Abstract
Streptococcus constellatus (S. constellatus) is a well-known part of the normal flora in humans. Pyogenic spondylitis (PS) induced by S. constellatus is very rare. In this case, a 46-year-old male patient presented to our hospital with a 2-month history of low back pain and weakness in both legs. Based on his clinical manifestations, laboratory findings, blood culture results, imaging and histopathological findings, the patient was diagnosed with PS caused by S. constellatus. One unique aspect of this case is the acute and severe course of infection, which resulted in multiple organ dysfunction syndrome and septic shock in the first week and rapid vertebral destruction within 2 months of the patient’s admission. His obese status may be relevant to his presentation. This case report suggests that S. constellatus infections should not be overlooked, especially in patients with known risk factors and predispositions to infectious diseases.
Introduction
Pyogenic spondylitis (PS) is an uncommon but potentially life-threatening disease, which encompasses a broad range of clinical entities such as pyogenic spondylodiscitis, septic discitis and vertebral osteomyelitis.Citation1 Staphylococcus aureus is the most common causative pathogen of PS, whereas PS induced by Streptococcus constellatus (S. constellatus), which is found in the normal flora of the upper respiratory, digestive and reproductive tracts, is very rare.Citation2–5 Herein, we describe an extremely rare case of rapid progress of PS related to S. constellatus in an obese patient.
Case Presentation
A 46-year-old male patient with body mass index of 40 kg/m2 presented to our hospital with the chief complaint of “two months history of low back pain with weakness in both legs”. On admission, physical examination revealed significant tenderness in the L3–5 intervertebral and paraspinal area, pain and decreased skin sensation in the right calf and dorsum, and weak muscle strength in both lower limbs (right side: grade 3 for tibialis anterior muscle, extensor hallucis longus and triceps surae, and grade 4 for quadriceps femoris; left side: grade 3 for quadriceps femoris, and grade 4 for tibialis anterior muscle, extensor hallucis longus and triceps surae). No obvious kyphosis or scoliosis deformity was found in his whole spine. Laboratory findings and serological examination revealed inflammation. Renal and liver dysfunction was also identified (). X-ray ( and ), magnetic resonance imaging (MRI) ( and ) and computed tomography (CT) ( and ) scans showed abnormality in his L3–5 lumbar segment.
Table 1 Laboratory Findings of This Case at Different Time-Points
Figure 1 X-ray radiographs at admission. Lateral (A) and anteroposterior (B) radiographs show grade I L4 spondylolisthesis and loss of height of L4–5 disc (red arrow).
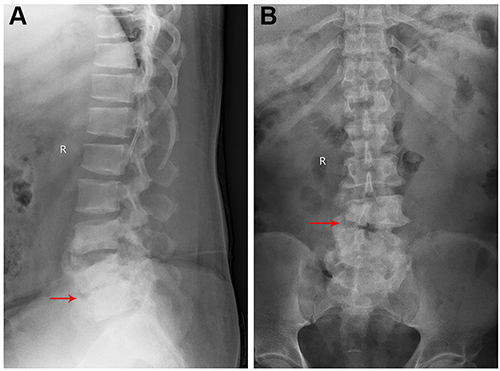
Figure 2 Fat-suppressed T2-weighted MRI images at different time-points. (A) Sagittal MRI image at admission; (B) axial MRI image of L4–5 disc space at admission; (C) sagittal MRI image at 78 days after admission; (D) axial MRI image of L4–5 disc space at 78 days after admission; (E) sagittal MRI image at re-examination; (F) axial MRI image of L4–5 disc space at re-examination. Red arrows indicate hyperintense signal in vertebral bodies and paravertebral tissue. Pink arrow indicates spinal canal abscess. Blue arrow indicates paravertebral soft tissue abscess. The damaged area was extended at 78 days after admission but relieved at re-examination.
