Abstract
Gordonia is a recognized pathogen in patients with immunodeficiency and a normal immune response, which can cause bacteremia, endocarditis, peritonitis and pulmonary infection. We report a case of wound infection after pacemaker implantation caused by Gordonia crocea. Matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS) was routinely used to identify the pathogen, and the results showed that the pathogen could not be accurately identified in the MALDI-TOF database at present. The 16S rRNA gene of the pathogen was further sequenced, and the result was Gordonia crocea. To the best of our knowledge, this is the first reported case of human infection caused by Gordonia crocea.
Introduction
Gordonia was first described by Tsukamura in 1971.Citation1 In 1977, Gordonia was classified as Rhodococcus,Citation2 However, through the analysis of mycolic acid of bacterial cell wall, it was found that Rhodococcus spp. usually contained mycolic acid with 34 to 52 carbon atoms, while members of the genus Gordonia contained mycolic acid with 48–66 carbon atoms.Citation3 Coupled with the rise of 16sRNA sequencing technology, in 1988, Stackebrandt et al reintroduced the genus Gordonia. The latest taxonomy shows that genus Gordonia belongs to Nocardiaceae, suborder Corynebacterineae, order Actinomycetales. The members of the genus Gordonia are a group of aerobic, spore-free, nonmotile, Gram-positive, acid-fast negative and weakly acid-fast positive microorganisms that widely exist in nature and hospital environments. There are limited clinical reports of infection caused by Gordonia. Because genus Gordonia is easy to be mistakenly identified as Nocardia and Rhodococcus, an unknown number of Gordonia infections may be omitted in clinicCitation4 Here, we report a case of wound infection after pacemaker implantation caused by Gordonia crocea, and look forward to deepening the clinical understanding of this pathogen.
Case Report
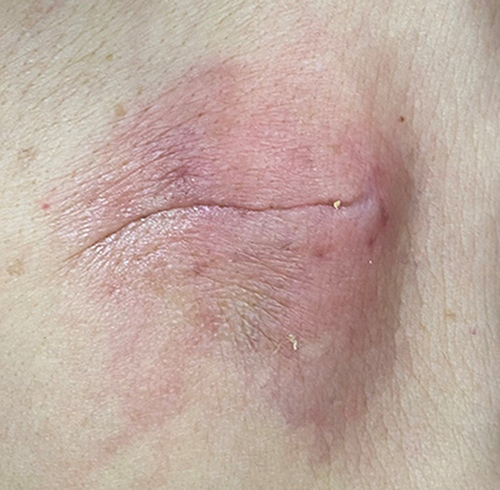
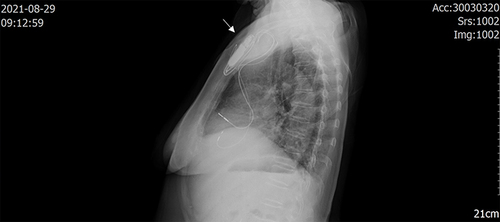
An 81-year-old female patient, who had no obvious cause for symptoms of dizziness, distension, headache, general weakness, vertigo, visual rotation, palpitation and discomfort without inducement, went to the nearby health service center for medical treatment. Past history: one year ago, the patient had repeated symptoms of dizziness and vertigo, and was diagnosed as Meniere's disease in another hospital. Two years ago, the patient underwent surgical treatment for right inguinal hernia, without obvious immune deficiency. According to the history of present illness, the doctor diagnosed sick sinus syndrome according to the results of a dynamic electrocardiogram and was admitted to our hospital during July 2021. Permanent pacemaker implantation and coronary angiography were performed under local anesthesia on July 26. The results showed that the proximal and middle segment of the anterior descending branch had mild stenosis, the middle part of the circumflex branch had moderate stenosis, and the proximal and middle segment of the right coronary artery had mild stenosis. She was discharged in early August 2021 and was diagnosed as having: an artificial permanent pacemaker implantation; coronary atherosclerotic heart disease; and hypertension grade II. About 2 weeks after the operation, the patient’s pacemaker pouch began to show redness and elicited pain (). She went to the local hospital for a change of the dressing, but there was no obvious improvement. On August 24, 2021, she went to the outpatient clinic of our hospital and was given moxifloxacin 0.4 g p.o q.d. external treatment with mupirocin ointment, after treatment, the pain decreased, but skin redness was increased. For further diagnosis and treatment, the patient came to the outpatient clinic of our hospital again on August 28, 2021, and she was admitted to the hospital with a pacemaker pouch infection. Physical examination showed that the pacemaker pouch was red and the skin temperature of the patient had increased. On August 29, 2021, the patient underwent chest X-ray examination, which showed a high-density from the pacemaker pouch, suggesting infection (). Antibiotic treatment, with routine consideration to cover Gram-positive bacteria, vancomycin treatment after admission, vancomycin treatment for 3 days, exudation and ulcer, suggested that the effect of vancomycin was not satisfactory. Pacemaker electrode extraction and debridement suture were considered on August 31, 2021, after removal of the pacemaker, the necrotic tissue was excised, the drainage strip retained and vancomycin administered. No drainage was found in the drainage strip the next day, and the drainage strip was removed and examined by bacterial culture. The pacemaker wound recovered well on the second day after the pacemaker was removed. On September 2, 2021, pacemaker implantation was performed on the opposite side. After 10 days, the wound had no redness, swelling or pus and had healed well. Stitches were removed and the patient discharged from the hospital.
