Abstract
Purpose
The emergence of drug-resistant tuberculosis (DR-TB) represents a threat to the control of tuberculosis. This study aimed to estimate the patterns and trends of DR-TB in patients with suspected DR-TB. In addition, risk factors for multidrug-resistant tuberculosis (MDR-TB) were identified among suspected DR-TB patients in Dalian, China.
Patients and Methods
A total of 5661 patients with suspected DR-TB from Jan 1, 2013 to Dec 31, 2020 were included in the final analysis. The resistance pattern of all resistant strains was determined by drug susceptibility testing (DST) using the conventional Lowenstein-Jensen Proportion Method (LJ). DR-TB trends were estimated from 2013 to 2020. During the research period, the chi-square test was employed to analyze the significance of linear drug-resistance trends across time. Bivariate and multivariate logistic regression were performed to assess factors associated with MDR-TB.
Results
From 2013 to 2020, the resistance rates of rifampicin (RFP) and isoniazid (INH) decreased significantly, whereas the resistance rates of ethambutol (EMB) and streptomycin (SM) increased in patients with suspected DR-TB. From 2013 to 2020, the prevalence of DR-TB decreased in all patients from 34.71% to 28.01% with an average annual decrease of 3.02%. Among new cases, from 2013 to 2020, the prevalence of DR-TB (from 26.67% to 24.75%), RFP-resistant TB (RR-TB) (from 15.09% to 3.00%) and MDR-TB (from 6.08% to 2.62%) showed a significant downward trend. Among patients with a previous treatment history, DR-TB (from 54.70% to 37.50%), RR-TB (from 44.16% to 11.49%) and MDR-TB (from 26.90% to 10.34%) showed a significant downward trend from 2013 to 2020. Males (AOR 1.28, 95% CI 1.035–1.585), patients 45 to 64 years of age (AOR 1.75, 95% CI 1.342–2.284), patients 65 years and older (AOR 1.65, 95% CI 1.293–2.104), rural residents (AOR 1.24, 95% CI 1.014–1.519) and a previous treatment history (AOR 3.94, 95% CI 3.275–4.741) were risk factors for MDR-TB.
Conclusion
The prevalence of DR-TB, RR-TB and MDR-TB was significantly reduced from 2013 to 2020. Considerable progress has been made in the prevention and treatment of DR-TB during this period. However, the increasing rate of drug resistance in EMB and SM should be taken seriously. Suspected DR-TB patients who are male, older than 45 years of age, live in rural areas, and have a history of TB treatment should be given priority by health care providers.
Introduction
Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis (MTB) and is one of the leading causes of death from a single infectious agent worldwide, ranking above HIV/AIDS.Citation1 Drug-resistant tuberculosis (DR-TB) remains a public health concern worldwide.Citation2,Citation3 According to the Global Tuberculosis Report 2021, it is estimated that approximately 9 million people developed TB and that 1.5 million died from TB in 2020.Citation4 The emergence of DR-TB, specifically multidrug-resistant TB (MDR-TB), which is defined as TB resistant to at least isoniazid (INH) and rifampicin (RFP), and extensively drug-resistant TB (XDR-TB), which is defined MDR-TB with additional resistance to any fluoroquinolone and at least one of the three second-line injectable drugs, is a threat to the control of this disease.Citation5 It is estimated that there were greater than 500,000 patients with RFP-resistant TB (RR-TB) globally in 2019, 78% of whom were MDR-TB patients.Citation6 Ofloxacin has a higher resistance rate than moxifloxacin in MDR-TB.Citation7
China is one of the countries with the heaviest DR-TB burden.Citation8,Citation9 In 2019, an estimated 833,000 new cases of TB were diagnosed in China.Citation6 Among them, MDR/RR-TB accounted for 7.1% of new cases and 21% of previous cases.Citation10 Despite progress in TB control, China still faces a serious epidemic of DR-TB.Citation11 MDR-TB is generally caused by incorrect or inadequate treatment by doctors or by patients not taking their medication consistently or not completing the entire course of treatment.Citation12 Many studies now demonstrate that primary DR-TB is more likely to be caused by person-to-person transmission.Citation13 In addition, gene mutations may also lead to the emergence of DR-TB.Citation14 Patients with DR-TB often experience catastrophic medical expenditures. As a result of these high costs, patients often discontinue treatment; thus, the disease worsens.