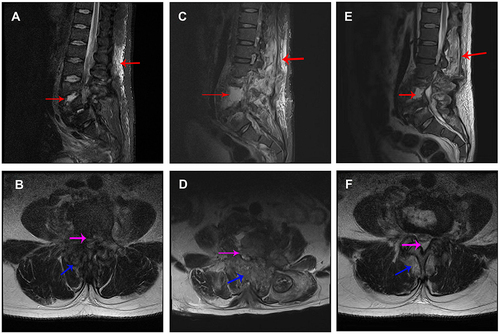
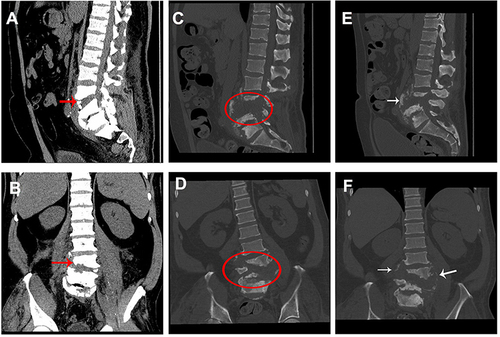
Figure 3 CT images at different time-points. (A) Sagittal CT image at admission; (B) axial CT image at admission; (C) sagittal CT image at 78 days after admission; (D) axial CT image at 78 days after admission; (E) sagittal CT image at re-examination; (F) axial CT image at re-examination. Red arrow indicates slight L3–5 vertebral body destruction with grade I L4 spondylolisthesis. Oval indicates broader and more severe L3–5 damage including grade II L4 spondylolisthesis, L4–5 disc space disappearance and L3–5 vertebral body destruction. White arrow indicates new bone formation.
Two days after admission, the patient experienced high fever (39.8°C), and complained of abdominal distension, low back pain and oliguria (excretion of about 300 mL of urine per day). Blood culture was then performed. His renal and liver function had further deteriorated. Abnormal myocardial injury markers and myocardial enzyme profiles were observed (). Tuberculous spondylitis was suspected. Empirical antibiotics (isoniazid 0.3 g, ivggt, qd; pyrazinamide 0.75 g, po, qd; rifampicin 0.45 g, po, qd; and ethambutol, 0.75 g, po, qd) and symptomatic treatments such as diuresis and albumin supplements were given to the patient.
Four days after admission, the patient experienced sudden loss of consciousness and unstable circulation. His inflammation and liver dysfunction were aggravated compared with 2 days before (). He was transferred to ICU for further treatment. Streptococcus constellatus was identified in his blood culture. Antimicrobial susceptibility tests were then performed. The results showed that isolates from the blood of the patient were susceptible to imipenem, penicillin G, ertapenem, cefepime, cefotaxime, teicoplanin, levofloxacin, chloramphenicol and linezolid, but resistant to erythrocin, tetracycline and clindamycin. During the period in the ICU (lasting for 46 days), systemic antibiotic therapy (imipenem: 1 g, q6h, ivgtt; teicoplanin: 1.2 g, q24h, ivgtt, for the first 3 days, followed by 0.6 g, q24h, ivgtt) and symptomatic treatments such as fluid supplementation, maintenance of water–electrolyte balance, and nutritional support were given to the patient. Lumbar puncture and biopsy of L3–5 disc space tissues were performed on the 31st day after the patient was transferred to ICU. Tissue culture and Mycobacterium tuberculosis (TB)-PCR test results were negative. Pathological results revealed fibrous tissue hyperplasia accompanied by acute and chronic inflammatory cell infiltration; locally, degeneration, necrosis and dead bone formation were also noticed (). Based on all of the test results, TB infection and tumor were ruled out and the patient was finally diagnosed with PS caused by S. constellatus.
Figure 4 (A) H&E staining of L4–5 disc space tissues shows typical characteristics of acute and chronic inflammatory cell infiltration; (B) MRI image shows an obvious swelling (red arrow) in the patient’s thigh.
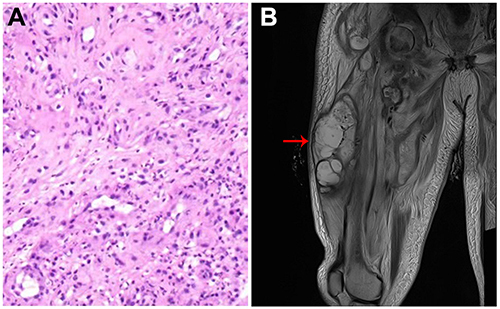
After the emergency had been brought under control, the patient was sent back to our department, where obvious swelling in the patient’s thigh was noticed (). Approximately 500 mL of brown liquid, i.e. paravertebral pus, which had been generated from the absorption of broken bone and soft tissue in the lumbar spine and descended into the muscle tissue of the thigh along the muscle gap, was suctioned out. No bacteria were found after culture, indicating that the antibiotic therapy had been effective.
A physical examination 78 days after admission revealed tenderness in the L3–5 intervertebral and paraspinal area, hypoesthesia in the inside and outside skin of the right calf, and weak muscle strength in both lower limbs (grade 1 for tibialis anterior muscle, extensor hallucis longus and triceps surae, and grade 3 for quadriceps femoris). His muscle tension was normal. Major lumbar spinal infection and vertebral destruction were noticed on MRI ( and ) and CT ( and ) scans. Immobilization surgery was planned for the patient; however, he refused surgery for economic reasons. He was discharged from our hospital 53 days later.
