Abstract
Purpose
The characteristics of patients with severe COVID-19 pneumonia who underwent direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP), in addition to steroids and immunomodulators, remain unclear.
Patients and Methods
We conducted a retrospective observational study on 31 patients with severe COVID-19 pneumonia treated with PMX-DHP in an intensive care unit (ICU) from December 2020 to September 2021.
Results
Outcomes 28 days after admission to the ICU were 20 in the survival group and 11 in the death group. Parameters significantly different between the survival and death group before PMX-DHP were percentage of invasive mechanical ventilation (25% vs 72.7%, P = 0.0209), PaO2/FIO2 (P/F) ratio (104.5 vs 75, P = 0.0317), and sequential organ failure assessment (SOFA) score (2 vs 3, P = 0.0356). Invasive mechanical ventilation avoidance rate was significantly different between the survival (100%) and death group (0%) (P = 0.0012). P/F ratio, respiratory ratio (RR), and lymphocyte counts improved significantly after PMX-DHP for all patients. The lymphocyte counts changed significantly in the survival (P < 0.0001), but not the death group (P = 0.7927).
Conclusion
PMX-DHP, in addition to steroids and immunomodulators, may improve oxygenation and alleviate tachypnea by modulating the lymphocyte numbers and levels of various mediator against severe COVID-19 pneumonia. It may be better to perform PMX-DHP before multi organ dysfunction and lung injury has progressed. Furthermore, the early increase in lymphocyte counts after PMX-DHP might be an indicate a positive outcome.
Introduction
A worldwide pandemic of the Coronavirus disease (COVID-19) started in 2019, with many deaths from severe pneumonia.Citation1 Patients with severe COVID-19 patients in intensive care units (ICU) often need ventilator management,Citation2 which results in long stays in the ICU, and a shortage of ICU beds becomes a serious problem. Systemic inflammation involving high levels of various cytokines, cytokine storms, might be associated in the mechanism underlying the aggravation of COVID-19.Citation3 Therefore, inhibition of cytokine activity may aid the recovery from severe COVID-19. At present, steroids are often used for systemic inflammation during COVID-19 pneumonia.Citation4 Additionally, immunomodulators, such an interleukin (IL)-6 receptor blockade (tocilizumab and sarilumab)Citation5 and Janus kinase (JAK) inhibitors (baricitinib),Citation6 may be effective against severe COVID-19 pneumonia. However, even if these immunomodulators are administered, ventilator management is needed; sometimes, this leads to fatal outcomes. Therefore, further treatment options are needed for severe COVID-19 pneumonia.
Direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) is a blood purification therapy used to treat septic shock and peritonitis.Citation7,Citation8 The main mechanism of action of PMX-DHP is endotoxin adsorption. In addition, another supposed mechanism underlying its action is its ability to change the leukocyte ratio in various manners, such as by increasing the lymphocyte numbers, decreasing the regulatory T cell ratio,Citation9 and removing monocytes.Citation10 PMX-DHP therapy may improve acute respiratory diseases, such as rapidly progressive interstitial pneumoniaCitation11 and acute exacerbation of idiopathic pulmonary fibrosis,Citation12 by suppressing the activity of mediators, including various cytokines. Severe COVID-19 pneumonia causes pulmonary fibrosis.Citation13,Citation14 Therefore, PMX-DHP therapy may be effective for severe COVID-19 pneumonia. However, there are only a few case reports regarding the treatment of severe COVID-19 pneumonia using PMX-DHP.Citation15–17 The characteristics of patients with severe COVID-19 pneumonia that were treated using PMX-DHP and the patient characteristics that are supportive of recovery remain unclear.
We performed an observational study to explore the overall characteristics of patients who underwent PMX-DHP treatment for severe COVID-19 pneumonia.
Materials and Methods
We conducted a retrospective observational study at the Nihon University Itabashi Hospital, Tokyo. All patients in this study were admitted to the ICU from December 2020 to September 2021 and were diagnosed with severe COVID-19 pneumonia by positive SARS-CoV-2 real-time reverse transcription-polymerase chain reaction (RT-PCR) and pneumonia on chest X-ray or chest computerized tomography (CT). Severe COVID-19 was defined as a P/F ratio under 200 with respiratory support (use of an oxygen mask with a reservoir, high-flow nasal cannula (HFNC), or invasive mechanical ventilation) and ICU admission. All patients included in the study underwent PMX-DHP during ICU admission. Patients who received PMX-DHP for the amelioration of septic shock were excluded.