Dalian is located in Northeast China, covers an area of 12,574 square kilometers, and has a registered population of 6.95 million people. There is a high prevalence of TB in Dalian.Citation15 The incidence of TB in the region is 52/100,000, which is well above the global average.Citation16 However, the Directly Observed Treatment and Short Course (DOTS) strategy and the Global Fund for MDR-TB Prevention and Treatment project have been implemented in this region since 2012.Citation15 Many patients with suspected DR-TB are diagnosed in Dalian every year, and these patients can easily develop MDR-TB or even XDR-TB.
Understanding the patterns and trends of DR-TB in suspected DR-TB patients is essential for the prevention and treatment of DR-TB. However, to our knowledge, no study has focused on the patterns and trends of DR-TB in suspected DR-TB patients in Dalian, China. Therefore, the purpose of this study was to determine the patterns and trends of DR-TB in suspected DR-TB patients. In addition, to determine the risk of MDR-TB, we explored the association between demographic characteristics and MDR-TB among suspected DR-TB patients in Dalian, northeast China.
Patients and Methods
Study Population and Data Collection
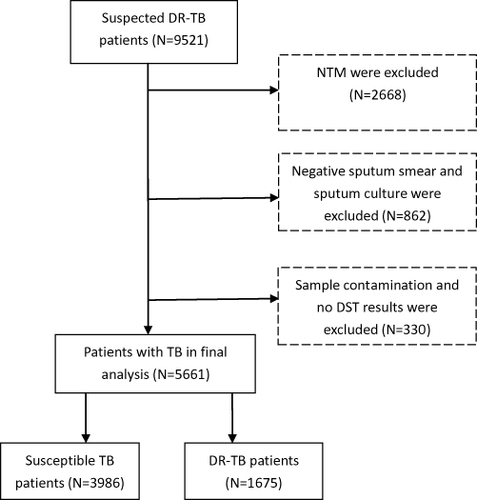
This study was performed at a public health institution in Dalian, China. Sociodemographic data and drug susceptibility data were extracted periodically from the China Disease Control and Prevention Information System from a public health institution. Patients with suspected DR-TB include those who have received previous TB treatment, relapse cases, loss to follow-up and treatment failures, or TB patients infected with DR-TB strains. In the current study, a total of 9521 suspected DR-TB patients were considered for inclusion from Jan 1, 2013 to Dec 30, 2020. All patients were diagnosed by sputum smear examination and sputum culture. Based on the results of the p-nitrobenzoic acid (PNB) culture method, nontuberculous mycobacteria (NTM) were excluded. Moreover, negative sputum smears and sputum cultures were excluded. In addition, patients without drug susceptibility testing (DST) results and contaminated samples were excluded from the study. Finally, 5661 eligible patients were included in this study. The details of the inclusion or exclusion criteria for patients are shown in .