Then, 32 days after discharge, the patient returned to our hospital for re-examination. Physical examination showed no tenderness in his whole spine. Hypoesthesia still existed in the inside and outside skin of the right calf. His muscle strength (grade 2 for both right and left side tibialis anterior muscle, extensor hallucis longus and triceps surae, and grade 4 for both right and left side quadriceps femoris) had improved and muscular tension was normal. Laboratory findings were almost normal (). The MRI results showed that the lumbar spinal infection was alleviated compared with the previous observation ( and ). New bone formation and osteosclerotic changes were observed on CT images ( and ).
Discussion
Streptococcus constellatus, together with Streptococcus anginosus and Streptococcus intermedius, is part of the Streptococcus anginosus group (SAG). Although S. constellatus has been reported in a series of diseases, such as liver abscess,Citation6 empyemaCitation7 and descending necrotizing mediastinitis,Citation8 its pathogenic potential is sometimes overlooked because of its commensal nature. In this rare case, S. constellatus characteristically behaved as a virulent bacterium and caused the rapid progression of PS infection.
Spondylodiscitis, spondylitis or vertebral osteomyelitis is a disease specified based on the infection site. The term spondylodiscitis is usually used when the infection affects the intervertebral disc, whereas vertebral osteomyelitis or spondylitis is usually used if the infection invades the vertebral body. However, in many cases, the infection has already compromised the intervertebral disc and vertebral body structures at the time of diagnosis; therefore, these terms are frequently used without clear distinction.Citation9 To our knowledge, S. constellatus is an extremely rare pathogen of PS. Only a few cases have been reported. For instance, Ahn et al reported one case of S. constellatus-induced PS with a spinal epidural abscess in a 49-year-old male patient.Citation2 Gangone et al reported another case of spontaneous group C S. constellatus spondylodiscitis in a 72-year-old man.Citation3 In addition, Lim et al reported one case of S. constellatus-induced spondylodiscitis in a 14-year-old male,Citation4 and Potsios et al reported another case of S. constellatus-induced PS in a 64-year-old immunocompromised male patient.Citation5 All of these cases recovered after surgery or 6–8 weeks of antibiotic treatment.
Correct diagnosis is the first step in the treatment of PS; however, the clinical symptoms (such as local pain, fever, limitation of motion and localized tenderness near the affected area) and laboratory findings (such as elevated ESR, WBC and CRP) of PS are non-specific, as can also be seen in other diseases such as tuberculous spondylitis.Citation1 The correct diagnosis of PS should be made based on the patient’s overall information, including clinical symptoms, laboratory findings, blood and/or tissue cultures, and imaging findings.Citation1
Conservative treatment with systemic antibiotic therapy is the first choice for PS treatment.Citation1 The choice of antibiotic should based on bacterial culture results. If the patients are quite ill and the organism remains undetermined, empirical antibiotics are usually used. The duration of antibiotic therapy is not well established. In some studies, it is recommended that antibiotic therapy should be administered for 6–8 weeks. Surgical intervention is indicated when there is severe pain, spinal cord or radicular compression, extensive bone destruction, epidural abscess or failure of conservative management.Citation1 In our case, the patient received antibiotic therapy in the ICU for 6 weeks. When he had left the ICU, internal immobilization surgery was planned for him; unfortunately, he refused surgery for economic reasons.
Bone destruction is not rare but is less serious in PS. In a study performed by Chang et al, the authors compared MRI parameters in 33 tuberculous spondylitis patients and 33 PS patients. They found that 23 out of 33 PS patients had grade ≤2 vertebral body destruction, while only six tuberculous spondylitis patients had grade ≤2 vertebral body destruction.Citation10 Severe and rapidly progressing bone destruction is rarer in PS because most PS patients respond positively to 6–8 weeks of systemic antibiotic therapy. Only a few cases that focused on the rapid progression of PS have been reported. For instance, in a study performed by Ogasawara et al, the authors reported a case of PS with an acute course caused by Corynebacterium simulans. In that case, MRI images showed a high signal only at the intervertebral disc on days 1 and 10, but a high signal at the 4th and 5th lumbar vertebra bodies across the intervertebral disc 40 days later.Citation11 In another study, performed by Karakida et al, the authors reported a case of PS with rapid bone destruction after chemoradiotherapy for tongue cancer. In that case, no obvious abnormality was observed on CT imaging at the time of PS onset, but pathological fractures in C3 were seen 2 months later.Citation12 The patient developed multiple organ dysfunction syndrome (renal and liver dysfunction, myocardial injury) and septic shock in the first week and rapid vertebral destruction within 2 months after admission. Such a rapidly progressing PS condition involving severe vertebral destruction makes our case unique.