PMX-DHP therapy was considered when the PaO2/FIO2 (P/F) ratio did not improve (less than 200) 24 to 72 hours after treatment with steroids and tocilizumab or baricitinib as the immunomodulatory therapy. After the careful consideration of patient parameters, the clinical COVID-19 team comprising healthcare professionals would decide whether to perform PMX-DHP therapy on each patient.
PMX-DHP therapy was performed using Toraymyxin PMX-20R (Toray Industries, Tokyo, Japan) at a blood flow rate of 80–120 mL/min for 6 h for over two continuous days on each patient. Nafamostat mesylate (30–50 mg/h) or unfractionated heparin (50 U/kg/h) was used for anticoagulation therapy.
The following data were collected from clinical records: age, gender, BMI, past history, whether they survived in the ICU, blood sampling data, chest radiograph score,Citation18 respiratory rate (RR), sequential organ failure assessment (SOFA) score,Citation19 onset to admission/ICU and admission/PMX-DHP treatment days, duration of ICU stay, treatment profile (antiviral agent, steroid, immunomodulatory therapy, anticoagulant therapy), respiratory support when PMX-DHP treatment was initiated, invasive mechanical ventilation avoidance rate, and variant type of SARS-CoV-2. The SOFA score refers to the organ dysfunction score and is calculated as a point score based on the function of six organ systems (respiratory, coagulation, hepatobiliary, cardiovascular, central nervous, and renal systems). A higher score (0–24) is associated with increased ICU mortality.Citation19 In order to determine the chest radiography score, the chest radiograph was divided into four zones, the extent of abnormal lung parenchyma was visually scored as 0, no involvement; 1, mild to moderate involvement (estimated involvement 0–50% of lung parenchyma); and 2, severe involvement (estimated involvement, 50% of lung parenchyma) per zone, resulting in a score between 0 and 8.Citation18
As a guide of our facility for respiratory support, treatment using a high-flow nasal cannula (HFNC) was performed for patients when the SpO2 was less than 93% after about 8–10 L/min an oxygen mask with a reservoir was used for respiratory support. Invasive mechanical ventilation, while performing muscle relaxation and deep sedation, was performed when tachypnea (RR, 30/min or higher) progressed, hypoxemia progressed, and PaCO2 increased, even after the use of an HFNC. Veno-venous extracorporeal membrane oxygenation (V–V ECMO) was performed following the ELSO guidelines.Citation20 However, our facility is not a high-volume center for V–V ECMO.
All statistical analyses were performed using JMP 13.0.0 (SAS Institute Inc., North Carolina, USA). Normality tests were performed and showed that the data had a non-normal distribution. The Mann–Whitney U-test was used to compare the results between the two groups (survival group and death group). Categorical variables were compared using Fisher’s exact test. The Kruskal–Wallis test was used to assess three timepoints: day 1, before PMX-DHP therapy was performed; day 3, the day after PMX-DHP therapy was performed twice; and day 7. The possible pairwise comparisons for the three points were conducted using the Steel-Dwass test. Results with a p-value < 0.05 were considered statistically significant. The data are shown as the median and interquartile range because the data were not normally distributed.
Results
The flowchart for patient selection is shown . We excluded 3 cases for which PMX-DHP therapy was used to ameliorate septic shock. The characteristics of all 31 patients are shown in . The outcomes 28 days after admission to the ICU were 20 patients in the survival group and 11 in the death group. A period of 6 days passed from disease onset to admission, 10 days to ICU admission, and 11 days (median days) to PMX-DHP therapy. The treatments for COVID-19 were antivirals (97%), steroids (100%), immunomodulatory therapies (100%; tocilizumab or baricitinib), and anticoagulants (93.6%). The respiratory support used before PMX-DHP therapy was as follows: O2 supplementation (not ventilated), 9.7%; HFNC, 48.4%; invasive mechanical ventilation, 41.9%; and V–V ECMO, 0%. The invasive mechanical ventilation avoidance rate was 15/18 (83.3%), differing significantly between the survival (15/15) and death groups (0/3) (P = 0.0012). The variant type B.1.617.2 (Delta) was detected in 15/18 (83%) of participants overall: 10/13 (76.9%) in the survival group and 5/5 (100%) in the death group. No mutant variant other than the Delta variant was tested. No Omicron variant was detected in Japan during the study period. The age (P = 0.0021) and percentage of invasive mechanical ventilation (P = 0.0209) were significantly different between the survival and death groups; other characteristics were not significantly different.