Drug Susceptibility Test
Sputum samples were collected from suspected DR-TB patients with labeled plastic bottles in this study before the initiation of TB therapy.Citation17 Samples were sent to the national reference laboratory of Tuberculosis Prevention and Control Center for DST. Sputum samples were cultured on Lowenstein-Jensen (LJ) culture media.Citation18 DST was conducted for first-line anti-TB drugs (FLDs), including INH, RFP, ethambutol (EMB) and streptomycin (SM). The following concentrations of anti-TB drugs were administered according to the proportion method: 0.2 μg/mL for INH, 40 μg/mL for RIF, 2 μg/mL for EMB, and 4 μg/mL for SM.Citation19 Resistance to a specific drug was determined if the growth rate was greater than 1% compared to the control.Citation20,Citation21
Definitions
New cases refer to a patient who has never been treated for TB or has taken TB treatment for less than one month.Citation22
Previously treated cases referred to patients who had received one month or more of TB treatment in the past.Citation22
Mono-resistant TB (MR-TB) is defined as resistance to one FLDs only.Citation22
Polydrug-resistant TB (PDR-TB) is defined as resistance to more than one FLDs (other than both INH and RFP).Citation22
RR-TB is defined as resistance to RFP with or without resistance to other anti-TB drugs.Citation22
Statistical Analysis
All data were entered into the database established by MS Excel 2010 (Microsoft Corporation, Redmond, WA, USA), and DR-TB trends were plotted. We estimated the drug resistance trends of different patterns of drug resistance types between new and previously treated patients. The chi-square test for trends was used to determine the changes and temporal trend in the different resistance patterns over time. To identify the link between a collection of independent variables and dependent variables, multivariate logistic regression was performed on all variables with p values < 0.2 in univariate logistic regression. Two-sided P<0.05 was considered statistically significant. Statistical analyses were performed using the SPSS 22.0 statistical package (IBM Corporation, Armonk, NY, USA).
Ethical Considerations
This study received ethical approval from the Ethics Committee of Dalian Medical University and Tuberculosis Specialist Hospital. Permission to use the data were obtained from the hospital administration. Patient consent for this retrospective study was waived because all data were from the China Information System for Disease Prevention and Control. All data were deidentified and analyzed. The research methods used in this article are in full compliance with the Helsinki Declaration.
Results
Patient Characteristics
In this retrospective study, a total of 5661 patients were eligible for inclusion and were enrolled between Jan 1, 2013 and Dec 31, 2020. Of the 5661 patients, the median age was 54 years (IQR 41–65), and approximately three-quarters were men (76.79%). A total of 3986 (70.41%) were drug-susceptible patients, and 1675 (29.59%) were DR-TB patients who were resistant to at least one anti-TB drug. The majority of patients were 45–64 years old (62.37%). Among drug-susceptible and DR-TB patients, the number and proportion of patients with a previous treatment history were 905 (22.70%) and 704 (42.03%), respectively. The basic sociodemographic characteristics of suspected DR-TB patients are shown in .
Table 1 Sociodemographic Characteristics of Suspected DR-TB Patients
Drug Resistance Patterns
In this retrospective study, the DST results of all 5661 suspected DR-TB patients were analyzed. Of these, the prevalence of DR-TB was 29.59%. Among 4052 new cases, the highest proportion of any resistance to four FLDs was observed in INH 504 (12.44%) followed by RFP 372 (9.18%), SM 357 (8.81%), and EMB 74 (1.83%). Among 1609 previously treated cases, the highest proportion of any resistance to four FLDs was observed in INH 464 (28.84%) followed by RFP 440 (27.35%), SM 203 (12.62%), and EMB 95 (5.90%).
Of the patients with MR-TB, the number and proportion of MR-TB to FLDs, including INH, RFP, EMB, and SM, were 159 (3.92%), 74 (1.83%), 8 (0.20%), and 191 (4.71%) among new cases and 103 (6.40%), 78 (4.85%), 6 (0.37%), and 47 (2.92%) among previously treated cases, respectively. The number and proportions of MDR-TB among new and previously treated cases were 251 (6.19%) and 394 (24.49%), respectively ().