Obesity is one of predisposing factors to PS.Citation13 In a study performed by Schoof et al, the authors found that obesity is a risk factor for a severe course of spondylodiscitis.Citation14 In this case, we suspected that the rapid progression of PS may be related to his obese status, because other well-known predisposing factors to PS, such as diabetes mellitus, long-term steroid therapy, malignancy, liver cirrhosis, chronic renal failure, malnutrition, substance abuse, HIV infection and septicemia, had not been identified in this patient.
Conclusion
In this report, we present a rare case of PS caused by S. constellatus. A unique aspect of this case is the acute and severe course of infection, which resulted in rapid vertebral destruction within 2 months after admission. His obese status may be relevant to his presentation. Streptococcus constellatus infections should not be overlooked, especially in patients with known risk factors and predispositions to infectious diseases.
Patient Consent and Ethics Statement
The patient has provided informed consent for publication of the case. No ethical committee approval was required because this case was analyzed in a retrospective manner.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
Additional information
Funding
References
- Sato K, Yamada K, Yokosuka K, et al. Pyogenic spondylitis: clinical features, diagnosis and treatment. Kurume Med J. 2019;65(3):83–89. doi:10.2739/kurumemedj.MS653001
- Ahn HD, Kim JY, Kim SY, Park YS, Seo YH, Cho YK. Pyogenic spondylitis with spinal epidural abscess caused by Streptococcus constellatus. Korean J Med. 2006;71(1):397.
- Gangone R, Findlay I, Lakkireddi PR, Marsh G. A very rare spontaneous group-C Streptococcal constellatus spondylodiscitis: a case report. J Orthopaedics. 2009;6(3):e7.
- Lim SW, Lim HY, Kannaiah T, Zuki Z. Streptococcus constellatus spondylodiscitis in a teenager: a case report. Malays Orthop J. 2017;11(3):50–52. doi:10.5704/MOJ.1711.004
- Potsios C, Xaplanteri P, Zoitopoulos V, et al. Pyogenic spondylodiscitis due to Streptococcus constellatus in an immunocompromised male patient: a case report and review of the literature. Case Rep Infect Dis. 2019;2019:9364951. doi:10.1155/2019/9364951
- Akuzawa N, Hatori T, Kitahara Y, Kurabayashi M. Multiple liver abscesses and bacteremia caused by Streptococcus constellatus infection: a case report. Clin Case Rep. 2016;5(1):69–74. doi:10.1002/ccr3.774
- Xia J, Xia L, Zhou H, Lin X, Xu F. Empyema caused by Streptococcus constellatus: a case report and literature review. BMC Infect Dis. 2021;21(1):1267. doi:10.1186/s12879-021-06955-2
- Ye RH, Yang JC, Hong HH, et al. Descending necrotizing mediastinitis caused by Streptococcus constellatus in an immunocompetent patient: case report and review of the literature. BMC Pulm Med. 2020;20(1):43. doi:10.1186/s12890-020-1068-3
- Algrmi SEA, Taha MMMT, Fattah IMA, Youssef EME. Management of patients with Spondylodiscitis: an overview. Eur J Mol Clin Med. 2021;8(3):3023–3034.
- Chang MC, Wu HTH, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine. 2006;31(7):782–788. doi:10.1097/01.brs.0000206385.11684.d5
- Ogasawara M, Matsuhisa T, Kondo T, et al. Pyogenic spondylitis with acute course caused by Corynebacterium simulans. J Infect Chemother. 2020;26(3):294–297. doi:10.1016/j.jiac.2019.10.012
- Karakida K, Uchibori M, Nakanishi Y, et al. Pyogenic spondylitis with rapid bone destruction after chemoradiotherapy for tongue cancer: a case report and literature review. Tokai J Exp Clin Med. 2020;45(4):182–188.
- Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105(10):181–187. doi:10.3238/arztebl.2008.0181
- Schoof B, Stangenberg M, Mende KC, Thiesen DM, Ntalos D, Dreimann M. Obesity in spontaneous spondylodiscitis: a relevant risk factor for severe disease courses. Sci Rep. 2020;10(1):21919. doi:10.1038/s41598-020-79012-8