Table 1 Baseline Characteristics of Patients Treated with PMX-DHP Therapy
The results of the patient evaluations immediately before PMX-DHP therapy are summarized in . The medians of each evaluated parameter were as follows: P/F ratio, 100; RR, 25; neutrocyte count, 7970 cells / L; lymphocyte count, 354 cells/ L; C-reactive protein (CRP), 1.57 mg/dL; D-dimer, 2.6 ug/mL; ferritin, 718.7 ng/mL; lactate dehydrogenase (LDH), 491 U/L; surfactant protein D (SP-D), 247 ng/mL; thymus and activation-regulated chemokine (TARC), 118.5 pg/mL; interleukin (IL)-6, 126 pg/mL; IL-10, 2 pg/mL; SOFA score 3; and chest radiograph score, 7. The parameters that differed significantly between the survival and death groups were P/F ratio (P = 0.0317), D-dimer (P = 0.0015), LDH (P = 0.0258), IL-6 (P = 0.0430), and SOFA score (P = 0.0356). No data for 3 cases of ferritin, for 1 case of SP-D, TARC, IL-6 and IL-10, respectively.
Table 2 Results of the Baseline Examinations of Patients Treated with PMX-DHP Therapy (Median)
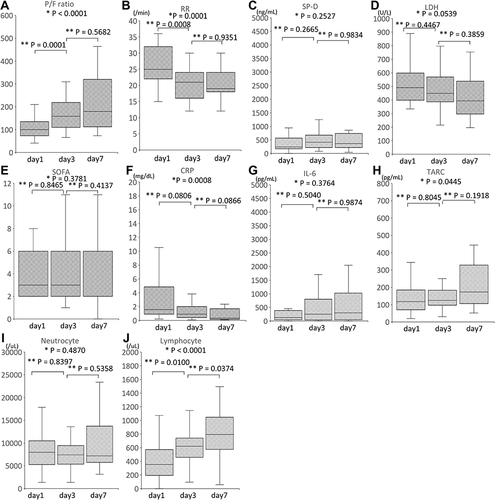
The time course of the clinical parameter measurements for all patients are presented in . The measurements were taken at three timepoints: day 1, before PMX-DHP therapy was performed; day 3, the day after PMX-DHP therapy was performed twice; and day 7. The P/F ratio (A, P < 0.0001), RR (B, P = 0.0001), CRP (F, P = 0.008), TARC (H, P = 0.0445), and Lymphocyte counts (J, P < 0.0001) changed significantly over time. The SP-D (C, P = 0.2527), LDH (D, P = 0.0539), SOFA (E, P = 0.3781), IL-6 (G, P = 0.3764), and Neutrocyte count (I, P = 0.4870) did not change significantly over time.
Figure 2 Time course of the clinical parameters for all patients. Median and interquartile ranges of P/F ratio (A), RR (B), SP-D (C), LDH (D), SOFA (E), CRP (F), IL-6 (G), TARC (H), Neutrocyte (I) and Lymphocyte (J) count. P/F ratio, RR, SP-D, LDH, SOFA, CRP, IL-6, TARC, Neutrocyte and Lymphocyte count were measured before the start of PMX-DHP (day 1), after PMX-DHP was administered 2 times (day 3) and on day 7. Since 2 patients died on day 5, there were no data for 2 cases on day 7. There were no data regarding the SP-D, TARC, and IL-6 for 1 case. CRP, C-reactive protein; IL, interleukin; LDH, lactate dehydrogenase; P/F, PaO2/FIO2; RR, Respiratory ratio; SOFA, sequential organ failure assessment; SP-D, Surfactant Protein-D; TARC, thymus and activation-regulated chemokine; *Kruskal–Wallis test; **Steel-Dwass test.