Isolates Culture and Identification
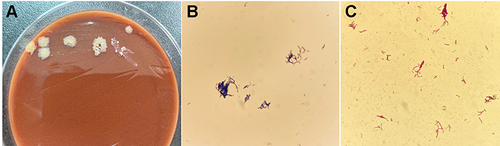
Drainage strips were inoculated in Columbia blood agar, MacConkey agar medium, non-inhibitor chocolate agar and Sabouraud’s agar plates and cultured in a constant temperature incubator in a CO2 enhanced atmosphere at 35°C. After 24 h culture, small colonies could be seen on the blood and chocolate plates. Seventy-two-hour later, yellowish, wrinkled and dry colonies were observed on the non-inhibitor chocolate agar plates, with colony sizes about 2–3 mm (). The colony on the blood plate did not hemolyze and did not produce aerial hyphae. The results of Gram staining showed that the strain was Gram-positive bacilli () and had weak acid-fast staining positive (). The modified strain was subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (VITEK® MS MALDI-TOF, BioMérieux Corporate, France) with a peak and high resolution, but no identification results were obtained. After the genome was extracted from the isolated strain using a bacterial genome extraction kit (Tiangen, Beijing). A DNA was amplified with primers (upstream primer: 27F: AGAGTTTGATCMTGGCTCAG. The downstream primer: 1492R: GGTTACCTTGTTACGACTT), the amplification length was 1500 bp, and then it was purified and sent to Shanghai Maipu Biotechnology Co., Ltd. (Shanghai MAP Biotech Co., Ltd.) for sequencing (sequencer ABI3730xl, ABI company). The sequencing results (Sample ID:260902407705) were analyzed by Sequencing Analysis (ver. 5.2) software and compared in http://blast.ncbi.nlm.nih.gov/Blast.cgi. The result of alignment was Gordonia crocea (GenBank:LC488830.1) and the coverage was 100%.
Results of Antimicrobial Susceptibility Testing
The strains isolated from this case were detected by the non-standardized disk diffusion method according to the CLSI M100 (Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition) and CLSI M24 (Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 3rd Edition). Briefly, the study isolate was cultured in 5% CO2 for 24 hours at 35 °C in Columbia blood plate and the turbidity was adjusted to 0.5McFarland, the bacterial suspension was evenly coated on MH Agar. After the suspension was fully absorbed, Amikacin Amoxicillin-clavulanate, Ceftriaxone, Ciprofloxacin, Clarithromycin, Vancomycin, Imipenem, Linezolid, Minocycline, Moxifloxacin, Trimethoprim-sulfamethoxazole, Tobramycin (ThermoFisher, Oxoid) were applied respectively. After incubating in 5% CO2 incubator at 35°C for 20–24 hours, measure the diameter of the zones of complete inhibition, including the diameter of the disk by vernier caliper. Results revealed that the strains had a high level of sensitivity in vitro to all kinds of common antibiotics. The antimicrobial susceptibility testing results are shown in .