Table 2 Drug Resistance Patterns in New and Previously Treated Patients with Suspected DR-TB
Annual Drug Resistance Trends
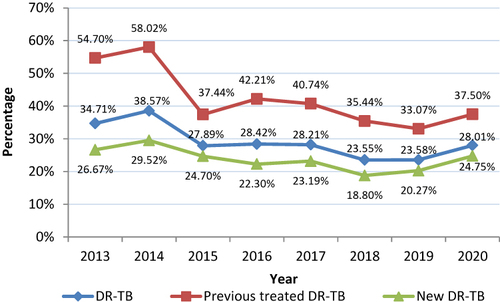
From 2013 to 2020, the prevalence of DR-TB decreased in all patients with suspected DR-TB from 34.71% to 28.01% with an average annual decrease of 3.02% and a significant downward trend (χ2 = 44.916, P<0.001). Among new cases, DR-TB decreased from 26.67% to 24.75% from 2013 to 2020 with an average annual decrease of 1.06% and a significant downward trend (χ2 = 12.242, P<0.001). Among previously treated cases, DR-TB decreased from 54.70% to 37.50% from 2013 to 2020 with an average annual decrease of 5.25% and a significant downward trend (χ2 = 33.147, P<0.001) ().
First-Line Drug Resistance Rates Among New and Previously Treated Patients with Suspected DR-TB, 2013 to 2020
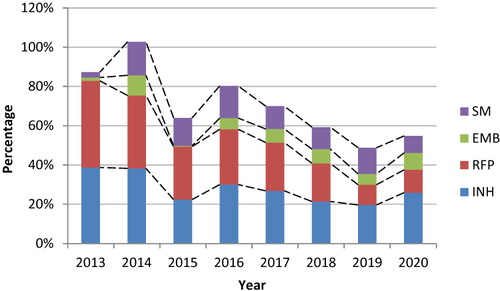
Among new cases with suspected DR-TB, the resistance rate of INH decreased significantly from 17.11% in 2013 to 11.84% in 2020 (χ2 = 20.388, P<0.001). The resistance rate of RFP decreased significantly from 16.00% in 2013 to 3.62% in 2020 (χ2 = 39.609, P<0.001). The resistance rate of EMB increased significantly from 0.22% in 2013 to 1.97% in 2020 (χ2 = 4.420, P=0.036). The resistance rate of SM increased significantly from 0.22% in 2013 to 13.82% in 2020 (χ2 = 21.123, P<0.001) ().
Figure 3 Rates and trends of resistance to four first-line drugs in new TB patients from 2013 to 2020.
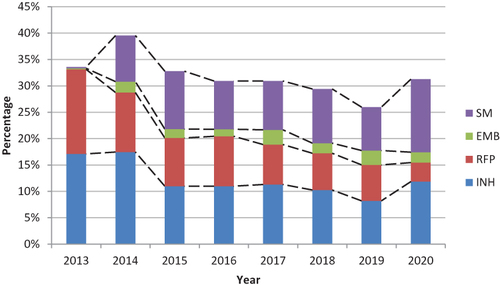
Among previously treated cases with suspected DR-TB, the resistance rate of INH decreased significantly from 38.67% in 2013 to 25.96% in 2020 (χ2 = 22.030, P<0.001). The resistance rate of RFP decreased significantly from 44.20% in 2013 to 11.54% in 2020 (χ2 = 74.140, P<0.001). The resistance rate of EMB increased from 1.66% in 2013 to 8.65% in 2020 (χ2 = 1.975, P=0.160), but the upward trend was not statistically significant. The resistance rate of SM increased from 2.76% in 2013 to 8.65% in 2020 (χ2 = 0.007, P=0.934), but the upward trend was not statistically significant ().
Drug Resistance Trends in RR-TB and MDR-TB Among New and Previously Treated Cases with Suspected DR-TB, 2013 to 2020
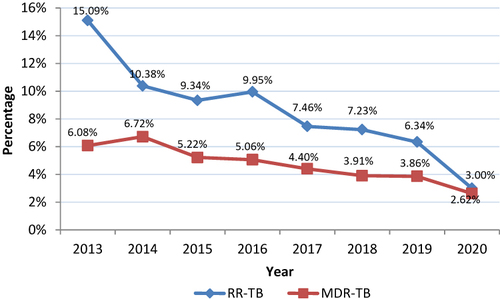
Among new cases with suspected DR-TB, the rate of RR-TB decreased from 15.09% in 2013 to 3.00% in 2020 with an average annual decrease of 20.61%, showing a significant downward trend (χ2 = 35.429, P<0.001). The rate of MDR-TB decreased from 6.08% in 2013 to 2.62% in 2020 with an average annual decrease of 11.33%, showing a significant downward trend (χ2 = 10.040, P=0.002) ().
