in the graphs the recovery rate varied with sex, age, comorbidity and severity. A Log rank test supported the graphically suggested difference in recovery rate among strata of variables (–).
Figure 6 Cumulative survival distribution by age among Covid-19 patients admitted to treatment centers of Southern Ethiopia from May 30, 2020–October 15, 2021.
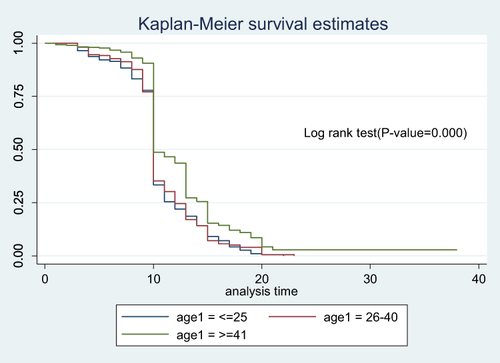
Figure 7 Cumulative survival distribution by sex among Covid-19 patients admitted to treatment centers of Southern Ethiopia from May 30, 2020–October 15, 2021.
Predictors of Time to Recovery from Covid-19
In a bivariate Cox regression analysis covariates with p-value <0.25 such as age, sex, comorbidity, severity, oxygen supplement and treatment center were considered for multivariable analysis. In multivariable analysis severity, oxygen supplementation and treatment center were significantly associated with recovery rate. The proportional hazard assumption was satisfied for the significantly associated variables.
The hazard of recovery was 81% lower for patients who were critical compared with asymptomatic patients (aHR = 0.19, 95% CI: 0.12, 0.29). Severely ill patients had 60% lower hazard of recovery compared with asymptomatic patients (aHR = 0.40, 95% CI: 0.29, 0.56). The hazard of recovery was 80% lower for patients who were on mechanical ventilation compared with patients who did not use oxygen supplement (aHR = 0.20, 95% CI: 0.073, 0.56). The hazard of recovery was 32% lower for patients treated in Otona comprehensive specialized hospital compared with patients treated in Worabe comprehensive specialized hospital (aHR = 0.68, 95% CI: 0.51, 0.90) ().
Table 4 Cox Proportional Hazards Analysis of Predictors of Time to Recovery Among Covid-19 Patients Admitted to Treatment Centers of Southern Ethiopia from May 30, 2020–October 15, 2021
Model Goodness-of-Fit
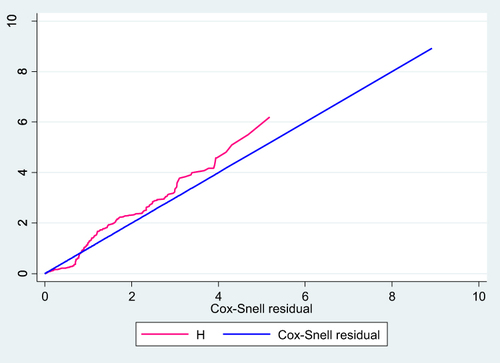
Adequacy of a fitted model was assessed by using Cox Snell residuals after fitting multivariable Cox Proportional Hazard Model. The graph of Nelson–Aalen cumulative hazard function and the Cox Snell residuals variable were compared with the hazard function to the diagonal line. As shown in the graph the hazard function follows the line which indicates that the model fitted the data well ().
Discussion
The aim of this study was to determine time to recovery, the incidence of recovery and its predictors among Covid-19 patients admitted at TCs of Southern Ethiopia from May 30, 2020–October 15, 2021. The median time to recovery from Covid-19 was 10 days (IQR: 8–16 days). This is approximately similar to the median recovery time found in studies done in Osaka City Juso Hospital, Japan (11 days),Citation22 Milan, Italy (14 days),Citation20 in China, Guangzhou Eighth People’s Hospital (12 days),Citation23 in Singapore (12 days),Citation24 in China, Zhejiang University and the Shenzhen Third People’s Hospital (15 days)Citation25 and in Ethiopia at Millennium Covid-19 Care Center (14 days)Citation26 and in Wollega University Referral Hospital (18 days).Citation27 This time to recovery is shorter than the findings in Israel (20–21 days),Citation28 Eka Kotebe General Hospital (19 days)Citation29 and in tertiary care hospital in Harar (44 days).Citation30 However, this finding is higher than the median recovery time reported from University of California San Diego Health (7 days).Citation31 The discrepancy in median recovery time among studies could be due to the difference in disease severity, sample size, background difference among patients and the difference in service quality among care centers and difference in time of disease identification (diagnosis). For example, in a study done in Harar, Ethiopia, 13.18% of the patients were asymptomatic which is very much smaller than the number of asymptomatic patients in this study (56.21%).Citation30 It is likely to take longer duration for patients with severe Covid-19 to recover than asymptomatic patients.Citation32
The incidence density of recovery was 8.24 per 100 person-days (95% CI: 7.67, 8.85). However, the incidence of recovery in this study was higher than the recovery rate in Wollega University Referral Hospital (4.38 per 100 person-days).Citation27 The difference in comorbidity distribution and severity level of disease could explain the disagreement among studies. Also the variation in quality of service and socio-demographic characteristics of the patients could be a reason for a discrepancy among studies. Obviously, settings are different in terms of availability of medical equipment and staff.