Table 1 Results of Gordonia crocea Antimicrobial Susceptibility Testing (Disk Diffusion Method)
Discussion
To our knowledge, this is the first reported case of a human infection caused by Gordonia crocea, characterized by wound infection after pacemaker implantation. Gordonia crocea was the only pathogen isolated and cultured. However, the shortcoming of this study is the use of non-standardized antimicrobial susceptibility testing method. In 2020, Tamura et al isolated and reported a new strain of Gordonia in the sludge of a sewage treatment plant and named it Gordonia crocea.Citation5 The presence of exogenous foreign bodies may be the promoting factors for Gordonia crocea infection. After the redness, pain and local skin temperature increased, the patient was treated with moxifloxacin and mupirocin. At the beginning of the treatment, pain level was decreased, after 3 days, the skin redness area was enlarged and the pain aggravated, indicating the failure of empirical treatment. After admission, the patient’s antibiotics were upgraded and vancomycin was given as an anti-infective treatment. Three days after vancomycin treatment, fluid exudation and local ulcers appeared in the pouch. The results showed that the therapeutic effect of administering vancomycin alone was not ideal. Although the in vitro sensitivity of fluoroquinolone antibiotics such as moxifloxacin and ciprofloxacin showed that the diameter of the bacteriostatic zone was 38mm and the minimum inhibitory concentration (MIC) of vancomycin in vitro was relatively low (0.5μg/mL, E-testing method), it did not control the expansion of the infection, which further led to pouch debridement and suture and cardiac pacemaker implantation. We speculate that these findings may be related to the fact that the local concentration of antibiotics in the abscess was too low to be effective, which suggests that debridement combined with antibiotics may be the best treatment for infections involving abscess formation. This case did not have obvious immunocompromise, so we speculate that the Gordonia crocea came from the hospital environment or surgical equipment, and the possibility of nosocomial infection cannot be ruled out.
A literature search revealed that the genus Gordonia was first reported by Tsukamura.Citation1 Gordonia has weakly acid-fast resistance because its cell wall composition is similar to that of Rhodococcus and Nocardia.Citation6 It may be mistakenly identified in the clinical microbiology laboratory,Citation7 which may be attributed to the backwardness of identification technology, and the relatively slow growth of bacteria in this genus.Citation8 If the culture time was not prolonged or microbiology laboratory workers did not carefully read the plate, it is likely that the presence of the pathogen will be overlooked. As a result, clinical microbiology laboratory staff may miss many infections caused by Gordonia bacteria when isolating and identifying pathogens. At present, more and more people realize that Gordonia is the pathogen that causes an infection of immunodeficiency and normal immune people. In phylogeny, the genus Gordonia belongs to Corynebacterium suborder. There are 47 species (officially and effectively named so far) (https://lpsn.dsmz.de/genus/gordonia) in the genus, in which Gordonia bronchialis can cause skin abscess.Citation9 Gordonia bronchialis isolated from sputum samples or bronchoalveolar lavage fluid of immunosuppressive patients is usually considered to be pathogenic bacteria, but it is usually necessary to isolate the same strain from two qualified sputum samples. Gordonia bronchialis has also been demonstrated to cause breast abscess,Citation10 secondary peritonitis after peritoneal dialysis,Citation11,Citation12 sternal incision infection,Citation13 endophthalmitisCitation14 and pacemaker-induced endocarditis.Citation15 Gordonia terrae has been reported to cause a catheter-related bloodstream infectionCitation16 and peritonitis.Citation17 Gordonia aichiensis can elicit bacteremia,Citation18 and Gordonia jacobaea has been reported to cause arthritis.Citation19
To date, about 50 strains of Gordonia have been isolated from different environments, and about 30% of Gordonia species are considered to be human opportunistic pathogens and the remaining 70% of species have been shown to play important roles in bioremediation or biodegradation of pollutants.Citation20–24 The reports of clinical infections caused by Gordonia are increasing year by year, which may be due to the progress of identification technology and the continuous improvement of molecular microbial detection technology in recent years, as well as the reduction of testing costs. We believe that more Gordonia bacteria will be isolated in the clinic in the near future, and therefore staff of the clinical microbiology laboratory should pay more attention to the detection of this pathogen in clinical samples and strive to improve the identification ability of this pathogen, thus reducing the cost of treatment for patients with infectious diseases.
Conclusion
We report a case of wound infection after pacemaker implantation in an elderly woman caused by Gordonia crocea, sp. nov. In this case, no underlying diseases were found except sick sinus syndrome, hypertension and Meniere's disease, and no obvious immunocompromise was observed in the patient, which reminds us to pay attention to the infection caused by Gordonia in the immunocompetent patients in clinical.