Figure 5 Drug resistance trends in RR-TB and MDR-TB among new TB patients from 2013 to 2020.
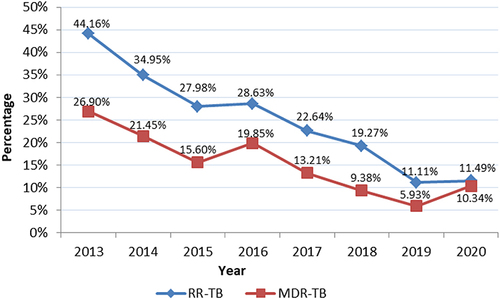
Between 2013 and 2020, the rate of RR-TB in cases with a prior treatment history decreased significantly from 44.16% to 11.49% with a mean annual decrease of 17.50% (χ2 = 70.382, P<0.001). Between 2013 and 2020, the rate of MDR-TB in cases with a prior treatment history decreased significantly from 26.90% to 10.34% with a mean annual decrease of 12.77% (χ2 = 37.401, P<0.001) ().
Association Between Sociodemographic Characteristics and MDR-TB in Patients with Suspected DR-TB
In a multifactorial analysis, men were more likely to develop MDR-TB than women (AOR 1.28, 95% CI 1.035–1.585). TB patients aged 45 to 64 years had a 1.75-fold risk of developing MDR-TB (AOR 1.75, 95% CI 1.342–2.284) compared with those younger than 45 years, and those aged 65 years and older had a 1.65-fold risk of developing MDR-TB (AOR 1.65, 95% CI 1.293–2.104). Patients living in rural areas were more likely to develop MDR-TB than those living in urban areas (AOR 1.24, 95% CI 1.014–1.519). Patients with a history of TB treatment were at higher risk of developing MDR-TB than new patients (AOR 3.94, 95% CI 3.275–4.741) ().
Table 3 Univariable and Multivariable Logistic Regression Analyses of Sociodemographic Characteristics for MDR-TB Among Suspected DR-TB Patients
Discussion
This is the first retrospective study to focus on drug resistance patterns and trends in suspected DR-TB patients in Dalian, China. Among patients with suspected DR-TB, the prevalence of DR-TB, RR-TB and MDR-TB showed a significant decrease in both new and previously treated patients with suspected DR-TB, from 2013 to 2020. We hypothesize that several reasons could explain this finding. First, the DR-TB control strategy, including the implementation of short-course chemotherapy strategies under DOTS and improved adherence to medication among TB patients at the site, is effective. Other studies have shown that poor adherence to medication is a risk factor for the development of DR-TB.Citation23–25 In addition, other prevention and control measures were also taken during this period, such as placing DR-TB patients in separate wards from susceptible TB patients for treatment. This separation policy effectively prevented the nosocomial transmission of DR-TB. The second is the continued government investment in special funds for TB treatment subsidies, such as regular transportation grants and nutrition payments. This policy helps to reduce the financial burden on patients. As a disease of poverty, poorer economic conditions are an important risk factor for the development of DR-TB.Citation26 Finally, the development of new rapid detection technologies, such as line probe assay (LPA) and Xpert MTB/RIF, has improved accessibility to DST, further reducing the development of DR-TB.
Regarding resistance to specific drugs, the rates of resistance to RFP and INH decreased significantly in both new and previously treated TB patients. However, the resistance rates of SM and EMB showed an increasing trend. This finding can be explained. The DST results of EMB and SM were less accurate than those of other FLDs tested in vitro for DST.Citation27,Citation28 This feature may have contributed to the findings in the study. These findings will help to adjust the control measures for DR-TB and further reduce the number of DR-TB patients.