Expectedly, severely and critically ill patients at the time of admission had lower hazard of recovery. The hazard of recovery was 81% and 60% lower for patients who were critically and severely ill compared with asymptomatic patients.Citation33 This is similar to the findings in studies done in Jakarta, IndonesiaCitation34 and Millennium Covid-19 Care Center, Ethiopia.Citation26 The possible reason for this finding could be that the lymphocyte count falls as Covid-19 clinical stages advance, thus, severe and critical clinical stages result in poor clinical outcome.Citation35,Citation36 Also, this outcome is expected because severely and critically ill patients develop refractory respiratory failure which requires aggressive management such as tracheal intubation and mechanical ventilation which are not generally available in health facilities of low-income settings like ours.
The hazard of recovery was lower for patients who were on mechanical ventilation compared with patients who did not use oxygen supplementation. This is in line with a study done in China.Citation37 The strength of this study is that it included a large sample size and considered multi centers which represent the Covid-19 cases of the region. The application of advanced statistical technique is another strength of this study. Use of secondary data makes it difficult to control some confounders such as laboratory markers. The data on comorbidity were oral reports which might not represent the real status of the patients.
Conclusion
The median time to recovery from Covid-19 was relatively short. The incidence density of recovery was 8.24 per 100 person-days. The hazard of recovery was lower for patients at higher levels of Covid-19 severity and for patients in need of mechanical ventilation. Early identification of severity levels of the patients is required at the time of admission. Special attention, critical follow–up and management is warranted for patients at higher levels of Covid-19 severity.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Consideration
Ethical approval was obtained from the Institutional Review Board (IRB reference number = 32/20) of Wachemo University. Letter of permission was obtained from hospital administration in each TC. Personal identifiers were not used on data collection checklist. All information collected from patient cards was kept strictly confidential and names of patients were not included in the checklist. All methods were performed in accordance with the relevant guidelines and regulations and were in compliance with the Declaration of Helsinki. Since, the study design was retrospective informed consent was waived by Institutional Review Board (IRB) of Wachemo University namely:
Aklilu Habte, Email: [email protected]
Alule Siyum, Email: [email protected]
Abera Mishamo, Email: [email protected]
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflicts of interests.
Acknowledgments
We would like to thank Wachemo University, College of Medicine and Health Sciences, Office of Research coordination for funding and giving the opportunity to conduct this study.
Additional information
Funding
References
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382 (18):1708–1720. doi:10.1056/NEJMoa2002032
- Ma X, Wang D, Xu W, et al. A novel coronavirus from patients with pneumonia in China, 2019. 2020;727–733.
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 (February):102433. doi:10.1016/j.jaut.2020.102433
- World Health Organaization. Transmission of SARS-CoV-2_ implications for infection prevention precautions; 2020.
- World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization; 2020.
- Prem K, Liu Y, Russell TW, Kucharski AJ, Eggo RM, Davies N. Articles the effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:261–270.
- Level R, Level G, High V. Novel Coronavirus (2019-nCoV). 2020.
- World Health Organaization. Coronavirus disease (COVID-19); 2020.
- Bezzio C, Variola A, Allocca M, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. doi:10.1136/gutjnl-2020-321411
- Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31 (6):1157–1165. doi:10.1681/ASN.2020030276
- Bellosta R, Luzzani L, Natalini G, Pegorer A. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72 (January):1864–1872. doi:10.1016/j.jvs.2020.04.483
- The World Economic Forum. COVID action platform _ world economic forum.
- Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, march-june 2020 [internet]; 2020: 69. Available from: https://www.cdc.gov/mmwr. Accessed June 10, 2022.