Patient Consent and Ethics Statement
Patient provided informed consent for the case details and images to be published. No ethics committee approval was required for this study as the data had been analyzed in a retrospective manner.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
Additional information
Funding
References
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68(1):15–26. doi:10.1099/00221287-68-1-15
- Goodfellow M, Alderson G. The actinomycete-genus Rhodococcus: a home for the “rhodochrous” complex. J Gen Microbiol. 1977;100(1):99–122. doi:10.1099/00221287-100-1-99
- Arenskotter M, Broker D, Steinbuchel A. Biology of the metabolically diverse genus Gordonia. Appl Environ Microbiol. 2004;70(6):3195–3204. doi:10.1128/AEM.70.6.3195-3204.2004
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45(4):483–486. doi:10.1086/520018
- Tamura T, Saito S, Hamada M, et al. Gordonia crocea sp. nov. and Gordonia spumicola sp. nov. isolated from sludge of a wastewater treatment plant. Int J Syst Evol Microbiol. 2020;70(6):3718–3723. doi:10.1099/ijsem.0.004225
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42(6):2870–2871. doi:10.1128/JCM.42.6.2870-2871.2004
- Zampella JG, Kwatra SG, Kazi N, et al. Madura foot caused by Gordonia terrae misdiagnosed as Nocardia. Australas J Dermatol. 2017;58(3):e129–e131. doi:10.1111/ajd.12516
- Blanc V, Dalle M, Markarian A, et al. Gordonia terrae: a difficult-to-diagnose emerging pathogen? J Clin Microbiol. 2007;45(3):1076–1077. doi:10.1128/JCM.02394-06
- Bartolome-Alvarez J, Barba-Rodríguez N, Galán-Ros J, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29(3):170–173.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43(6):3009–3010. doi:10.1128/JCM.43.6.3009-3010.2005
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37(1):217–220. doi:10.1007/s00467-021-05313-3
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14(2):109–111. doi:10.1016/j.nephro.2017.09.006
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3(5):e005067. doi:10.1099/jmmcr.0.005067
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis –associated endophthalmitis, Oregon, USA. Emerg Infect Dis. 2019;25(5):1017–1019. doi:10.3201/eid2505.180340
- Mormeneo Bayo S, Ruíz MP, Samper UA, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin. 2021;40(5):255–257.
- Grisold AJ, Roll P, Hoenigl M, et al. Isolation of Gordonia terrae from a patient with catheter-related bacteraemia. J Med Microbiol. 2007;56(Pt 12):1687–1688. doi:10.1099/jmm.0.47388-0
- Hou C, Yang Y, Li Z. A Chinese patient with peritoneal dialysis-related peritonitis caused by Gordonia terrae: a case report. BMC Infect Dis. 2017;17(1):179. doi:10.1186/s12879-017-2283-2
- Thomas E, Lejeune F, Caillon J, et al. [First case report of Gordonia aichiensis bacteremia]. Med Mal Infect. 2017;47(7):508–509. French. doi:10.1016/j.medmal.2017.07.009
- Guiraud J, Lescure M, Faganello D, et al. A case of prosthetic joint septic arthritis caused by Gordonia jacobaea. J Microbiol Immunol Infect. 2021;55(2):355–357. doi:10.1016/j.jmii.2021.08.001
- Zhang H, Lin Z, Liu B, et al. Bioremediation of di-(2-ethylhexyl) phthalate contaminated red soil by Gordonia terrae RL-JC02: characterization, metabolic pathway and kinetics. Sci Total Environ. 2020;733:139138. doi:10.1016/j.scitotenv.2020.139138
- Qi P, Sun D, Wu T, et al. Stress proteins, nonribosomal peptide synthetases, and polyketide synthases regulate carbon sources-mediated bio-demulsifying mechanisms of nitrate-reducing bacterium Gordonia sp TD-4. J Hazard Mater. 2022;422:126900. doi:10.1016/j.jhazmat.2021.126900
- Sarkar B, Mandal S. Gordonia sp. BSTG01 isolated from Hevea brasiliensis plantation efficiently degrades polyisoprene (rubber). 3 Biotech. 2021;11(12):508. doi:10.1007/s13205-021-03063-5
- Wang Y, Ren Q, Zhan W, et al. Biodegradation of di-n-octyl phthalate by Gordonia sp. Lff and its application in soil. Environ Technol. 2021:1–8. doi:10.1080/09593330.2021.1890839
- Guo Y, Huang Y, Pang S, et al. Novel mechanism and kinetics of tetramethrin degradation using an indigenous Gordonia cholesterolivorans A16. Int J Mol Sci. 2021;22(17):17. doi:10.3390/ijms22179242