Using multifactorial regression analysis, we found that among patients with suspected DR-TB, men were more likely to develop MDR-TB than women. A systematic review of studies confirmed our study results.Citation29 We suggest that this finding is potentially explained by the notion that men are more likely to consume more tobacco and alcohol than women.Citation30 Moreover, another reason may be that men are less compliant with TB treatment than women, thus increasing their risk of developing MDR-TB.Citation31 In our study, it was found that patients aged 45 years and older with suspected TB were more likely to develop MDR-TB than patients aged less than 45 years. This finding is similar to the results of other previous studies.Citation24,Citation32 In contrast to this finding, previous studies have found that MDR-TB is more common in patients younger than 45 years of age given that they are busier with work or study than older patients and subsequently more exposed to the risk of DR-TB.Citation33–35 This discrepancy may be related to the different study populations. Patients with suspected DR-TB living in rural areas are more likely to develop MDR-TB than those living in urban areas. This finding is similar to that reported in a previous study.Citation36 One possible reason for this is that patients living in rural areas usually have poorer adherence to TB treatment than those living in urban areas.Citation25 In addition, this finding is noted potentially because rural patients live in remote areas with insufficient socioeconomic development, low education and a lack of nutrition and sanitation facilities, which have an important impact on the development of MDR-TB.Citation35 TB patients with a history of treatment were significantly associated with MDR-TB (P<0.05).Citation37 A similar result was found in a study conducted in Ethiopia.Citation38 TB patients with a history of treatment often have poor medication habits, which then lead to suppression of the growth of susceptible bacilli and the development of resistance in the remaining strains.Citation39
Limitations
Some limitations of the current study should be noted. First, given that our study was conducted on patients with suspected DR-TB, our findings cannot be generalized to patients with other types of TB. Second, due to limited data, we only studied the status of INH, RFP, SM and EMB in suspected DR-TB, whereas the status of resistance to other anti-drugs (eg, pyrazinamide and ofloxacin) was not analyzed. Third, some important information, such as smoking, alcohol consumption, and nutritional status were not included.
Conclusion
This study identified a significant decrease in the proportion of DR-TB, RR-TB and MDR-TB among patients with suspected DR-TB from 2013 to 2020. This finding implies that the regional preventive and control measures for DR-TB are effective. An increasing trend of drug resistance against SM and EMB were noted among patients with suspected DR-TB. In addition, sex, age, residence and patient type were significantly associated with MDR-TB. The development of targeted measures among patients with suspected DR-TB would be beneficial for the control of DR-TB.
Consent for Publication
Not applicable as details like videos or images related to study subjects were not recorded for this study.
Author Contributions
YP contribute to conception and study design, analysis, interpretation of data and drafting the article. All authors made substantial contributions to acquisition of data, revising or critically reviewing the article; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are indebted to all study participants whose data were used in this study. We thank the Haoqiang Ji for revision suggestions. We also thank Dongping Pan for his support of this research.
Additional information
Funding
References
- World Health Organization. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. Geneva, Switzerland: World Health Organization; 2013.
- Wang MG, Wu SQ, He JQ. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1). doi:10.1186/s12879-021-06666-8
- Fox GJ, Nguyen TA, Coleman M, Trajman A, Velen K, Marais BJ. Implementing tuberculosis preventive treatment in high-prevalence settings. Int J Infect Dis. 2021;113:S13–S5. doi:10.1016/j.ijid.2021.02.094
- World Health Organization. Global Tuberculosis Report 2021. Geneva, Switzerland: World Health Organization; 2021.
- Zurcher K, Ballif M, Fenner L, et al. Drug susceptibility testing and mortality in patients treated for tuberculosis in high-burden countries: a multicentre cohort study. Lancet Infectious Dis. 2019;19(3):298–307. doi:10.1016/S1473-3099(18)30673-X
- World Health Organization. Global Tuberculosis Report 2020. Geneva, Switzerland: World Health Organization; 2020.