- Meng Y, Wu P, Lu W, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China_ A retrospective study of 168 severe patients _ enhanced reader. PLoS Pathog. 2020;16 (4):e1008520. doi:10.1371/journal.ppat.1008520
- Zhou B, She J, Wang Y, Ma X Clinical infectious diseases ® 2020;71 (16):2240-2Duration of viral shedding of discharged patients with severe COVID-19. Available from: www.gov.cn/zhengce/zhengceku/2020-01/28/5472673/files/0f96c10cc09d4d36a6. Accessed June 10, 2022.
- Wootton DG, Dickinson L, Pertinez H, et al. A longitudinal modelling study estimates pneumonia recover to baseline by 10 days. Eur Respir J. 2017;49. doi:10.1183/13993003.02170-2016
- Tiewsoh K, Lodha R, Pandey RM, Broor S, Kalaivani M, Kabra SK. Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatr. 2009;9:15. doi:10.1186/1471-2431-9-15
- Ababa A. Federal democratic republic of Ethiopia central statistical agency population projection of Ethiopia for all regions at wereda level from 2014 – 2017; 2017.
- ADDIS Standard. NEWS_ SNNPRS council approves legal framework which makes hawassa city accountable to future sidama regional state - Addis standard.
- Ciceri F, Castagna A, Rovere-querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217 (May):108509. doi:10.1016/j.clim.2020.108509
- Pintilie M. Power and Sample size for time to event analysis type I and II errors; 2013:1–28.
- Oda Y, Shimada M, Shiraishi S, Kurai O. Treatment and outcome of COVID-19 patients in a specialized hospital during the third wave: advance of age and increased mortality compared with the first/second waves. JA Clin Reports. 2021;7. doi:10.1186/s40981-021-00489-x
- Chen X, Zhu B, Hong W, Zeng J, He X, Chen J. Associations of clinical characteristics and treatment regimens with the duration of viral RNA shedding in patients with COVID-19. Int J Infect Dis. 2020;98:252–260. doi:10.1016/j.ijid.2020.06.091
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323 (15):1488–1494. doi:10.1001/jama.2020.3204
- Kaijin X, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19.
- Id TWL, Hassen IS, Maru EH, et al. Characteristics and outcome profile of hospitalized African patients with COVID-19: the Ethiopian context. PLoS One. 2021;71:1–15. doi:10.1371/journal.pone.0259454
- Id TT, Id BW, Seyoum D, Id G, Merdassa E. Time to recovery from COVID-19 and its predictors among patients admitted to treatment center of Wollega University Referral Hospital (WURH), Western Ethiopia: survival analysis of retrospective cohort study. PLoS One. 2021;8:1–12. doi:10.1371/journal.pone.0252389
- Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J Infect Dis. 2020;222 (6):910–918. doi:10.1093/infdis/jiaa388
- Abdella S, Id A, Tessema M, et al. Time to recovery and its predictors among adults hospitalized with COVID-19: a prospective cohort study in Ethiopia. PloS one. 2020;15:1–11.
- Mamo AG, Merga BT, Birhanu A, Alemu A, Belay Negash YD, Dessie Y. Predictors of mortality among hospitalized COVID-19 patients at a tertiary care hospital in in Ethiopia. Infect Drug Resist. 2021;14:5363–5373. doi:10.2147/IDR.S337699
- Lori Daniels B, Amy Sitapati M, Zhang J, et al. Relation of Statin use prior to admission to severity and recovery among COVID-19 inpatients – pubMed. Am J Cardiol. 2020;136:149–155. doi:10.1016/j.amjcard.2020.09.012
- Williams V, Jayashree M, Nallasamy K, et al. 0.9% saline versus plasma-lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial.. Critical Care. 2020;24 (1):1–11. doi:10.1186/s13054-019-2683-3
- Kaso AW, Hareru HE, Kaso T, Agero G. Factors associated with poor treatment outcome among hospitalized COVID-19 patients in South Central, Ethiopia. BioMed Res Int. 2022;2022. doi:10.1155/2022/4551132
- Laila D, Id R, Isnaini S. Treatment profiles and clinical outcomes of COVID-19 patients at private hospital in Jakarta. PLoS One. 2021;16 (4):e0250147. doi:10.1371/journal.pone.0250147
- Liu J, Sumeng L, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients - PubMed. EBioMed. 2020;55:102763. doi:10.1016/j.ebiom.2020.102763
- Li Y, Yang T, Wang S, et al. The value of lymphocyte count in determining the severity of COVID-19 and estimating the time for nucleic acid test results to turn negative - PubMed. Bosn J Basic Med Sci. 2021;21 (2):235. doi:10.17305/bjbms.2020.4868
- Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J Infect Dis. 2020;222 (6):910–918.