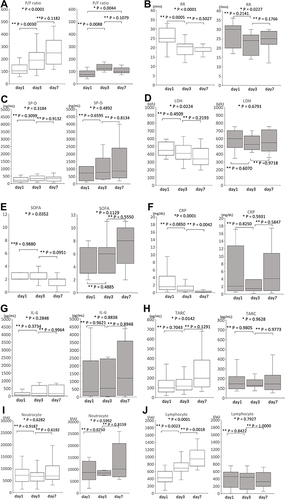
The time course of the clinical parameters for the survival and death groups are shown in . In the death group, 2 deaths occurred on day 5. The P/F ratio (A, P < 0.0001 in survival group, P = 0.0044 in death group) and RR (B, P < 0.0001 in survival, P = 0.0227 in death group) changed significantly over time in the survival and death groups. The LDH (D, P = 0.0224), SOFA (E, P = 0.0352), CRP (F, P < 0.0001), TARC (H, P = 0.0142) and Lymphocyte count (J, P < 0.0001) changed significantly in the survival group but remained unchanged in the death group. No data of SP-D, TARC, and IL-6 were obtained for 1 case.
Figure 3 Time course of the clinical parameters for the survival (white) and death (black) groups. Median and interquartile ranges of the P/F ratio (A), RR (B), SP-D (C), LDH (D), SOFA (E), CRP (F), IL-6 (G), TARC (H), Neutrocyte (I) and Lymphocyte (J) count. The P/F ratio, RR, SP-D, LDH, SOFA score, CRP, IL-6, TARC, and Neutrocyte and Lymphocyte count were measured before the start of PMX-DHP (day 1), after PMX-DHP was administered 2 times (day 3), and on day 7. Because 2 patients died on day 5, there were no data for 2 cases in the death group on day 7. There were no data regarding the SP-D, TARC, and IL-6 for 1 case in the death group. CRP, C-reactive protein; IL, interleukin; LDH, lactate dehydrogenase; P/F, PaO2/FIO2; RR, Respiratory ratio; SOFA, sequential organ failure assessment; SP-D, Surfactant Protein-D; TARC, thymus and activation-regulated chemokine; *Kruskal–Wallis test; **Steel-Dwass test.
Discussion
In the present study, we made two important clinical observations. First, in patients with severe COVID-19 receiving PMX-DHP therapy, the P/F ratio, RR, and lymphocyte counts improved at an early stage. An early increase in lymphocyte counts after PMX-DHP therapy may indicate a positive outcome. Second, at the time of PMX-DHP therapy, the P/F ratio and SOFA score were worse in the death group than in the survival group.
The IL-6 is lower in severe COVID-19 pneumonia than in sepsis.Citation21 Common treatments for severe COVID-19 pneumonia include anti-IL-6 receptor monoclonal antibodies, such as tocilizumab, and JAK inhibitors, in addition to steroids. The mortality of patients with severe COVID-19 pneumonia was significantly lower in the steroid plus tocilizumab combination group than in the steroid-increased group.Citation22 Thus, anti–IL-6 receptor monoclonal antibodies can clinically ameliorate severe COVID-19 pneumonia. However, because IL-6 levels are lower in severe COVID-19 pneumonia than in sepsis,Citation21 blocking IL-6 alone may not be effective in some severe COVID-19 cases; thus, in such cases, inhibiting mediators other than IL-6 may improve clinical outcomes. In the present study, the IL-6 levels did not change over time in all patients, although PMX-DHP therapy was performed for many cases using anti-IL-6 receptor antibodies. Ichiyasu et al reported that PMX-DHP therapy improved the mortality and the P/F ratio in patients with acute respiratory diseases, such as rapidly progressive interstitial pneumonia.Citation11 This study showed that the IL-6 levels were similar in the survivor and non-survivor groups; furthermore, the P/F ratio improved after PMX-DHP therapy in the survivor group but not in the non-survivor group. Therefore, PMX-DHP therapy for severe COVID-19 pneumonia may improve the P/F ratio and RR via some favorable effects against these disease-aggravating processes, including an anti-inflammatory effects. Another study suggested that the lymphocyte count in severe COVID-19 cases are lower than those in non-severe cases and may thus serve as a potential therapeutic target.Citation23 In yet another study regarding sepsis, increasing the lymphocyte counts was suggested as a proposed mechanism underlying the effectiveness of PMX-DHP.Citation9 In the present study, the lymphocyte count increased significantly in the survival group. Conversely, the lymphocyte count did not significantly change in the death group. It is unclear whether the PMX-DHP therapy increased the lymphocyte count; however, the early increase in lymphocyte count after PMX-DHP therapy may be indicative of a positive outcome. PMX-DHP therapy alleviated the acute exacerbation of idiopathic pulmonary fibrosis.Citation12 PMX-DHP therapy for the acute exacerbation of idiopathic pulmonary fibrosis significantly decreased the levels of IL-9, IL-10, IL-12, IL-17A, and profibrotic cytokines, including platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF).Citation24 COVID-19 patients can develop pulmonary fibrosis. Fibrotic changes in the lung were found after autopsy in patients who died of COVID-19.Citation25,Citation26 In severe COVID-19 pneumonia, PMX-DHP therapy in addition to steroids and anti-IL-6 receptor monoclonal antibodies, may improve oxygenation and ameliorate tachypnea by modulating the lymphocyte count and the levels of various mediators other than IL-6.