- Ali S, Khan MT, Khan AS, Abbas Q, Irfan M. Fluoroquinolone Resistance Among Isolates of Mycobacterium tuberculosis in Khyber Pakhtunkhwa, Pakistan. Microbial Drug Resistance. 2021;27(6):786–791. doi:10.1089/mdr.2020.0118
- Alene KA, Xu ZH, Bai LQ, et al. Spatial clustering of drug-resistant tuberculosis in Hunan province, China: an ecological study. BMJ Open. 2021;11(4). doi:10.1136/bmjopen-2020-043685.
- Song WM, Li YF, Ma XB, et al. Primary drug resistance of Mycobacterium tuberculosis in Shandong, China, 2004-2018. Respir Res. 2019;20(1). doi:10.1186/s12931-019-1199-3.
- Tantan R, Puxuan L, Guofang D. 2020WHO tuberculosis report: key data for China, the whole world. Electronic J Em Infect Dis. 2020;5(4):280.
- Ding C, Wang ST, Shangguan YW, et al. Epidemic Trends of Tuberculosis in China from 1990 to 2017: evidence from the Global Burden of Disease Study. Infect Drug Resist. 2020;13:1663–1672. doi:10.2147/IDR.S249698
- Tembo BP, Malangu NG. Prevalence and factors associated with multidrug/rifampicin resistant tuberculosis among suspected drug resistant tuberculosis patients in Botswana. BMC Infect Dis. 2019;19(1):1–8. doi:10.1186/s12879-019-4375-7
- Günther G. Multidrug-resistant and extensively drug-resistant tuberculosis: a review of current concepts and future challenges. Clin Med (Northfield Il). 2014;14(3):279. doi:10.7861/clinmedicine.14-3-279
- Khan MT, Ali S, Khan AS, et al. Insight into the drug resistance whole genome of Mycobacterium tuberculosis isolates from Khyber Pakhtunkhwa, Pakistan. Infect Genetics Evolution. 2021;92:104861. doi:10.1016/j.meegid.2021.104861
- Du L, Zhang Y, Lv X, et al. Prevalence of multidrug-resistant tuberculosis in Dalian, China: a Retrospective Study. Infect Drug Resist. 2021;14:1037. doi:10.2147/IDR.S294611
- Ji H, Xu J, Wu R, et al. Cut-off Points of Treatment Delay to Predict Poor Outcomes Among New Pulmonary Tuberculosis Cases in Dalian, China: a Cohort Study. Infect Drug Resist. 2021;14:5521. doi:10.2147/IDR.S346375
- Rasool G, Khan AM, Mohy-Ud-Din R, Riaz M. Detection of Mycobacterium tuberculosis in AFB smear-negative sputum specimens through MTB culture and GeneXpert(®) MTB/RIF assay. Int J Immunopathol Pharmacol. 2019;33:2058738419827174. doi:10.1177/2058738419827174
- Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Eng J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa1108789
- Wan L, Guo Q, Wei J-H, et al. Accuracy of a reverse dot blot hybridization assay for simultaneous detection of the resistance of four anti-tuberculosis drugs in Mycobacterium tuberculosis isolated from China. Infect Dis Poverty. 2020;9(1):1–10. doi:10.1186/s40249-020-00652-z
- Li X, Sheng L, Tu J, Lou L. A preliminary study on the synergistic effect of nano Ag and anti-tuberculosis drugs on drug-resistant Mycobacterium tuberculosis. J Nanosci Nanotechnol. 2020;20(10):6155–6160. doi:10.1166/jnn.2020.18597
- Rahman SM, Ather MF, Nasrin R, et al. Performance of WHO-Endorsed Rapid Tests for Detection of Susceptibility to First-Line Drugs in Patients with Pulmonary Tuberculosis in Bangladesh. Diagnostics. 2022;12(2):410. doi:10.3390/diagnostics12020410
- Definitions W. Reporting Framework for Tuberculosis–2013 Revision. Geneva: World Health Organization; 2013.
- Ullah I, Javaid A, Tahir Z, et al. Pattern of drug resistance and risk factors associated with development of drug resistant Mycobacterium tuberculosis in Pakistan. PLoS One. 2016;11(1):e0147529. doi:10.1371/journal.pone.0147529
- Pradipta IS, Forsman LD, Bruchfeld J, Hak E, Alffenaar J-W. Risk factors of multidrug-resistant tuberculosis: a global systematic review and meta-analysis. J Infection. 2018;77(6):469–478. doi:10.1016/j.jinf.2018.10.004
- Desissa F, Workineh T, Beyene T. Risk factors for the occurrence of multidrug-resistant tuberculosis among patients undergoing multidrug-resistant tuberculosis treatment in East Shoa, Ethiopia. BMC Public Health. 2018;18(1):1–6. doi:10.1186/s12889-018-5371-3
- Wang K, Chen SH, Wang XM, et al. Factors Contributing to the High Prevalence of Multidrug-Resistant Tuberculosis Among Previously Treated Patients: a Case-Control Study from China. Microbial Drug Resistance. 2014;20(4):294–300. doi:10.1089/mdr.2013.0145
- Guidelines ATC. Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations. J Assoc Physicians India. 2006;54:219–234.
- Jou R, Chiang C, Yu C, Wu M. Proficiency of drug susceptibility testing for Mycobacterium tuberculosis in Taiwan. Int j Tuberculosis Lung Dis. 2009;13(9):1142–1147.
- Rajendran M, Zaki RA, Aghamohammadi N. Contributing risk factors towards the prevalence of multidrug-resistant tuberculosis in Malaysia: a systematic review. Tuberculosis. 2020;122:101925. doi:10.1016/j.tube.2020.101925
- Baluku JB, Mukasa D, Bongomin F, et al. Gender differences among patients with drug resistant tuberculosis and HIV co-infection in Uganda: a countrywide retrospective cohort study. BMC Infect Dis. 2021;21(1):1–11. doi:10.1186/s12879-021-06801-5
- Zhang C, Wang Y, Shi G, et al. Determinants of multidrug-resistant tuberculosis in Henan province in China: a case control study. BMC Public Health. 2015;16(1):1–8. doi:10.1186/s12889-016-2711-z
- Suarez-Garcia I, Rodriguez-Blanco A, Vidal-Perez J, et al. Risk factors for multidrug-resistant tuberculosis in a tuberculosis unit in Madrid, Spain. Eur j Clin Microbiol Infect Dis. 2009;28(4):325–330. doi:10.1007/s10096-008-0627-y
- Soeroto AY, Pratiwi C, Santoso P, Lestari BW. Factors affecting outcome of longer regimen multidrug-resistant tuberculosis treatment in West Java Indonesia: a retrospective cohort study. PLoS One. 2021;16(2):e0246284. doi:10.1371/journal.pone.0246284
- Ahmad AM, Akhtar S, Hasan R, Khan JA, Hussain SF, Rizvi N. Risk factors for multidrug-resistant tuberculosis in urban Pakistan: a multicenter case–control study. Int j Mycobacteriol. 2012;1(3):137–142. doi:10.1016/j.ijmyco.2012.07.007
- Hameed S, Ahmad SR, Rahman MAU, Nazir H, Ullah I. Drug resistance profile of Mycobacterium tuberculosis and predictors associated with the development of drug resistance. J Global Antimicrobial Resistance. 2019;18:155–159. doi:10.1016/j.jgar.2019.03.009
- Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. doi:10.1016/j.ijid.2015.08.013
- Mehari K, Asmelash T, Hailekiros H, et al. Prevalence and factors associated with multidrug-resistant tuberculosis (MDR-TB) among presumptive MDR-TB patients in Tigray Region, Northern Ethiopia. Canadian J Infect Dis Med Microbiol. 2019;2019:548.
- Workicho A, Kassahun W, Alemseged F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist. 2017;10:91–96. doi:10.2147/IDR.S126274
- Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15. doi:10.1186/s12879-015-1202-7