In COVID-19 patients, a lower P/F ratio on ICU admission was an independent risk factor associated with mortality.Citation27 SP-D is a useful marker for acute lung injury and acute respiratory distress syndrome (ARDS) and indicates the degree of lung injury.Citation28 SP-D also shows severity in COVID-19 patients.Citation29 In the present study, the SP-D was relatively high in the death group before PMX-DHP therapy, although not significantly different; in the death group, as shown by the low P/F ratio and the relatively high SP-D, lung injury caused by COVID-19 had already progressed at the time of PMX-DHP therapy. In addition, the proportion of cases for whom invasive mechanical ventilation was performed before PMX-DHP therapy was significantly higher in the death group than in the survival group. This also suggests that lung injury was progressing. Even in a previously reported meta-analysis, the fatality rate for COVID-19 patients requiring invasive mechanical ventilation was high.Citation30 PMX-DHP therapy may be less effective in COVID-19 with progressed severe lung injury, including features such as lower oxygenation, higher SP-D, and requirement for invasive mechanical ventilation. The SOFA score was high in the death group before PMX-DHP therapy. In a systematic review regarding COVID-19, a high SOFA score was shown to increase the risk of death.Citation31 Thus, COVID-19 patients with a high SOFA score are likely to show poor outcomes. In the present study, the SOFA score improved after PMX-DHP therapy in the survival group but not in the death group, and the invasive mechanical ventilation avoidance rate in the survival and death groups differed significantly. Therefore, it may be better to perform PMX-DHP therapy before multi-organ dysfunction and lung injury have progressed.
This study has four limitations. First, the results of the present study cannot be compared with those of a non-PMX-DHP therapy group. In the future, a prospective study using the non-PMX-DHP therapy group as a control is needed. Second, patients subjected to respiratory management using invasive ventilators have poor outcomes, but it is unknown whether PMX-DHP therapy is ineffective when invasive ventilator management is performed. Thus, the effectiveness of PMX-DHP therapy for COVID-19 patients undergoing invasive ventilator management should be investigated. Third, it is unknown whether the effects observed in this study are due to PMX-DHP therapy alone or also due to the other treatments used in combination, such as steroids and immunomodulatory therapy. However, in the present study, baseline treatments for severe COVID-19 pneumonia, including antiviral agents, immunomodulatory therapies, and anticoagulants, were administered in most cases. Therefore, these data will help clarify whether PMX-DHP therapy is effective as an additional treatment in combination with the baseline treatments. Fourth, this study does not examine the superiority of each respiratory therapy (for eg, HFNC and invasive mechanical ventilation). Further research is needed to determine which combination of PMX-DHP and respiratory therapy is effective.
Conclusions
This study shows that the P/F ratio, RR, and lymphocyte counts were improved early in patients with severe COVID-19 receiving PMX-DHP therapy. PMX-DHP therapy, in addition to steroids and immunomodulators, may improve oxygenation and alleviate tachypnea by modulating the lymphocyte counts and the levels of various mediators. An early increase in lymphocyte counts after PMX-DHP therapy may indicate a positive clinical outcome. Thus, performing PMX-DHP therapy before multi-organ dysfunction and lung injury have progressed may improve disease outcomes in patients with COVID-19 pneumonia.
Ethics Approval and Informed Consent
This study complies with the Declaration of Helsinki. This study was approved by the Clinical Research Review Committee of the Nihon University School of Medicine (RK-211214-13). Since this is a retrospective observational study, we do not have individual patient consent. Information regarding disclosure posters are disclosed.
Disclosure
We have no conflict of interest to declare.
Additional information
Funding
References
- Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed J. 2020;43(4):334–340. doi:10.1016/j.bj.2020.05.023
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323(16):1574–1581. doi:10.1001/jama.2020.5394
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi:10.1016/S0140-6736(20)30628-0
- Horby P, Lim WS; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:10.1056/NEJMoa2021436
- Gordon AC, Mouncey PR; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi:10.1056/NEJMoa2100433
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi:10.1056/NEJMoa2031994
- Dellinger RP, Bagshaw SM, Antonelli M, et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the Euphrates randomized clinical trial. JAMA. 2018;320(14):1455–1463. doi:10.1001/jama.2018.14618
- Shimizu T, Miyake T, Kitamura N, Tani M, Endo Y. Endotoxin adsorption: direct hemoperfusion with the polymyxin B-immobilized fiber column (PMX). Transfus Apher Sci. 2017;56(5):682–688. doi:10.1016/j.transci.2017.08.015
- Ono S, Kimura A, Hiraki S, et al. Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery. 2013;153(2):262–271. doi:10.1016/j.surg.2012.06.023
- Nishibori M, Takahashi HK, Katayama H, et al. Specific removal of monocytes from peripheral blood of septic patients by polymyxin B-immobilized filter column. Acta Med Okayama. 2009;63(1):65–69. doi:10.18926/AMO/31855
- Ichiyasu H, Horio Y, Masunaga A, et al. Efficacy of direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) in rapidly progressive interstitial pneumonias: results of a historical control study and a review of previous studies. Ther Adv Respir Dis. 2017;11(7):261–275. doi:10.1177/1753465817708950
- Enomoto N, Mikamo M, Oyama Y, et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med. 2015;15:15. doi:10.1186/s12890-015-0004-4
- Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518–2520. doi:10.1001/jama.2020.8907
- Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi:10.1016/S2213-2600(20)30222-8
- Kuwana T, Kinoshita K, Hirabayashi M, et al. PMX-DHP therapy for dyspnea and deoxygenation in severe COVID-19 pneumonia: a case series. Infect Drug Resist. 2021;14:1305–1310. doi:10.2147/IDR.S299023
- Katagiri D, Ishikane M, Asai Y, et al. Direct hemoperfusion using a polymyxin B-immobilized polystyrene column for COVID-19. J Clin Apher. 2021;36(3):313–321. doi:10.1002/jca.21861
- De Rosa S, Cutuli SL, Ferrer R, Antonelli M, Ronco C; COVID-19 EUPHAS2 Collaborative Group. Polymyxin B hemoperfusion in coronavirus disease 2019 patients with endotoxic shock: case series from EUPHAS2 registry. Artif Organs. 2021;45(6):E187–E194. doi:10.1111/aor.13900
- Schalekamp S, Huisman M, van Dijk RA, et al. Model-based prediction of critical illness in hospitalized patients with COVID-19. Radiology. 2021;298(1):E46–E54. doi:10.1148/radiol.2020202723
- Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi:10.1097/00003246-199811000-00016
- Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi:10.1097/MAT.0000000000001193
- Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi:10.1016/S2213-2600(20)30404-5
- Naik NB, Puri GD, Kajal K, et al. High-dose dexamethasone versus tocilizumab in moderate to severe COVID-19 pneumonia: a randomized controlled trial. Cureus. 2021;13(12):e20353. doi:10.7759/cureus.20353
- Akbari H, Tabrizi R, Lankarani KB, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258:118167. doi:10.1016/j.lfs.2020.118167
- Oishi K, Mimura-Kimura Y, Miyasho T, et al. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine. 2013;61(1):84–89. doi:10.1016/j.cyto.2012.08.032
- Liu Q, Wang RS, Qu GQ, et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi:10.12116/j.issn.1004-5619.2020.01.005
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi:10.3760/cma.j.cn112151-20200312-00193
- Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi:10.1001/jamainternmed.2020.3539
- Eisner MD, Parsons P, Matthay MA, Ware L, Greene K; Acute Respiratory Distress Syndrome Network. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–988. doi:10.1136/thorax.58.11.983
- Tong M, Xiong Y, Zhu C, et al. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect Dis. 2021;21(1):737. doi:10.1186/s12879-021-06447-3
- Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021;203(1):54–66. doi:10.1164/rccm.202006-2405OC
- Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11):e0241955. doi:10.1371/journal.pone.0241955